Introduction
Post-cardiac arrest (CA) syndrome, or
post-resuscitation multiple organ dysfunction syndrome (PR-MODS),
has become an independent risk factor for the prognosis of patients
with successful resuscitation after CA (1). The pathogenesis of PR-MODS has not been
fully elucidated; however, it is currently considered that
intestinal mucosal ischemia-reperfusion injury triggered by CA is
an important factor leading to failure of multiple organs and
tissues, and even mortality in patients following cardiopulmonary
resuscitation (CPR) (1). The
mortality rate of patients with PR-MODS after CA was as high as 60%
in the United states in 2015 (2).
Current research on PR-MODS mainly focuses on the mechanism of
myocardial and brain injury after CA. The treatment options include
advanced respiratory cycle support and mild hypothermia for brain
protection (3,4). However, to the best of our knowledge,
research associated with the pathophysiological alterations
following CPR and corresponding prevention and treatment options
remain lacking.
Extracorporeal membrane oxygenation (ECMO) is an
extracorporeal technique of providing prolonged cardiac and
respiratory support to injured heart and lungs (5,6). This
intervention has been widely applied in children, but it is also
useful in adults with cardiac and respiratory failure (7,8). The
function of ECMO is to remove blood from the body of a patient and
to artificially remove carbon dioxide and oxygenating red blood
cells. ECMO is used either post-cardiopulmonary bypass or in late
stage treatment of a person with profound heart and/or lung failure
(9). This intervention is now
recognized as a treatment for CA (10,11),
whereas its effects after CA and resuscitation on intestinal
mucosal injury, and the potential mechanisms, to the best of our
knowledge, have not been studied.
Considering that ischemia-reperfusion injury is the
main pathophysiological mechanism of intestinal injury following
spontaneous CA (12), the present
study proposed to correct the acute hypoxic state of the intestinal
mucosa caused by hypoperfusion at the earliest time, which would
help to block or reverse intestinal mucosal ischemia-reperfusion
injury. The aim was to explore the protective effects of ECMO
following CPR on intestinal mucosal injury, and to assess the
potential mechanisms. The present findings may provide a basis for
an important strategy to improve the prognosis of patients that
have undergone CPR.
Materials and methods
Ethics statement
All experimental protocols followed in the present
study were approved by the Ethics Committee of Capital Medical
University (Beijing, China). The animals were kept in a
pathogen-free environment and were given free access to drinking
water and food. All procedures were performed strictly in
accordance with the Institutional Animal Care Instructions.
Animal preparation, tracheal
intubation and catheterization
Changbaishan domestic miniature swine purchased from
Beijing Huaxia Xingyang Biological Technology Co., Ltd. [license
no. SYXK (Beijing) 2017-0013] were used in the present study. A
total of 24 healthy male pigs (age, 11–13 weeks; weight, 28.3±2.5
kg) were selected and randomly divided into two groups: CPR group
(treated with CPR after CA; n=12) and CPR+ECMO group (treated with
ECMO during CPR after CA; n=12). Prior to the experimental
procedures, all animals were fasted of solids overnight, followed
by sedation using intramuscular injection with 10.0 mg/kg ketamine
and 0.5 mg/kg midazolam. Subsequently, anesthesia was induced by
ear vein injection with a bolus dose of 2.0 mg/kg propofol. The
swine were then secured on the operating table for the subsequent
procedures. All procedures were performed with aseptic surgical
techniques at room temperature (26°C), while the body temperature
of the animals was maintained at 37°C under an infrared lamp.
First, to maintain sufficient anesthetic and analgesic depth,
boluses of anesthetics (1.0 mg/kg propofol) and analgesics (4.0
µg/kg fentanyl) were administered intravenously. Propofol (9.0
mg/kg/h) and fentanyl (1.0 µg/kg/h) were delivered to maintain the
level of anesthesia and analgesia according to physiological
parameters, corneal and palpebral reflexes, and spontaneous
movement. There was no difference in body weight or other
characteristics, including the extra doses of propofol and fentanyl
administered during the preparatory phase between the CPR and
CPR+ECMO groups. At the end of the experiments (6 h after ROSC),
potassium chloride (74.5 mg/kg), in conjunction with general
anesthesia (9.0 mg/kg/h propofol and 1.0 µg/kg/h fentanyl), was
used to euthanize the animals.
The anesthetized animals were intubated with a
6.5-mm cuffed endotracheal tube into the trachea via tracheotomy,
and then mechanically ventilated with a volume-controlled
ventilator (Draeger vi 400; Draegerwerk AG & Co., KGaA) at a
tidal volume of 15.0 ml/kg and a respiratory frequency of 12–20/min
with an inspired oxygen fraction of 35% at the beginning. The
end-tidal capnography (CO2SMO Plus!® monitor;
Respironics, Inc.) was used to monitor the partial pressure of
carbon dioxide. Adequate baseline ventilation was determined using
an arterial blood gas analyzer (ABL80; Radiometer Medical ApS).
Three surface electrodes were placed on the fore
limbs of the animals to configure a standard lead II
electrocardiogram (ECG) as previously described (13). The ECG was recorded using a
multichannel physiological recorder (BL-420 F Data Acquisition and
Analysis system; Chengdu TME Technology Co., Ltd.).
To induce ventricular fibrillation (VF) and maintain
intravenous fluid therapy, a 5-Fr pacing catheter was placed into
the right ventricle through the right external jugular vein. To
measure the mean arterial pressure (MAP), a fluid-filled catheter
(5 Fr; Terumo Corporation) was inserted into the aortic arch
through the left femoral artery. A Swan-Ganz catheter (7 Fr;
Edwards Lifesciences Corporation) was placed in the pulmonary
artery through the left femoral vein to measure cardiac output (CO)
using the thermodilution method (12). For animals in the ECMO group, a 14-Fr
peripheral venous catheter (Dragon Laifu Medical Products Co.,
Ltd.) was additionally placed into the right atrium through the
left internal jugular vein, as well as a 12-Fr artery catheter into
the ascending aorta through the right femoral artery. All catheters
were filled with heparinized normal saline solution (5.0 U/ml) to
prevent clotting.
Hemodynamic parameters were monitored using an HP
monitor (M1165; Hewlett-Packard Co.). Normal saline solution (5.0
ml/kg/h) and colloidal fluid (5.0 ml/kg/h) were infused
intraoperatively to replenish fluid losses.
Extracorporeal life support
The ECMO circuit consisted of one arterial input
catheter, one venous output catheter, a Levitronix CentriMag
console (Levitronix GmbH), a centrifugal pump head (Maquet
Cardiopulmonary AG; Getinge AG) and a mechanical gas blender
(Thoratec Corporation). The circuit was driven by the centrifugal
pump head in line with the Adult Microporous Membrane Oxygenator
with Bioline Coating (Maquet Cardiopulmonary AG).
The left internal jugular vein and the right femoral
artery were cut. The ECMO circuit connected to the 16-Fr venous
outflow cannula was placed in the left internal jugular vein and
the 14-Fr arterial inflow cannula was placed in the right femoral
artery. The catheters were filled with heparinized saline solution
(5.0 U/ml) and clamped. The circuit was primed with colloid
solution (1,000 ml), preheparinized with 250 U/kg heparin, and had
50% oxygen air mix delivered to the oxygenator. The oxygen/air flow
was repeatedly adjusted to maintain pO2 and
pCO2 in the blood. A temperature of 37°C was maintained
with a water-circulating heat exchanger. VF was induced by a
programmed electrical stimulation instrument (GY-600A; Kaifeng
Huanan Instrument Co.) with mode S1S2 (300/200 ms), 40 V, 8:1
proportion and −10-ms step length. VF was confirmed by the presence
of a characteristic ECG tracing and an immediate drop in arterial
blood pressure.
Experimental protocol
Animals were prepared according to the
aforementioned procedure and the ECMO circuit was placed. Then, the
animals were permitted to rest for 30 min to achieve a steady
resting level and the baseline values of heart rate, MAP and CO
were measured. Subsequently, VF was induced in all animals in the
right ventricular apex until it was verified by the ECG trace,
accompanied by a drop of arterial blood pressure. Mechanical
ventilation was stopped when VF was induced successfully. After 12
min, CPR was performed manually for 2 min in animals from the
CPR+ECMO and CPR groups according to the 2015 guidelines for CPR
(14). The depth of chest
compression was ~1/3 of the anteroposterior diameter. A
HeartstartMRx monitor/defibrillation with Q-CPR (Philips Medical
Systems, Inc.) was used to ensure the quality of the compressions.
A bag respirator with room air was used to ventilate the pigs, and
the compression-to-ventilation ratio was 30:2. Subsequently, CPR
was performed for 4 min followed by shock attempts in the CPR group
as previously described (15,16).
After the shock attempts, CPR was continuously performed in the CPR
group and CPR combined with ECMO was continuously performed in the
CPR+ECMO group until the end of the experiment. Defibrillation was
performed using diphase 4.0 J/kg using a Heartstart M3535A
defibrillator (Philips Medical Systems, Inc.) for the first attempt
between the right infraclavicular electrode and the apical
electrode. When necessary, additional epinephrine (0.02 mg/kg) was
injected into the right atrium for the restoration of spontaneous
circulation (ROSC).
The endpoint of the aforementioned experiments was 6
h after ROSC or the death of the animals in both groups. ROSC was
considered to occur when MAP was >60 mmHg for 20 min or systolic
blood pressure was >80 mmHg. CPR was stopped in the CPR group
when ROSC was achieved. Otherwise, CPR and defibrillation were
performed alternately in animals in the CPR group. For the CPR+ECMO
group, the flow rate of ECMO was maintained between 3 and 5 l/min
to maintain MAP >60 mmHg. To maintain the level of
pO2 within 100–120 mmHg, the oxygen flow was adjusted
using the oxygenator. Animals were ventilated during the
reperfusion of ECMO to keep end-tidal pCO2 within 35–45
mmHg by adjusting the respiratory frequency. The activated clotting
time was maintained at >250 sec. CPR was considered to have
failed if spontaneous circulation was not restored within 30 min.
Animals that were alive 6 h post-ROSC were sacrificed using
potassium chloride (74.5 mg/kg), in conjunction with general
anesthesia (9.0 mg/kg/h propofol and 1.0 µg/kg/h fentanyl),
administered intravenously.
Hemodynamic parameters
Hemodynamic parameters, including heart rate (HR),
MAP and CO, were monitored. HR was recorded by the standard lead II
ECG. MAP was measured with a fluid-filled catheter advanced from
the left femoral artery into the aortic arch through a pressure
transducer with pulse indicator continuous CO system. A Swan-Ganz
catheter (7 Fr; Edwards Lifesciences Corporation) was advanced from
the left femoral vein and flow directed into the pulmonary artery
to measure CO by the thermodilution method (17). All values were recorded prior to VF
for the baseline value, at ROSC (ROSC0), and various time points
after ROSC, including 1 (ROSC1), 2 (ROSC2), 3 (ROSC3), 4 (ROSC4)
and 6 h (ROSC6).
Serum and intestinal tissue acute
injury biomarkers
Blood samples (2.5 ml) were collected in 0.109 mol/l
trisodium citrate (9:1 v/v) concomitantly at the following time
points: Before VF (baseline), ROSC0, ROSC1, ROSC2, ROSC4 and ROSC6.
Blood samples were acquired from the left femoral by intravenous
cannulation. The initial 5 ml blood sample was discarded due to
possible inclusion of heparinized normal saline solution.
Subsequently, the samples were immediately centrifuged at 6,000 × g
for 10 min at 4°C, and the supernatants were stored at −80°C until
further analysis.
The levels of interleukin (IL)-1 (cat. no. DY6226),
IL-6 (cat. no. P6000B), tumor necrosis factor α (TNF-α; cat. no.
DY690B) in the blood samples as well as in the intestinal tissue
homogenates were measured using an ELISA kit (R&D Systems,
Inc.) according to manufacturer's protocols.
Acute injury biomarkers in the blood samples and the
intestinal tissue homogenates were detected using commercial
analysis kits, according to the manufacturers' protocols. Primary
analysis kits were used to measure the levels of malondialdehyde
(MDA; cat. no. ab233471; Abcam), superoxide dismutase (SOD, cat.
no. ab65354; Abcam) and myeloperoxidase (MPO; cat. no. ab105136,
Abcam).
ELISA (Beijing Solarbio Science & Technology
Co., Ltd.) was used to measure the expression of Na/K-ATPase and
Ca-ATPase (cat. nos. BC0065 and BC0965, respectively) in the
intestinal mucosa homogenate.
Intestine histology examination
Samples from the intestine were fixed with 10%
formalin at 4°C overnight, dehydrated and embedded in paraffin, cut
into sections (10 µm), stained with hematoxylin solution for 5 min
and with eosin for 3 min at room temperature, and observed under a
light microscope (magnification, 200×; CX41; Olympus
Corporation).
Intestine ultrastructure
examination
Fresh intestine tissues were cut into pieces
(1.0×1.0×1.0 mm), treated with 3% glutaraldehyde at 4°C for 30 min,
flushed with PBS, fixed with 1% perosmic acid (4°C, 30 min) and
dehydrated with acetone. The tissues were infiltrated with a
mixture of propylene oxide and epoxy resin overnight and embedded
in resin. The tissues were then cut into serial ultrathin sections
(70 nm) and stained with 4% uranyl acetate for 20 min followed by
0.5% lead citrate for 5 min. The sections were examined using
transmission electron microscopy (magnification, ×10,000; JEM-1010;
JEOL, Ltd.). The ultrastructure of the intestinal mucosal
epithelial cells, including microvilli and mitochondria, was
observed.
Intestine immunostaining
Immunostaining was performed on the fixed intestine
tissues using a standard protocol with primary antibodies (18). Briefly, the intestine tissue was
fixed in 10% formalin at 4°C overnight, embedded in paraffin and
sliced into 5-µm sections. The slides were deparaffinized and
incubated with 20.0 mg/ml proteinase K for 15 min at 4°C.
Endogenous peroxidase activity was inhibited using 3% hydrogen
peroxide at room temperature for 10 min. The slides were incubated
with the primary antibodies, including monoclonal anti-Bax and
anti-Bcl-2 antibodies (both 1:10,000 dilution; Bcl-2: cat. no.
2870; Bax, cat. no. 5023; Cell Signaling Technology, Inc.) at 4°C
overnight followed by incubation with the secondary horseradish
peroxidase-conjugated goat anti-rabbit IgG antibody (1:1,000
dilution; cat. no. A16104SAMPLE; Thermo Fisher Scientific, Inc.).
The staining results were observed under a light microscope
(magnification, ×200; CX41; Olympus Corporation). Two investigators
blinded to the groups semi-quantitatively scored the slides by
evaluating the staining intensity in four fields of each image as
described (18).
TUNEL assay
Apoptosis was examined using a TUNEL apoptosis
detection kit (EMD Millipore), according to the manufacturer's
protocol. In brief, the intestine tissue was fixed in 10% formalin
at 4°C overnight, embedded in paraffin and sliced into 5-µm
sections. The slides were deparaffinized, rehydrated and incubated
with 20.0 mg/ml proteinase K for 15 min at 4°C. Endogenous
peroxidase activity was inhibited using 3% hydrogen peroxide.
Sections were then incubated with terminal deoxynucleotidyl
transferase enzyme at 37°C for 1 h. Staining was revealed using the
3,3-diaminobenzidine chromogen and the nucleus was counterstained
by hematoxylin for 5 min at room temperature. Mounting Medium (cat.
no. ab64230; Abcam) was applied to mount the slide and the staining
was observed under a light microscope (CX41; Olympus Corporation).
Four fields in each slide were randomly analyzed (magnification,
×200). The grey value was analyzed using ImageProPlus software
(version 7.0; Media Cybernetics, Inc.) as previously described
(18). At ≥4 fields of view were
randomly selected from each slide and analyzed.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software (version 5; GraphPad Software, Inc.). All
quantitative data are expressed as the mean ± SD from 12
experimental repeats. Comparisons between two groups of
quantitative data were performed by Student's t-test when both sets
of data fit the normal distribution and the homogeneity of variance
was accepted. A non-parametric Mann-Whitney test was used when any
set of data did not fit the normal distribution. Comparisons among
multiple groups of quantitative data were performed using repeated
one-way ANOVA when the data fit normal distribution, followed by
rank-sum test (Mann-Whitney rank) and Bonferroni post hoc test.
Log-rank (Mantel-Cox) test was used to analyze survival rates.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Basic characteristics and variables of
the animals
No significant differences were identified in body
length, body weight, dose of anesthetics and animal preparation
time between the two groups (P>0.05; Table I).
 | Table I.Variables of the animals in the CPR
and CPR+ECMO groups. |
Table I.
Variables of the animals in the CPR
and CPR+ECMO groups.
| Index | CPR group | CPR+ECMO group | P-value |
|---|
| Body length,
cm | 107.2±2.9 | 105.8±3.6 | 0.3 |
| Body weight,
kg | 34.17±4.4 | 35.42±3.2 | 0.4 |
| Propofol, mg | 202.8±3.9 | 203.9±4.5 | 0.5 |
| Fentanyl, µg | 125.4±6.2 | 130.2±11.7 | 0.2 |
| Preparation time,
min | 135.4±10.3 | 135.4±13.7 | 1.0 |
CPR+ECMO treatment and mortality
As shown in Fig. 1,
two pigs died 2 h after CPR in the CPR group due to irreversibly
decreased blood pressure and blood oxygen. At 4 h, two more pigs
died. The overall mortality rate in the CPR group was 33.3%. On the
other hand, no rapid mortality was observed in the CPR+ECMO group.
The 6 h-survival rate was 100%, suggesting that ECMO may improve
the prognosis of animals with CA.
ECMO treatment improves hemodynamic
parameters
The basal HR and CO of the animals in the two groups
were not significantly different (CPR vs. CPR+ECMO; HR, 119.2±6.7
vs. 121.1±6.4 bpm; CO, 3.7±0.5 vs. 4.0±0.8 l/min; both P>0.05;
Fig. 2). When compared with that in
the CPR group, the HR of the animals in the CPR+ECMO group was
significantly decreased at 0, 1, 2, 3, 4 and 6 h after successful
resuscitation (all P<0.01), and CO was increased significantly
at each time point (all P<0.01).
 | Figure 2.Comparison of hemodynamic parameters
between the two groups. (A) Comparison of ventricular rate between
animals from the CPR and the CPR+ECMO groups at baseline and at 0,
1, 2, 3, 4 and 6 h after successful resuscitation. (B) CO was
compared between animals from the CPR and CPR+ECMO groups at
baseline, as well as 0, 1, 2, 3, 4 and 6 h after successful
resuscitation. Data at each time point are presented as the mean ±
SD. n=12 in both groups. **P<0.01 and ***P<0.001 vs. CPR. CO,
cardiac output; CPR, cardiopulmonary resuscitation; ECMO,
extracorporeal membrane oxygenation; HR, heart rate; ROSC,
restoration of spontaneous circulation. |
ECMO decreases the levels of
inflammatory factors in the circulation and intestinal mucosa
On a tissue level, compared with levels in the CPR
group, TNF-α, IL-1, IL-6 and MPO levels in the tissue homogenates
of mucosa specimens 6 h after ROSC were significantly decreased in
the CPR+ECMO group, whereas the levels of
Na+/Ca2+-ATPase in the tissue were
significantly increased (Fig. 3A-F).
On a serum level, compared with those recorded in the CPR group,
the levels of TNF-α, IL-1 and IL-6 measured in the serum of animals
from the CPR+ECMO group appeared to be decreased, though the
difference was not statistically significant (Fig. 3G-I).
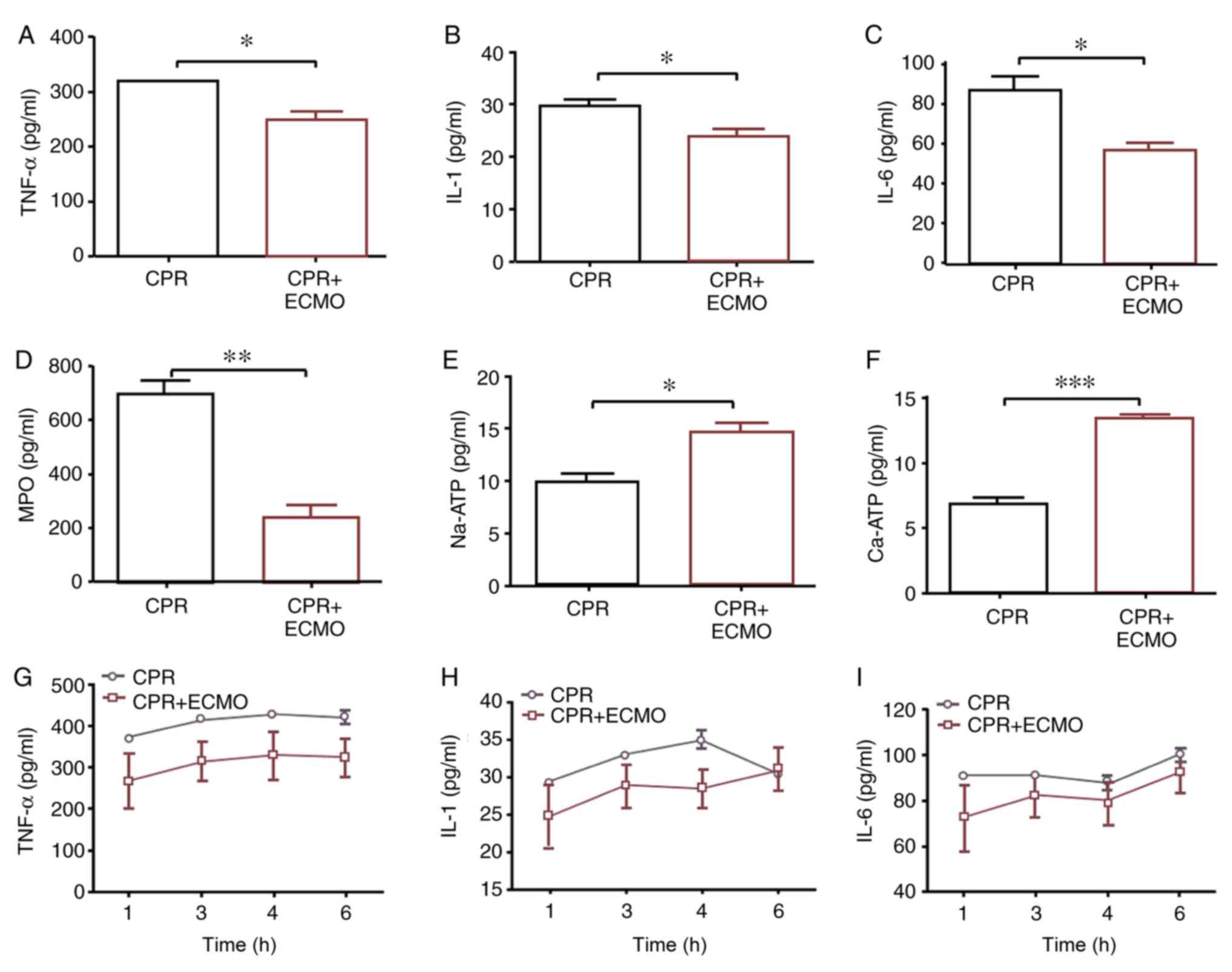 | Figure 3.Comparison of expression levels of
inflammation-associated mediators in circulation or intestinal
mucosa between the two groups. Expression levels of (A) tissue
TNF-α, (B) tissue IL-1, (C) tissue IL-6, (D) tissue MPO, (E) tissue
Na-ATP and (F) tissue Ca-ATP, and (G) serum TNF-α, (H) serum IL-1
and (I) serum IL-6. Data are expressed as the mean ± SD. n=12.
*P<0.05; **P<0.01; ***P<0.001. CPR, cardiopulmonary
resuscitation; ECMO, extracorporeal membrane oxygenation; IL,
interleukin; MPO, myeloperoxidase; TNF-α, tumor necrosis factor
α. |
ECMO enhances SOD and decreases MDA in
intestinal mucosa
Compared with those in the CPR group, the SOD levels
were significantly higher in the CPR+ECMO group, whereas the levels
of tissue MDA were significantly lower (Fig. 4).
ECMO reduces intestinal mucosal
epithelial barrier damage
HE staining demonstrated that the intestinal mucosal
epithelial cells in the CPR+ECMO group exhibited significantly
reduced congestion and edema compared with those in the CPR group.
The mucosal barrier was almost intact in the CPR+ECMO group, and
the infiltration of sub-epithelial inflammatory cells was
significantly reduced (Fig. 5A),
suggesting that ECMO can reduce intestinal epithelial mucosal
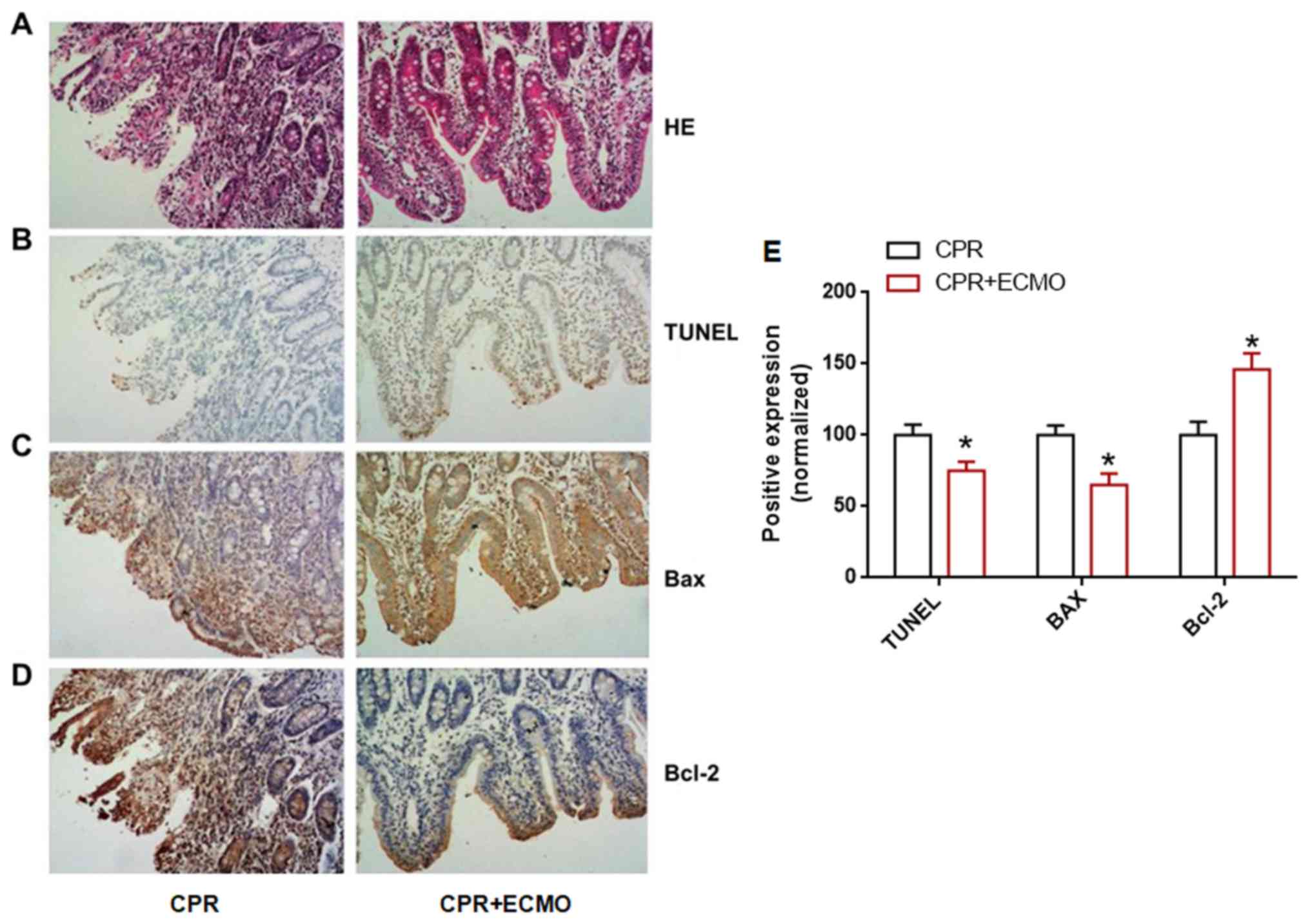
barrier damage. TUNEL staining revealed that the number of
TUNEL-positive cells in the intestinal mucosal epithelium of the
CPR+ECMO group was significantly lower than that of the CPR group
(Fig. 5B and E), suggesting that
ECMO treatment can inhibit intestinal epithelial cell apoptosis
after CA. In addition, immunohistochemistry revealed that the
expression levels of anti-apoptotic protein Bcl-2 in the intestinal
mucosa of the CPR+ECMO treatment group were higher than those in
the CPR group, whereas the expression of apoptosis-associated
protein Bax was significantly lower than that in the CPR group
(Fig. 5C-E). This further supports
the hypothesis that ECMO treatment inhibits apoptosis in intestinal
mucosal epithelial cells. Finally, electron microscopy revealed
that the degree of apoptosis and necrosis of the intestinal mucosa
in the CPR+ECMO group was markedly lower than that in the CPR group
(Fig. 6).
 | Figure 6.Electron microscopy results of
intestinal mucosa tissues from animals in the two groups. (A)
Representative electron microscopy images of intestinal mucosa
specimens from the CPR group (left panels) and the CPR+ECMO group
(right panels). The intestinal diffuse epithelial cells of
intestinal mucosa in CPR group had severe degeneration and
necrosis, basement membrane rupture, unclear demarcation, lysosome
increase, (B) cell gap enlargement, tight junction disappearance,
and breakdown of microvilli on the surface of epithelial cells. (C)
The nuclei of epithelial cells were concentrated, chromatin edges
were gathered, and the nuclear membrane was invaded to form
apoptotic bodies, showing apoptotic state in CPR group. Arrows
indicate injury of the tissue. Scale bar, 5 µm. CPR,
cardiopulmonary resuscitation; ECMO, extracorporeal membrane
oxygenation. |
Discussion
The present study induced VF in pigs and examined
whether CPR+ECMO could help improve CA outcome compared with
regular CPR. The results demonstrated that ECMO could significantly
reduce acute mortality after CA, and improve the hemodynamic
parameters. Pathophysiological results revealed that the intestinal
mucosa of the CPR group exhibited obvious congestion and edema,
cell shedding and villous necrosis at 6 h after resuscitation.
Patients who have suffered CA have ineffective
circulatory blood pressure, and their intestines are in a
non-perfused state (19,20). A previous study reported that a 4-min
CA can lead to intestinal hypoperfusion for up to 60 min (21). Due to insufficient effective
perfusion, the intestinal tissue is severely ischemic and hypoxic,
resulting in ischemic and hypoxic damage to the epithelial villus
of the intestinal mucosa, which leads to intestinal mucosal
epithelial edema, cell necrosis, shedding, and even complete
mucosal shedding and ulceration (22). In addition to intestinal mucosal
damage caused by direct ischemia and hypoxia, ischemia-reperfusion
injury is an important pathophysiological mechanism of intestinal
mucosal injury following CA (23).
When ischemia-reperfusion occurs, intestinal mucosa tissue may be
enriched with inflammatory cells that secrete a large number of
inflammatory mediators, cytokines, toxins, oxygen free radicals,
endotoxins and intestinal bacteria into the bloodstream, which
eventually leads to apoptosis and multiple organ failure (24). ECMO is an emerging in vitro
life-support system that allows the body to perform extracorporeal
blood circulation and effective gas exchange in the absence of its
own cardiopulmonary circulation, thereby maintaining the effective
perfusion and oxygenation of organs (25). Therefore, ECMO has been used for
acute breathing for patients with acute respiratory distress
syndrome, cardiopulmonary failure, sepsis and cardiogenic shock
(26,27). The results of the present study
suggest that early CPR with ECMO treatment can quickly halt the
acute ischemia and hypoxia in the intestinal mucosa, improving the
prognosis of patients. Furthermore, ECMO can block intestinal
ischemia-reperfusion injury in CA model animals to some extent.
Propofol has been reported to exhibit an anti-arrhythmic effect
(28,29). In the present study, the difference
between the dosage of propofol used in the two groups of animals
was not statistically significant. Thus, the dosage administered in
the two groups was comparable. It can be concluded that the
influence of propofol in the two groups of animals was similar, or
that the effect was not statistically different even if propofol
had an anti-arrhythmic effect. Therefore, the dosage of propofol
would not have affected the mortality or other results between the
two groups.
Currently, the majority of studies in the field of
CPR treatment focus on whether hypothermia treatment can improve
intestinal mucosal barrier damage (30,31).
However, the mechanism of intestinal mucosal injury after CA is not
fully understood and, therefore, research on associated
interventions is still in progress (32). Preventing acute ischemia and hypoxia
as well as acute ischemia-reperfusion injury is fundamental to
prevent intestinal mucosal barrier damage after CPR and to prevent
PR-MODS (33). The present study
revealed that timely initiation of ECMO treatment after CA could
significantly improve hemodynamic disorders, restore tissue
perfusion and oxygen supply, correct hypoxemia, reduce the systemic
inflammatory response and the oxidative stress response, and
thereby reduce the ischemia-reperfusion injury of intestinal mucosa
and the incidence and mortality of PR-MODS. The results suggest
that ECMO treatment can effectively prevent PR-MODS from occurring
in model animals after CPR in the acute phase of CA. However, it
remains unclear whether ECMO can improve the prognosis of patients
after CA and resuscitation. Furthermore, the long-term efficacy of
ECMO and the mechanisms of reducing the systemic inflammatory
response and intestinal mucosal barrier damage require additional
investigation.
In the animals in the CPR group, the expression
levels of MPO in circulating plasma and the intestinal mucosa were
significantly increased, suggesting an increase in neutrophil
infiltration (34). The inflammatory
mediators (TNF-α, IL-1 and IL-6) and acute phase-associated protein
(MPO) were significantly upregulated, suggesting that ‘inflammation
storms’ occurred systemically and locally (35). Overall, the intestinal mucosal
epithelial cells in CPR animals experienced excessive apoptosis and
the mucosal barrier was destructed. The timely treatment with ECMO
after CA significantly improved the circulation of the animals and
prevented inflammatory injury in the intestinal mucosa,
characterized by decreased neutrophil infiltration, decreased
inflammatory mediators, decreased cytokine expression, and reduced
apoptosis of intestinal mucosal epithelial cells. As previously
reported, SOD and MDA are opposing factors of cell survival
(36). SOD has a protective role,
while MDA has the opposite effect (36). The present study demonstrated that
ECMO increased the SOD level and reduced the MDA level. These data
reveal the protective effect of ECMO.
The intestinal mucosal epithelial layer is composed
of a single layer of columnar epithelial cells, including
absorbing, goblet and Paneth cells. The normal intestinal mucosal
barrier is composed of heterogeneous elements, including a
mechanical barrier that is made of tight junctions between the
absorbed cells, an immunological barrier composed of defensing and
lysozyme secreted by Paneth cells, and a chemical barrier composed
of mucus secreted by the goblet cells (37). In this present study, typical
morphological changes were observed in CPR group according to the
images obtained from TEM, but not in those from the CPR+ECMO
group.
The present study had several additional
limitations. First, the physiology of the swine model may not
reflect the situation in patients suffering from CA, and the
results should be confirmed in clinical practice. Secondly, all the
catheters of ECMO circuits were placed in advance; however, in the
clinical practice, there exists some difficulty in cannulating.
Thirdly, the experimental endpoint was 6 h after ROSC and the
observation duration was relatively short. Fourthly, miniature
swine were selected in the present study. The animal size may also
affect the outcomes of CPR and ECMO, which may cause bias. Finally,
the sample size in the present study was limited and a study with a
larger sample size should be performed in the future.
In conclusion, ECMO treatment may correct intestinal
mucosal ischemia-reperfusion injury in a timely manner, reduce the
incidence of PR-MODS after CPR in CA, and thereby improve the
prognosis of patients and reduce acute mortality.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural
Science Foundation of China (grant no. 81372025).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL, CSL, BL, QZ, XY, YZ, JL and LZ performed the
experiments and analyzed the data. YL and LZ designed the study and
wrote the manuscript.
Ethics approval and consent to
participate
All experimental protocols followed in the present
study were approved by the Ethics Committee of Capital Medical
University (Beijing, China). All procedures were performed strictly
in accordance with the Institutional Animal Care Instructions.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mentzelopoulos SD and Zakynthinos SG:
Post-cardiac arrest syndrome: Pathological processes, biomarkers
and vasopressor support, and potential therapeutic targets.
Resuscitation. 121:A12–A14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Callaway CW, Donnino MW, Fink EL, Geocadin
RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM
and Zimmerman JL: Part 8: Post-cardiac arrest care: 2015 American
heart association guidelines update for cardiopulmonary
resuscitation and emergency cardiovascular care. Circulation 132
(18 Suppl 2). S465–S482. 2015.
|
|
3
|
Perman SM, Grossestreuer AV, Wiebe DJ,
Carr BG, Abella BS and Gaieski DF: The utility of therapeutic
hypothermia for post-cardiac arrest syndrome patients with an
initial nonshockable rhythm. Circulation. 132:2146–2151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jahandiez V, Cour M, Bochaton T, Abrial M,
Loufouat J, Gharib A, Varennes A, Ovize M and Argaud L: Fast
therapeutic hypothermia prevents post-cardiac arrest syndrome
through cyclophilin D-mediated mitochondrial permeability
transition inhibition. Basic Res Cardiol. 112:352017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bacon MK, Gray SB, Schwartz SM and Cooper
DS: Extracorporeal membrane oxygenation (ECMO) support in special
patient populations-the bidirectional glenn and fontan
circulations. Front Pediatr. 6:2992018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu XY, Zhuang ZQ, Zheng RQ and Liu SQ:
Extracorporeal membrane oxygenation as salvage therapy for acute
massive pulmonary embolism after surgery for tibiofibular
fractures. Chin Med J (Engl). 131:2611–2613. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hernandez Conte AT, Ng D, Ramzy D,
Dilibero D, LaBounty TM, Gaultier C and Behringer EC:
Extracorporeal membrane oxygenation in a 29-year-old man with
Pneumocystis jirovecii respiratory failure and AIDS. Tex
Heart Inst J. 45:254–259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim H, Paek JH, Song JH, Lee H, Jhee JH,
Park S, Yun HR, Kee YK, Han SH, Yoo TH, et al: Permissive fluid
volume in adult patients undergoing extracorporeal membrane
oxygenation treatment. Crit Care. 22:2702018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Panda BR, Prabhu A, Provenzano S and Karl
T: Use of extracorporeal life support for emergency coronary artery
bypass grafting. Interact Cardiovasc Thorac Surg. 16:897–899. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan BK: Extracorporeal membrane
oxygenation in cardiac arrest. Singapore Med J. 58:446–448. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conrad SA and Rycus PT: Extracorporeal
membrane oxygenation for refractory cardiac arrest. Ann Card
Anaesth. 20 (Suppl 1):S4–S10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan H, Chen D, Liu B, Xie X, Zhang J and
Yang G: Effects of sodium hydrosulfide on intestinal mucosal injury
in a rat model of cardiac arrest and cardiopulmonary resuscitation.
Life Sci. 93:24–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meek S and Morris F: ABC of clinical
electrocardiography. Introduction. I-Leads, rate, rhythm, and
cardiac axis. BMJ. 324:415–418. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maudet L, Carron PN and Trueb L:
Cardiopulmonary resuscitation: The essential of 2015 guidelines.
Rev Med Suisse. 12:313–317. 2016.(In French). PubMed/NCBI
|
|
15
|
Hang CC, Li CS, Wu CJ and Yang J: Acute
kidney injury after cardiac arrest of ventricular fibrillation and
asphyxiation swine model. Am J Emerg Med. 32:208–215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu CJ, Li CS, Zhang Y and Yang J:
Application of positron emission tomography in the detection of
myocardial metabolism in pig ventricular fibrillation and
asphyxiation cardiac arrest models after resuscitation. Biomed
Environ Sci. 27:531–536. 2014.PubMed/NCBI
|
|
17
|
Gawlinski A: Measuring cardiac output:
Intermittent bolus thermodilution method. Crit Care Nurse.
20:118–120, 122–114. 2000.PubMed/NCBI
|
|
18
|
Chen Y, Huang Y, Lu X, Wang G and Chi P:
Antitumor effects of the silencing of programmed cell death ligand
1 in colorectal cancer via immunoregulation. Oncol Rep.
40:3370–3380. 2018.PubMed/NCBI
|
|
19
|
MacFie J: Enteral versus parenteral
nutrition: The significance of bacterial translocation and
gut-barrier function. Nutrition. 16:606–611. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schroeder DC, Maul AC, Mahabir E, Koxholt
I, Yan X, Padosch SA, Herff H, Bultmann-Mellin I, Sterner-Kock A,
Annecke T, et al: Evaluation of small intestinal damage in a rat
model of 6 Minutes cardiac arrest. BMC Anesthesiol. 18:612018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wurm R, Cho A, Arfsten H, van Tulder R,
Wallmüller C, Steininger P, Sterz F, Tendl K, Balassy C,
Distelmaier K, et al: Non-occlusive mesenteric ischaemia in out of
hospital cardiac arrest survivors. Eur Heart J Acute Cardiovasc
Care. 7:450–458. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Korth U, Krieter H, Denz C, Janke C,
Ellinger K, Bertsch T, Henn C and Klein J: Intestinal ischaemia
during cardiac arrest and resuscitation: Comparative analysis of
extracellular metabolites by microdialysis. Resuscitation.
58:209–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patil KD, Halperin HR and Becker LB:
Cardiac arrest: Resuscitation and reperfusion. Circ Res.
116:2041–2049. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Parks DA and Granger DN: Contributions of
ischemia and reperfusion to mucosal lesion formation. Am J Physiol.
250:G749–G753. 1986.PubMed/NCBI
|
|
25
|
Millar JE, Fanning JP, McDonald CI,
McAuley DF and Fraser JF: The inflammatory response to
extracorporeal membrane oxygenation (ECMO): A review of the
pathophysiology. Crit Care. 20:3872016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tu Y, Jin Q, Sun R and Li Q:
Extracorporeal membrane oxygenation support for a multitrauma
patient with ARDS: A case report and literature review. Exp Ther
Med. 15:2062–2065. 2018.PubMed/NCBI
|
|
27
|
Tillmann BW, Klingel ML, Iansavichene AE,
Ball IM and Nagpal AD: Extracorporeal membrane oxygenation (ECMO)
as a treatment strategy for severe acute respiratory distress
syndrome (ARDS) in the low tidal volume era: A systematic review. J
Crit Care. 41:64–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Q, Kong AL, Chen R, Qian C, Liu SW,
Sun BG, Wang LX, Song LS and Hong J: Propofol and arrhythmias: Two
sides of the coin. Acta Pharmacol Sin. 32:817–823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Warpechowski P, dos Santos AT, Pereira PJ
and de Lima GG: Effects of propofol on the cardiac conduction
system. Rev Bras Anestesiol. 60:438–444. 2010.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Hauwermeiren F, Vandenbroucke RE,
Grine L, Lodens S, Van Wonterghem E, De Rycke R, De Geest N, Hassan
B and Libert C: TNFR1-induced lethal inflammation is mediated by
goblet and Paneth cell dysfunction. Mucosal Immunol. 8:828–840.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xiong W, Xu S, Li H and Liang K: Moderate
hypothermia ameliorates enterocyte mitochondrial dysfunction in
severe shock and reperfusion. J Surg Res. 200:250–259. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stub D, Bernard S, Duffy SJ and Kaye DM:
Post cardiac arrest syndrome: A review of therapeutic strategies.
Circulation. 123:1428–1435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Oliveira THC, Souza DG, Teixeira MM and
Amaral FA: Tissue dependent role of PTX3 during
ischemia-reperfusion injury. Front Immunol. 10:14612019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lowe PP, Gyongyosi B, Satishchandran A,
Iracheta-Vellve A, Ambade A, Kodys K, Catalano D, Ward DV and Szabo
G: Alcohol-related changes in the intestinal microbiome influence
neutrophil infiltration, inflammation and steatosis in early
alcoholic hepatitis in mice. PLoS One. 12:e01745442017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
D'Elia RV, Harrison K, Oyston PC,
Lukaszewski RA and Clark GC: Targeting the ‘cytokine storm’ for
therapeutic benefit. Clin Vaccine Immunol. 20:319–327. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu G, Wang X, Wu S, Li X and Li Q:
Neuroprotective effects of puerarin on
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson's
disease model in mice. Phytother Res. 28:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Martens EC, Neumann M and Desai MS:
Interactions of commensal and pathogenic microorganisms with the
intestinal mucosal barrier. Nat Rev Microbiol. 16:457–470. 2018.
View Article : Google Scholar : PubMed/NCBI
|