Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection, and it has been proven that there are bacteria
or highly suspicious focus of infection in patients' blood
(1). Currently, the incidence of
sepsis is extremely high worldwide and, according to statistics,
there are 270 patients with the disease per 100,000 people
(2). Moreover, the incidence is
increasing year by year (3). Sepsis
is dangerous and has a very high fatality rate, with a mortality of
15.00% (4). Due to its high
incidence and mortality, more effective diagnosis and treatment
schemes for the disease have been explored (5–7). With
the deepening of research and the development of medical technology
in recent years, remarkable progress has been made in
anti-infection treatment and organ support therapy, but the
fatality rate of sepsis remains high (8). Therefore, the effective treatment of
sepsis is still a major research difficulty.
At present, sepsis is mostly common in intensive
care unit (ICU) and usually complicated with organ dysfunction, so
the treatment of the disease is difficult (9). ICU patients with sepsis are clinically
treated by sedative treatment, because of the great discomfort and
pain caused by invasive operation for ICU patients (10). Studies have shown that traditional
anesthetics propofol, midazolam and dexmedetomidine have strong
sedative, analgesic and certain anti-inflammatory effects on ICU
patients with sepsis, and they can relieve the anxiety and
discomfort of the patients (11,12).
According to Fernando et al (13), propofol has a better
anti-inflammatory effect on rats with pneumonia. Yamamura et
al (14) have reported that
midazolam and dexmedetomidine have high safety during mechanical
ventilation of patients with sepsis. Studies worldwide are usually
limited to the sedative treatment with anesthetics, and which drug
is the most suitable for ICU patients with sepsis remains
controversial. Therefore, the effects of propofol, midazolam and
dexmedetomidine on the sedative treatment of ICU patients with
sepsis and on arterial blood gas (ABG) were analyzed in this study,
in order to determine the most suitable drug for the sedative
treatment and provide reliable guidance for clinical practice.
Patients and methods
General information
In total 429 ICU patients with sepsis, admitted to
Renji Hospital, School of Medicine, Shanghai Jiaotong University
(Shanghai, China) from May 2015 to January 2019, were selected as
research subjects for a prospective analysis. There were 308 males
and 121 females, 43–76 years of age with an average age of
56.15±10.84 years. The study was approved by the Ethics Committee
of Renji Hospital, School of Medicine, Shanghai Jiaotong
University. Patients who participated in this research had complete
clinical data and signed informed consents were obtained from the
patients or their guardians. Inclusion criteria: patients who met
the diagnostic criteria for sepsis (15); patients who needed sedative
treatment; patients who had complete medical records; patients who
had good compliableness; patients with complete medical records;
patients who cooperated with the arrangement of the medical staff
in this hospital; and ICU patients. Exclusion criteria: pregnant
women; patients who were allergic to drugs; patients with heart
failure (≥ grade III); patients with renal failure (Rifle criteria
≥F); patients with hepatic failure (total plasma protein, <30
g/l; bilirubin, >85 mol/l); patients with brain death; patients
who were transferred to another hospital; patients who were
bedridden for a long time; patients with mental illness; or
patients with physical disability.
Methods
All patients received basic treatment, such as,
anti-infection treatment, correction of shock and improvement of
microcirculation. On this basis, 152 patients who were treated with
propofol (Aspen Pharmacare; 1% w/v) for sedation served as group A,
146 patients who were treated with midazolam (Jiangsu Nhwa
Pharmaceutical Co., Ltd.; SFDA approval no. H10980025) for sedation
served as group B, and 131 patients who were treated with
dexmedetomidine (Jiangsu Hengrui Medicine Co., Ltd.; SFDA approval
no. H20090248) for sedation served as group C. Patients in group A
were continuously pumped with propofol using a micro pump at 0.05
mg/kg/min for analgesia, during which the patients were
intravenously injected with propofol with a loading dose of 1–3
mg/kg for 30–60 sec. After that, the patients were continuously
pumped with propofol using the micro pump and the dosage of
medication was 0.5–4 mg/kg/h. Analgesic treatment for patients in
group B was the same as for group A. After that, the patients were
continuously pumped with midazolam using the micro pump at 0.05
mg/kg/h with a speed of 1 ml/h. Analgesic treatment for patients in
group C was the same as for group A. After that, the patients were
intravenously pumped with dexmedetomidine with a loading dose of 1
µg/kg/h for 10 min, and then continuous intravenous pumping of
dexmedetomidine for sedative treatment was carried out at 0.2–0.7
µg/kg/h. Diastolic blood pressure (DBP), systolic blood pressure
(SBP) and heart rate (HR) were recorded before and after treatment
in all three groups. Arterial blood was extracted before and after
treatment from the patients of the three groups for blood gas
analysis. Venous blood was also extracted from the patients of the
three groups, before and after treatment, to detect cardiac
troponin T (cTnT) and creatine kinase-MB (CK-MB).
Observation indexes
For blood pressure function, DBP, SBP and HR were
recorded before and after treatment; for blood gas function, the
arterial partial pressure of oxygen (PaO2) and arterial
partial pressure of carbon dioxide (PaCO2) were recorded
before and after treatment; for cardiac function, cTnT and CK-MB
were recorded before and after treatment; the sedative effect was
evaluated using APACHE II score (16); the wake-up time, the length of ICU
stay, and the adverse reactions during the sedative treatment were
recorded. Incidence of adverse reactions = (number of adverse
reactions)/(total number) ×100%
Statistical analysis
SPSS 24.0 (Beijing Strong-Vinda Information
Technology Co., Ltd.) was used to statistically analyze the
experimental results, and GraphPad 8 (Softhead Inc.) to plot the
figures and check the results. Count data, such as sex, were
expressed as rate and Chi-square test was used for their comparison
within groups. Measurement data, such as DBP and SBP, were
expressed as the mean ± standard deviation and t-test was used for
their comparison within groups. One-way analysis of variance and
LSD post hoc test were used for comparisons between multiple
groups. P<0.050, was considered to indicate a statistically
significant difference.
Results
Comparison of general information
There was no significant difference among groups A,
B and C in age, body mass index (BMI), white blood cells (WBC), red
blood cells (RBC), platelet (PLT), sex, place of residence,
ethnicity, combined diseases, surgery and chemotherapy
(P>0.050), indicating that the three groups of patients were
comparable (Table I).
 | Table I.Comparison of general information [n
(%)]. |
Table I.
Comparison of general information [n
(%)].
| Factor | Group A (n=152) | Group B (n=146) | Group C (n=131) | χ2 or F
value | P-value |
|---|
| Age (years) | 55.27±11.26 | 54.98±11.57 | 55.09±10.84 | 0.025 | 0.975 |
| BMI
(kg/cm2) | 21.87±5.37 | 21.26±5.08 | 22.05±5.86 | 0.824 | 0.440 |
| WBC
(×109/l) | 9.24±2.85 | 9.63±3.04 | 9.55±2.77 | 0.754 | 0.471 |
| RBC
(×1012/l) | 5.83±1.26 | 5.98±1.53 | 5.77±1.30 | 0.879 | 0.416 |
| PLT
(×109/l) | 284.62±36.24 | 278.66±35.25 | 280.69±31.09 | 1.157 | 0.316 |
| Sex |
|
|
| 0.846 | 0.655 |
| Male | 110 (72.37) | 101 (69.18) | 97 (74.05) |
|
|
|
Female | 42
(27.63) | 45
(30.82) | 34 (25.95) |
|
|
| Place of
residence |
|
|
| 0.891 | 0.640 |
| City | 127 (83.55) | 116 (79.45) | 108 (82.44) |
|
|
|
Countryside | 25
(16.45) | 30
(20.55) | 23
(17.56) |
|
|
| Ethnicity |
|
Han | 144 (94.74) | 136 (93.15) | 126 (96.18) |
|
|
|
Minority | 8
(5.26) | 10 (6.85) | 5
(3.82) |
|
|
| Combined
diseases |
|
|
| 2.141 | 0.995 |
|
Tumor | 74 (48.68) | 66 (45.21) | 65 (49.62) |
|
|
| Severe
pneumonia | 32 (21.05) | 27 (18.49) | 26 (19.85) |
|
|
| Severe
trauma | 15 (9.87) | 16 (10.96) | 12 (9.16) |
|
|
| Severe
cholangitis | 12 (7.89) | 15 (10.27) | 11 (8.40) |
|
|
| Severe
pancreatitis | 8 (5.26) | 10 (6.85) | 9 (6.87) |
|
|
| Diffuse
peritonitis | 11 (7.24) | 12 (8.22) | 8 (6.11) |
|
|
| Surgery |
|
|
| 0.597 | 0.742 |
|
Yes | 75 (49.34) | 69 (47.26) | 68 (51.91) |
|
|
| No | 77 (50.66) | 77 (52.74) | 63 (48.09) |
|
|
| Chemotherapy |
|
|
| 0.523 | 0.770 |
|
Yes | 34
(22.37) | 29
(19.86) | 25
(19.08) |
|
|
| No | 118 (77.63) | 117 (80.14) | 106 (80.92) |
|
|
Comparison of blood pressure
function
There was no significant difference among groups A,
B and C in HR, SBP and DBP before or after treatment (P>0.050).
After treatment, DBP and SBP in the three groups were significantly
higher than those before treatment (P<0.001); HR in the three
groups after treatment was significantly lower than that before
treatment (P<0.001) (Figs.
1–3).
Comparison of blood gas function
Among groups A, B and C there was no significant
difference in PaO2 and PaCO2 before or after
treatment (P>0.050). Also, there was no significant difference
before and after treatment in the three groups in terms of
PaO2 or PaCO2 (P>0.050; Table II).
 | Table II.Comparison of blood gas function. |
Table II.
Comparison of blood gas function.
| Blood gas | Group A
(n=152) | Group B
(n=146) | Group C
(n=131) | F value | P-value |
|---|
| PaO2
(mmHg) |
| Before
treatment | 75.23±6.84 | 76.18±7.05 | 74.39±6.97 | 2.300 | 0.102 |
| After
treatment | 76.52±6.79 | 75.18±6.84 | 75.33±7.14 | 1.669 | 0.190 |
| PaCO2
(mmHg) |
| Before
treatment | 38.94±6.85 | 39.14±7.04 | 39.20±6.90 | 1.368 | 0.256 |
| After
treatment | 39.08±6.54 | 38.68±6.97 | 37.84±7.10 | 1.180 | 0.308 |
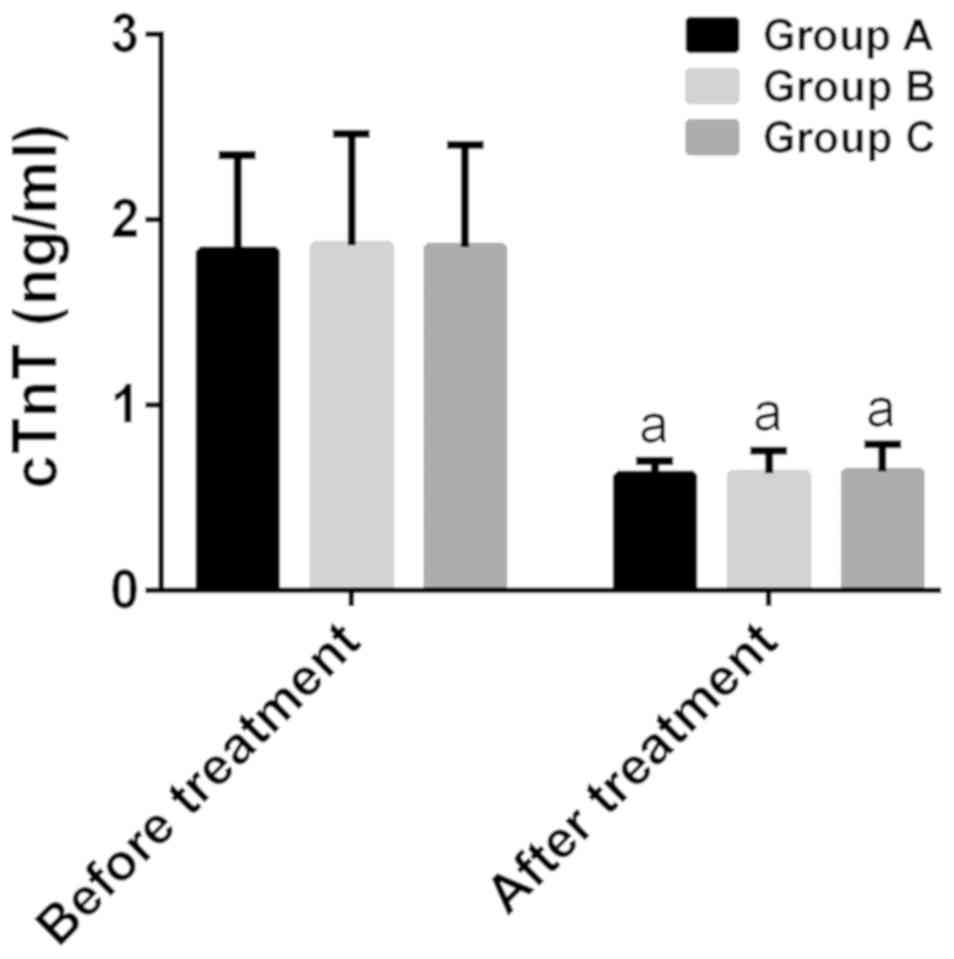
Comparison of cardiac function
There was no significant difference among groups A,
B and C in cTnT and CK-MB before treatment, as well as cTnT after
treatment (P>0.050). After treatment, cTnT and CK-MB in group A
were 0.62±0.08 and 2.68±0.88 ng/ml, respectively, in group B were
0.63±0.12 and 2.63±0.84 ng/ml, respectively, and in group C were
0.64±0.15 and 2.16±0.44 ng/ml, respectively. After treatment, cTnT
and CK-MB in the three groups were significantly lower than those
before treatment (P<0.001), and there was a statistically
significant difference among the three groups in CK-MB
(P<0.001). After treatment, there was no statistically
significant difference between groups A and B in CK-MB
(P>0.050), while in group C the value of CK-MB was significantly
lower than that in groups A and B (P<0.001) (Figs. 4 and 5).
Comparison of sedative effects
There was no significant difference among groups A,
B and C in APACHE II score before treatment and length of ICU stay
(P>0.050), whereas after treatment, there were statistically
significant differences among the three groups in the wake-up time
and APACHE II score (P<0.001). After treatment, there was no
significant difference between groups A and B in APACHE II score
(P>0.050), while in group C the score was significantly lower
than that in groups A and B (P<0.001). The wake-up time in group
A was significantly longer than that in groups B and C
(P<0.001), and the time in group B was longer than that in group
C (P<0.001) (Table III).
 | Table III.Comparison of sedative effects. |
Table III.
Comparison of sedative effects.
| Variable | Group A
(n=152) | Group B
(n=146) | Group C
(n=131) | F value | P-value |
|---|
| APACHE II before
treatment | 18.67±4.26 | 19.02±4.63 | 18.87±4.30 | 0.237 | 0.789 |
| APACHE II after
treatment | 14.82±2.05 | 14.63±2.28 |
11.67±1.69a,b | 103.324 | <0.001 |
| Wake-up time
(min) | 120.37±28.15 |
98.75±21.39c |
71.57±22.36c,d | 142.308 | <0.001 |
| Length of ICU stay
(days) | 7.82±3.25 | 7.93±3.52 | 7.75±3.35 | 0.101 | 0.904 |
Comparison of adverse reactions
The incidence of adverse reactions was 3.95% in
group A, 4.79% in group B and 3.82% in group C, without a
statistically significant difference among the three groups
(P>0.050) (Table IV).
 | Table IV.Comparison of adverse reactions [n
(%)]. |
Table IV.
Comparison of adverse reactions [n
(%)].
| Adverse
reaction | Group A
(n=152) | Group B
(n=146) | Group C
(n=131) | χ2
value | P-value |
|---|
| Heat | 2 (1.32) | 3 (2.05) | 2 (1.53) |
|
|
| Chill | 0 (0.00) | 1 (0.68) | 2 (1.53) |
|
|
| Fast heart
rate | 2 (1.32) | 2 (1.37) | 1 (0.76) |
|
|
| Nausea and
vomiting | 2 (1.32) | 1 (0.68) | 0 (0.00) |
|
|
| Total incidence
(%) | 3.95 | 4.79 | 3.82 | 0.200 | 0.905 |
Discussion
Sepsis is an extremely common malignant disease,
which poses a great threat to the life of ICU patients (17). Its main pathogenesis is
infection-induced severe systemic inflammatory response, which
usually causes multiple organ damage and failure (18). Sepsis complicated with myocardial
damage is very common due to the extremely significant effects of
toxins rich in septic bacteria and inflammatory mediators induced
by the bacteria on myocardial cells (19). Sepsis bacteria can cause
mitochondrial structure damage, mitochondrial calcium overload and
oxygen-free radical damage of myocardial cells, which may cause
decline of the cardiac function, vascular endothelial damage and
blood flow reduction in the early stages of the disease (20). Therefore, it is essential to evaluate
the influence on cardiac function in the sedative treatment of
sepsis. Propofol, midazolam and dexmedetomidine, as clinically
common anesthetics, are widely used in surgical anesthesia and
important for the sedative treatment of sepsis. Their therapeutic
values have been recognized, but their differences in the sedative
treatment of sepsis remain unclear. A study by Abdelmalik and
Rakocevic has shown that propofol is a protective factor for septic
respiratory failure (21), and the
study of Zamani et al has revealed that dexmedetomidine can
improve the survival time of ICU patients with sepsis (22). However, there are few comparative
studies among the three drugs. The present study explored the most
suitable drug for the sedative treatment of ICU patients with
sepsis through rigorous inclusion and exclusion criteria and
in-depth comparison of observation indexes, and therefore it is of
clinical significance. In the present study, there was no
significant difference among groups A, B and C in HR, SBP, DBP,
PaO2, PaCO2, cTnT, CK-MB, and APACHE II score
before treatment, and in DBP, SBP, HR, cTnT, and length of ICU stay
after treatment. After treatment, there was no significant
difference between groups A and B in APACHE II score. APACHE II
score in group C was significantly lower than that in groups A and
B. In the three groups, the indexes after treatment were
significantly better than those before treatment. These findings
suggest that propofol, midazolam and dexmedetomidine are effective
and safe for the sedative treatment of ICU patients with sepsis,
but dexmedetomidine has the best effect. This is similar to the
findings of Chen et al (23)
and Sonneville et al (24),
both of which support the results of this study. There was no
difference in HR among the three groups, suggesting that the three
sedation methods had the same effect on patients' cardiac function.
As an α2 adrenergic receptor agonist that mainly acts on the α2
receptor in the locus nucleus ceruleus of brainstem,
dexmedetomidine has sedative and analgesic effects on the nerve
activity and an inhibitory effect on the sympathetic nerve through
activating the α2 receptor (25).
Sepsis damages the myocardial cells mainly through mitochondria.
Therefore, the mechanism of action of dexmedetomidine may be that
the activation of adrenergic pathways through the α2 receptor,
accelerates the metabolism and production of glucose in the body,
and then supplements and reconstructs damaged mitochondria in time,
thereby relieving patients' pain and anxiety and protecting the
myocardial function. Propofol plays the role of analgesia mainly
through inhibiting the hypothalamus-pituitary-adrenal axis and
reducing cortisol secretion (26).
Cortisol is closely related to the human perception
and immune function. According to a study, cortisol binds to the
surface receptors of immune cells and blocks the downstream signal
transduction, causing the secretion and metabolism of inflammatory
cytokines (27). Therefore, the
protective effect of propofol on myocardial function may be related
to the inhibition of cortisol. The inhibition of cortisol secretion
reduces the activity of inflammatory cytokines, and other
mechanisms of action do not arise through mitochondria, so the
protective effect of propofol on patients with sepsis is less
significant than that of dexmedetomidine. This is also the reason
for the differences in the present study. Midazolam has typical
pharmacological activity of benzodiazepine and anxiolytic,
sedative, hypnotic, anticonvulsant and muscle relaxing effects,
with a very high plasma protein binding rate. It is mainly
metabolized by binding to glucose and then excreted by the kidney
in the human body (28). The liver
and kidney functions of ICU patients are more likely to be inferior
to those of other patients, so the effect of midazolam on the ICU
patients may be poorer. Therefore, midazolam is not suitable for
the sedative treatment of ICU patients with sepsis. The results of
this study revealed that the myocardial function of patients in
group B are poorer than that in group C, which has been confirmed.
There was no significant difference among groups A, B and C in the
incidence of adverse reactions, which indicated that propofol,
midazolam and dexmedetomidine are safe and feasible for the
sedative treatment of sepsis.
This study compared the values of propofol,
midazolam and dexmedetomidine in the sedative treatment of ICU
patients with sepsis. However, the exact mechanisms of action of
the three drugs were not verified due to limited experimental
conditions. Therefore, further exploration is needed. Also,
although there are various ICU patients, only a few ICU patients
with sepsis were included in this study, so there may be
differences with other types of patients.
In summary, propofol, midazolam and dexmedetomidine
are effective and safe in the sedative treatment of ICU patients
with sepsis, but dexmedetomidine has the best effect on protecting
the blood pressure and cardiac functions, which is worthy of
promotion.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JD and YC conceived and designed the study. YG
acquired the patients' data. JD and YC analyzed and interpreted the
data. JD was a major contributor in writing the manuscript. YG
reviewed the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Renji Hospital, School of Medicine, Shanghai Jiaotong University
(Shanghai, China). Patients who participated in this research had
complete clinical data. Signed informed consents were obtained from
the patients or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K;
International Forum of Acute Care Trialists, : International Forum
of Acute Care Trialists: Assessment of global incidence and
mortality of hospital-treated sepsis. Current estimates and
limitations. Am J Respir Crit Care Med. 193:259–272. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fleischmann C, Thomas-Rueddel DO, Hartmann
M, Hartog CS, Welte T, Heublein S, Dennler U and Reinhart K:
Hospital incidence and mortality rates of sepsis: An analysis of
hospital episode (DRG) statistics in Germany from 2007 to 2013.
Dtsch Arztebl Int. 113:159–166. 2016.PubMed/NCBI
|
|
4
|
Rhee C, Dantes R, Epstein L, Murphy DJ,
Seymour CW, Iwashyna TJ, Kadri SS, Angus DC, Danner RL, Fiore AE,
et al CDC Prevention Epicenter Program, : Incidence and trends of
sepsis in US hospitals using clinical vs claims data, 2009–2014.
JAMA. 318:1241–1249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Polat G, Ugan RA, Cadirci E and Halici Z:
Sepsis and septic shock: Current treatment strategies and new
approaches. Eurasian J Med. 49:53–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kadri SS, Rhee C, Strich JR, Morales MK,
Hohmann S, Menchaca J, Suffredini AF, Danner RL and Klompas M:
Estimating ten-year trends in septic shock incidence and mortality
in United States Academic Medical Centers using clinical data.
Chest. 151:278–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marik PE, Khangoora V, Rivera R, Hooper MH
and Catravas J: Hydrocortisone, vitamin C, and thiamine for the
treatment of severe sepsis and septic shock: A retrospective
before-after study. Chest. 151:1229–1238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stoller J, Halpin L, Weis M, Aplin B, Qu
W, Georgescu C and Nazzal M: Epidemiology of severe sepsis:
2008–2012. J Crit Care. 31:58–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson BJ, Reilly JP, Shashaty MGS,
Palakshappa JA, Wysoczanski A, Dunn TG, Kazi A, Tommasini A,
Mikkelsen ME, Schweickert WD, et al: Admission plasma levels of the
neuronal injury marker neuron-specific enolase are associated with
mortality and delirium in sepsis. J Crit Care. 36:18–23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steingrub JS, Lagu T, Rothberg MB,
Nathanson BH, Raghunathan K and Lindenauer PK: Treatment with
neuromuscular blocking agents and the risk of in-hospital mortality
among mechanically ventilated patients with severe sepsis. Crit
Care Med. 42:90–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marler J, Mohrien K, Kimmons LA, Vandigo
JE, Oliphant CS, Boucher AN and Jones GM: Effects of propofol on
vasopressor use in patients with sepsis and severe sepsis: A pilot
study. J Crit Care. 35:155–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawazoe Y, Miyamoto K, Morimoto T,
Yamamoto T, Fuke A, Hashimoto A, Koami H, Beppu S, Katayama Y, Itoh
M, et al Dexmedetomidine for Sepsis in Intensive Care Unit
Randomized Evaluation (DESIRE) Trial Investigators, : Effect of
dexmedetomidine on mortality and ventilator-free days in patients
requiring mechanical ventilation with sepsis: A randomized clinical
trial. JAMA. 317:1321–1328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernando SM, Rochwerg B and Seely AJE:
Clinical implications of the Third International Consensus
Definitions for Sepsis and Septic Shock (Sepsis-3). CMAJ.
190:E1058–E1059. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamura H, Kawazoe Y and Morimoto T:
Dexmedetomidine in patients with sepsis requiring mechanical
ventilation-reply. JAMA. 318:480. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seymour CW, Liu VX, Iwashyna TJ,
Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM,
Shankar-Hari M, Singer M, et al: Assessment of clinical criteria
for sepsis: For the Third International Consensus Definitions for
Sepsis and Septic Shock (Sepsis-3). JAMA. 315:762–774. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Godinjak A, Iglica A, Rama A, Tančica I,
Jusufović S, Ajanović A and Kukuljac A: Predictive value of SAPS II
and APACHE II scoring systems for patient outcome in a medical
intensive care unit. Acta Med Acad. 45:97–103. 2016.PubMed/NCBI
|
|
17
|
van Vught LA, Klein Klouwenberg PM,
Spitoni C, Scicluna BP, Wiewel MA, Horn J, Schultz MJ, Nürnberg P,
Bonten MJ, Cremer OL, et al MARS Consortium, : Incidence, risk
factors, and attributable mortality of secondary infections in the
intensive care unit after admission for sepsis. JAMA.
315:1469–1479. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Braun D: A retrospective review of the
sepsis definition after publication of sepsis-3. Am J Med.
132:382–384. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim KS, Suh GJ, Kim K, Kwon WY, Shin J, Jo
YH, Lee JH and Lee H: Quick Sepsis-related Organ Failure Assessment
score is not sensitive enough to predict 28-day mortality in
emergency department patients with sepsis: A retrospective review.
Clin Exp Emerg Med. 6:77–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vaez H, Rameshrad M, Najafi M, Barar J,
Barzegari A and Garjani A: Cardioprotective effect of metformin in
lipopolysaccharide-induced sepsis via suppression of toll-like
receptor 4 (TLR4) in heart. Eur J Pharmacol. 772:115–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdelmalik PA and Rakocevic G: Propofol as
a risk factor for ICU-acquired weakness in septic patients with
acute respiratory failure. Can J Neurol Sci. 44:295–303. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zamani MM, Keshavarz-Fathi M,
Fakhri-Bafghi MS, Hirbod-Mobarakeh A, Rezaei N, Bahrami A and Nader
ND: Survival benefits of dexmedetomidine used for sedating septic
patients in intensive care setting: A systematic review. J Crit
Care. 32:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Zhang X, Zhang B, He G, Zhou L and
Xie Y: Dexme-detomidine reduces the neuronal apoptosis related to
cardiopulmonary bypass by inhibiting activation of the JAK2-STAT3
pathway. Drug Des Devel Ther. 11:2787–2799. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sonneville R, de Montmollin E, Poujade J,
Garrouste-Orgeas M, Souweine B, Darmon M, Mariotte E, Argaud L,
Barbier F, Goldgran-Toledano D, et al: Potentially modifiable
factors contributing to sepsis-associated encephalopathy. Intensive
Care Med. 43:1075–1084. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren X, Ma H and Zuo Z: Dexmedetomidine
postconditioning reduces brain injury after brain hypoxia-ischemia
in neonatal rats. J Neuroimmune Pharmacol. 11:238–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barnes J, Hunter J, Harris S, Shankar-Hari
M, Diouf E, Jammer I, Kalkman C, Klein AA, Corcoran T, Dieleman S,
et al StEP- COMPAC group, : Systematic review and consensus
definitions for the Standardised Endpoints in Perioperative
Medicine (StEP) initiative: Infection and sepsis. Br J Anaesth.
122:500–508. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McGregor BA, Murphy KM, Albano DL and
Ceballos RM: Stress, cortisol, and B lymphocytes: A novel approach
to understanding academic stress and immune function. Stress.
19:185–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shankar-Hari M, Phillips GS, Levy ML,
Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD and
Singer M; Sepsis Definitions Task Force, : Developing a new
definition and assessing new clinical criteria for septic shock:
For the Third International Consensus Definitions for Sepsis and
Septic Shock (Sepsis-3). JAMA. 315:775–787. 2016. View Article : Google Scholar : PubMed/NCBI
|