Introduction
Alcoholic liver disease (ALD) is a major cause of
morbidity and mortality worldwide amongst people who abuse alcohol
(1). The spectrum of ALD ranges from
simple steatosis to alcoholic hepatitis, fibrosis, cirrhosis and
ultimately hepatocarcinoma (2). To
date, few satisfactory advances have been made in the management of
ALD. Thus, novel and more reliable therapeutic approaches are
urgently required.
Several factors have been demonstrated to contribute
to the progression of ALD in humans, including race, gender,
ethnicity and comorbidities such as hepatitis B and C virus
infection (3). Amongst these, gut
flora serves a pivotal role in the pathogenesis of ALD and is
closely associated with the liver in ALD via the gut-liver axis
(4). Alcohol induces quantitative
and qualitative alterations in the gut microbiota and increases gut
permeability. This results in the translocation of endotoxins such
as lipopolysaccharide (LPS) into the liver through the portal vein
(5) and stimulates the release of
pro-inflammatory mediators such as tumor necrosis factor (TNF)-α
and interleukin (IL)-1β that ultimately contribute to liver damage
in alcohol abuse patients (6).
Probiotics are live microorganisms that have
demonstrated promise in ameliorating liver injury by the
suppression of hepatic inflammation, regulation of intestinal
microbiota and improvement of intestinal barrier integrity
(7). Therefore, probiotics likely
have therapeutic potential for ALD (8). Therapeutic strategies targeting the
intestinal flora may be effective for ALD treatment (9). In addition, as the main source of
energy for small intestinal cell metabolism, glutamine regulates
intestinal barrier function by reducing its permeability, thereby
preventing intestinal bacterial and endotoxin translocation
(10). Glutamine also has research
and application value in the treatment of diseases related to
intestinal barrier damage (11).
Most studies report the protective effect of glutamine on the
intestinal barrier function based on the study of tight junctions;
however, few studies have focused on the effect of the amino acid
on intestinal microbiota (12,13).
It is therefore important to analyze intestinal
flora to assess the effects of glutamine and probiotics, and
clarify the mechanisms associated with ALD. The present study
investigated alterations in the gut microbiota in response to
chronic alcohol feeding followed by glutamine, Golden Bifido
(probiotic mixture containing live Lactobacillus bulgaricus,
Bifidobacterium and Streptococcus thermophilus) and
Medilac-S® (probiotic mixture containing live
Bacillus subtilis and Enterococcus faecium) treatment
in ALD rats. The present results may help further understanding of
glutamine and probiotics in ALD as well as the complexity of the
interplay amongst probiotics, the gut flora, inflammation and
ALD.
Materials and methods
Animals and treatments
A total of 60 male Sprague-Dawley rats were obtained
from the Laboratory Animal Center of Fuzhou Wushi Animal Center
(Fuzhou, China). Rats weighing 140±10 g and aged 8 weeks were
housed at three per cage in an specific pathogen free animal room.
Rats were housed in controlled conditions at a temperature of
20±2°C, a relative humidity of 55±5% and 12-h of light/dark cycle
with free access to food and water.
For the experiment, following one week of adaptive
feeding with a normal chow diet, the 60 rats were randomly divided
into six groups as follows (n=10; Fig.
1): i) C group: Normal chow diet and 1 ml/kg/day saline via
intragastric administration for 20 weeks; ii) M group: Normal chow
diet and intragastric ethanol (8 ml/kg/day; 56% ethanol) on the
first day, followed by a gradual increase to 18 ml/kg/day until the
end of the experiment with a 2 ml/kg/day interval; iii) T group:
Same method as the M group with intragastric administration of 1
ml/kg/day Golden Bifido suspension (total concentration, 500 mg/1
ml saline; cat. no. S1998004; Inner Mongolia Shuang Qi
Pharmaceutical Co., Ltd) for the last 8 weeks; iv) G group: Same
method as the M group with the intragastric administration of 1
ml/kg/day glutamine suspension (total concentration of 1 g/1 ml
saline; cat. no. 62010836; Sinopharm Chemical Reagent Co., Ltd.)
for the last 8 weeks; v) N group: Same method as the M group with
the intragastric administration of 1 ml/kg/day
Medilac-S® suspension (total concentration of 140 mg/1
ml saline; cat. no. S20030087; Beijing Hanmei Pharmaceutical Co.,
Ltd.) for the last 8 weeks; and vi) the L group: Same method as the
M group with the intragastric administration of a Golden Bifido
suspension (500 mg/kg/day) and a glutamine suspension (140
mg/kg/day) for the last 8 weeks. Doses of the treatment agents were
chosen on the basis of previous studies (14,15).
Subjects were weighed and then anesthetized using an
intraperitoneal injection of 10% chloral hydrate (300 mg/kg) at the
end of 20 weeks then rats were sacrificed by cervical dislocation.
Blood, liver, intestinal tissues and feces were collected for
subsequent biochemical analysis. All procedures were approved by
the Institutional Animal Care and Use Committee of Fujian Medical
University (registration number: 2015-CX-1).
Serum biochemical estimation
Blood samples were kept at room temperature for 1 h
and then centrifuged at 1,500 × g for 15 min at 4°C. The serum was
stored at −80°C. Serum aspartate aminotransferase (AST), alanine
aminotransferase (ALT) and triglyceride (TG) levels were measured
using an automatic biochemical analyzer (Konelab 20; Thermo Fisher
Scientific, Inc.). Tumor necrosis factor (TNF)-α (cat. no.
SEA133Ra), interleukin (IL)-6 (cat. no. SEA079Ra), diamine oxidase
(DAO) (cat. no. SEA656Ra), endotoxin (cat. no. CEB526Ge), occludin
(cat. no. SEC228Ra) and D-lactate (cat. no. CEV643Ge) levels were
detected by the relevant ELISA kits according to the manufacturer's
instructions (All from Wuhan USCN Business Co., Ltd.). Each
experiment was performed at least three times.
Pathologic evaluation
Each liver was fixed in 10% formalin solution for 36
h at 37°C, embedded in paraffin, sectioned into 4 µm-thick slices
and stained with hematoxylin for 10 min at 37°C. Stained sections
were imaged using a light microscope (Leica DM200; Leica
Microsystems Ltd.) using a 40X objective lens and a color
camera.
Gut microbiota analysis
Fresh feces (3–5 g) was obtained by a sterile swab,
placed in an anaerobic dilution solution (4.5 g/l
KH2PO4; 6 g/l Na2HPO4;
0.5 g/l L-cysteine-HCl; 2 g/l gelatin; 1 ml/l Tween-20) and
analyzed by Hangzhou Jinghang Biotechnology Technology Co., Ltd.
(Hangzhou, China) for 16S rDNA sequencing.
Western blot analysis
A total of 0.1 g of intestinal tissue sample were
ground, homogenized and then treated with radioimmunoprecipitation
assay buffer (Thermo Fisher Scientific, Inc.). Protein content was
determined by the bicinchoninic acid method. Equal amounts of
protein (30 µg) were separated via 10% SDS-PAGE and transferred to
a polyvinylidene difluoride membrane. PVDF membranes were blocked
with 5% fat-free milk powder diluted in TBS-T (0.05% Tween 20) at
37°C for 2 h. The membranes were incubated with the primary
antibodies against occludin (1:1,000; cat. no. ab167161) and
β-actin (1:1,000; cat. no. ab8227; both Abcam, Cambridge, UK) at
4°C overnight. After rinsing three times with Tris-buffered saline
and Polysorbate 20, the membranes were incubated with the
corresponding horseradish peroxidase-labeled secondary antibody
(1:1,000; cat. no. 31470; Thermo Fisher Scientific, Inc.) at 37°C
for 2 h in the dark. Protein bands were detected using the Pierce™
ECL Western Blotting Substrate (cat. no. 32209; Thermo Fisher
Scientific, Inc.) and Image lab software 3.0 (Bio-Rad Laboratories,
Inc.). Relative protein expression levels were normalized to that
of β-actin. Each experiment was performed at least three times.
Statistical analysis
Data are expressed as the mean ± standard deviation.
SPSS 16.0 (SPSS, Inc.) statistical software was used for
statistical analysis. One-way ANOVA was used to compare differences
amongst multiple groups followed by Dunnett's post hoc test.
P<0.05 was considered to indicate statistical significance.
Results
Glutamine and probiotic treatments
elevate the body weight (BW) of rats with ALD
As shown in Fig. 2,
the BW of ALD rats was obviously lower than the control
(P<0.05). However, the BW of rats in the T, G and L groups was
significantly higher compared with rats in the M group (P<0.01,
P<0.01 and P<0.05, respectively). There was no difference in
body weight between the N and M groups (P>0.05).
Glutamine and probiotic treatments
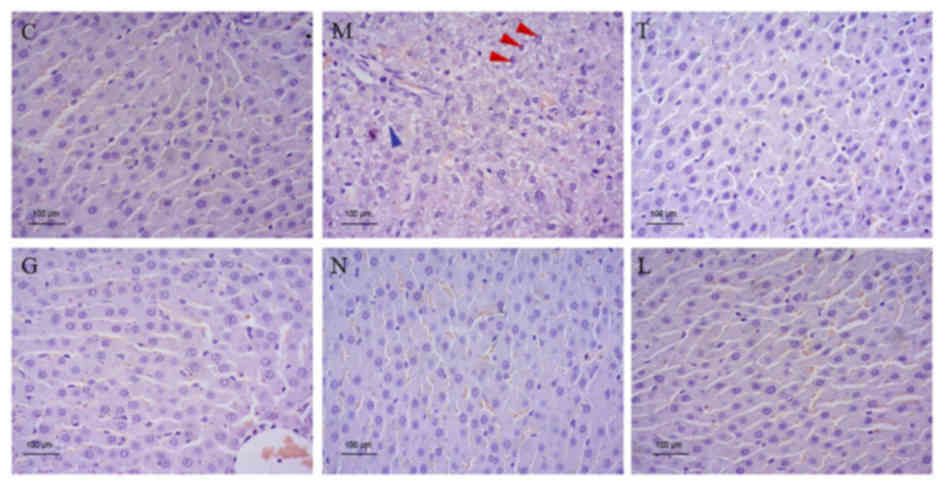
attenuate hepatic histopathological injury in rats with ALD
H&E staining of rat livers demonstrated serious
hepatic fatty changes, necrosis and inflammation in ALD rats.
However, rat liver tissues from the T, G, N and L groups exhibited
distinctly reduced alcohol-induced hepatic histopathological injury
in ALD rats (Fig. 3).
Glutamine and probiotic treatments
reduce serum ALT, TG and AST levels in rats with ALD
ALD rats had significantly higher ALT, TG and AST
serum levels compared with the normal controls (P<0.01; Table I). In contrast, serum AST, ALT, and
TG levels in T, G, L and N groups were significantly lower compared
with the ALD group (P<0.01; Table
I).
 | Table I.AST, ALT and TG levels in each
group. |
Table I.
AST, ALT and TG levels in each
group.
| Group | ALT (U/l) | TG (mmol/l) | AST (U/l) |
|---|
| C | 19.92±4.98 | 0.74±0.03 | 59.26±6.23 |
| M |
43.59±0.61a |
1.95±0.06a |
65.38±2.24a |
| T |
36.66±1.12b |
1.62±0.36b |
52.94±2.92b |
| G |
34.56±4.05b |
1.52±0.04b |
47.62±4.56b |
| N |
35.42±3.53b |
1.47±0.04b |
49.62±1.22b |
| L |
23.38±4.91b |
0.90±0.14b |
37.61±3.37b |
Glutamine and probiotic treatments
decrease serum DAO, endotoxin and D-lactic acid levels in rats with
ALD
As shown in Table
II, the ALD rats had significantly higher serum DAO, endotoxin
and D-lactate levels compared with the controls (P<0.01).
Following 20 weeks of various glutamine and probiotic treatments,
the serum DAO, endotoxin and D-lactic acid levels in the T, G, N
and L groups were significantly decreased compared with the M group
(P<0.01; Table II).
 | Table II.Serum DAO, endotoxin and D-lactic
acid levels in each group. |
Table II.
Serum DAO, endotoxin and D-lactic
acid levels in each group.
| Group | DAO (mg/ml) | Endotoxin
(EU/ml) | D-lactic acid
(mg/l) |
|---|
| C | 6.11±0.07 | 0.66±0.018 | 8.73±0.04 |
| M |
7.88±1.10a |
0.86±0.05a |
15.46±0.31a |
| T |
6.51±0.59b |
0.76±0.01b |
13.47±0.13b |
| G |
6.72±0.19b |
0.74±0.02b |
13.86±0.07b |
| N |
6.54±0.40b |
0.77±0.004b |
14.20±0.17b |
| L |
6.34±1.21b |
0.70±0.01b |
10.43±0.15b |
Glutamine and probiotic treatments
elevate occludin levels and reduce serum inflammatory factor levels
in rats with ALD
After chronic alcohol feeding, the M group exhibited
decreased occludin expression and elevated serum TNF-α and IL-6
levels compared with those in the C group (P<0.01; Fig. 4; Table
III). However, rats in the T, G, L, and N groups demonstrated
significantly reduced TNF-α and IL-6 levels (P<0.01; Table III) and increased occludin protein
expression (P<0.05; Fig. 4)
compared with the M group.
 | Table III.IL6, occludin and TNF-α levels in
each group. |
Table III.
IL6, occludin and TNF-α levels in
each group.
| Group | IL-6 (ng/l) | Occludin
(ng/l) | TNF-α (ng/l) |
|---|
| C | 3.47±0.07 | 48.22±5.58 | 1.92±0.12 |
| M |
5.53±0.13a |
35.24±9.55a |
3.70±0.14a |
| T |
4.82±0.17b |
39.03±3.23b |
3.07±0.20b |
| G |
4.64±0.39b |
40.51±1.32b |
2.74±0.10b |
| N |
4.91±0.37b |
41.07±8.39b |
2.69±0.14b |
| L |
3.86±0.48b |
41.76±4.55b |
2.22±0.12b |
Glutamine and probiotic treatments
modulate gut microbiota in rats with ALD
At the phylum level, the abundance of
Firmicutes was notably decreased in the ALD group compared
with the healthy controls (P<0.05; Fig. 5), whilst the proportion of
Bacteroidetes was not significantly different in ALD and
control rats. The proportion of Proteobacteria (P<0.05;
Fig. 5) and Actinobacteria
(P<0.01; Fig. 5) in the ALD group
was significantly higher compared with the control group. However,
treatment with glutamine and probiotics increased the abundance of
Firmicutes (P<0.05) and decreased the abundance of
Proteobacteria (P<0.05) and Actinobacteria
(P<0.05 P<0.01, P<0.05 and P<0.01, respectively) in T,
G, L, and N groups compared with the M groups (Fig. 5).
At the genus level, Prevotellaceae was the
most prevalent genus in healthy controls and significantly
decreased in the ALD group (P<0.05; Fig. 6). Moreover, the proportion of
Prevotellaceae in the N group was significantly higher
compared with the ALD group (P<0.01; Fig. 6). Porphyromonadaceae was the
second most prevalent genus in the control group. Alcohol infusion
induced a significant increase in Porphyromonadaceae in
comparison with the control group (P<0.05; Fig. 6). However, Porphyromonadaceae
abundance was reduced in T, G, L, and N groups when compared with
the ALD group (P<0.01; Fig. 6).
Prevotella was the third most prevalent genus in healthy
controls, whereas their relative abundance was reduced by alcohol
treatment (P<0.01; Fig. 6).
Following glutamine and probiotic treatments, Prevotella
abundance in the T and N groups were significantly decreased
compared with the M group (P<0.01; Fig. 6).
Discussion
Chronic ethanol feeding destroys the integrity of
the intestinal barrier and disturbs the gut microbiota (16,17).
These alterations increase the translocation of endotoxins such as
LPS from the intestinal lumen to the portal blood (18). LPS then binds to toll like receptor 4
on the surface of hepatic Kupffer cells to produce inflammatory
cytokines which ultimately results in ALD (19). Therefore, the restoration of
intestinal homeostasis could be a potential therapy for ALD. Many
studies have reported that alcohol exposure decreased final BW in
chronic ALD (20,21). The present study identified that BW
was lower in the ALD group compared with the control group at 20
weeks. However, treatments with Golden Bifido and glutamine
attenuated the reduction in BW induced by alcohol feeding.
Epithelial tight junctions are the primary component
of the intestinal mucosal barrier (12). Occludin is an integral membrane
protein localized at tight junctions (22) that has an important role in the
maintenance of the basic structure and function of tight junctions.
Chaudhry et al (23) reported
that chronic alcohol consumption caused the redistribution of
occludin from the intercellular junctions of the colonic epithelium
and disrupted colonic epithelial tight junctions and intestinal
epithelial barrier function; however, Glutamine supplementation
protected tight junctions in the colonic epithelium of mice fed
with alcohol and thus maintained intestinal epithelial barrier
function. Moreover, serum ALT and AST levels can be used to
indicate liver injury (24).
D-Lactate, endotoxin and DAO levels reflect the severity of
intestinal mucosa injury (25). Li
et al (26) identified that
an increase in these parameters occurred in rats that underwent
chronic alcohol feeding. In the present study, abnormally decreased
occludin levels and abnormally elevated plasma ALT, AST, TG, DAO,
endotoxin and D-lactate levels were detected in the ALD group,
indicating alcohol-induced liver injury and intestinal mucosa
injury. Significant changes in these indexes in the sera of rats in
all intervention groups revealed that probiotics and glutamine
alleviated liver damage and intestinal mucosa injury caused by
chronic alcohol use. Moreover, various inflammatory cytokines, such
as TNF-α and IL-6, have been reported to be involved in the
occurrence and development of ALD (27,28).
Previous studies have shown that glutamine and probiotics reduce
inflammation, promote the secretion of anti-inflammatory cytokines
and maintain intestinal barrier functions (29,30). The
present results demonstrated that probiotic and glutamine
treatments reduced the abnormally elevated serum IL-6 and TNF-α
levels following chronic ethanol consumption, demonstrating that
probiotics and glutamine likely alleviated hepatic inflammation via
the suppression of inflammatory cytokines. Moreover, liver injury
induced by alcohol at the histopathological level was completely
reversed after probiotic and glutamine treatments, indicating the
protective effect of probiotics and glutamine on liver damage.
Alcohol-induced intestinal dysbiosis is involved in
the pathogenesis of ALD (4,31). In the present study, at the phylum
level, Firmicutes was the most dominant phyla in healthy
controls, which was consistent with previous studies (32). The present results demonstrated
decreased Firmicutes and higher Actinobacteria and
Proteobacteria abundance at the phylum level in the ALD
group, which were in agreement with the results obtained by
Bull-Otterson et al (33). At
the genus level, increased Porphyromonadaceae and decreased
Prevotellaceae and Prevotella abundance were observed
in ALD mice compared with the controls. The increase in the
abundance of Porphyromonadaceae, elevated plasma endotoxin
levels and hepatic inflammation are strongly associated with
complications in chronic liver disease (34).
Previous studies have demonstrated that probiotics
ameliorate alcohol-induced gut dysbiosis and prevent alcoholic
liver injury (7,35). In the present study, probiotics and
glutamine notably elevated the abundance of Firmicutes and
reduced Actinobacteria, Proteobacteria and
Porphyromonadaceae abundance following continuous alcohol
consumption, indicating that probiotic and glutamine treatments may
attenuate gut dysbiosis.
In conclusion, the present study demonstrated that
probiotic and glutamine treatments ameliorated ALD in rats via the
suppression of inflammation and the regulation of intestinal
microbiota. Results suggested that these interventions may
potentially serve as inexpensive therapies for the prevention and
treatment of ALD.
Acknowledgements
Not applicable.
Funding
The present study study was supported by Fujian
Medical Innovation Foundation (grant no. 2015-CX-1).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
ZL and YZ designed the study; HH, ZL and YZ
performed the experiments; HH and XL collected the data; HH, XL and
YZ analyzed the data; and YZ and XL prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Institutional
Animal Care and Use Committee of Fujian Medical University
(registration number: 2015-CX-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singal AK, Bataller R, Ahn J, Kamath PS
and Shah VH: ACG clinical guideline: Alcoholic liver disease. Am J
Gastroenterol. 113:175–194. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Beier JI and Mcclain CJ: Mechanisms and
cell signaling in alcoholic liver disease. Biol Chem.
391:1249–1264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Shea RS, Dasarathy S and Mccullough AJ:
Alcoholic liver disease. Hepatology. 51:227–229. 1995.
|
|
4
|
Szabo G: Gut-liver axis in alcoholic liver
disease. Gastroenterology. 148:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bode C and Bode JC: Effect of alcohol
consumption on the gut. Best Pract Res Clin Gastroenterol.
17:575–592. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lucey MR, Mathurin P and Morgan TR:
Alcoholic hepatitis. N Engl J Med. 360:2758–2769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malaguarnera G, Giordano M, Nunnari G,
Bertino G and Malaguarnera M: Gut microbiota in alcoholic liver
disease: Pathogenetic role and therapeutic perspectives. World J
Gastroenterol. 20:16639–16648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komatsuzaki N and Shima J: Effects of live
lactobacillus paracasei on plasma lipid concentration in rats fed
an ethanol-containing diet. Biosci Biotechnol Biochem. 76:232–237.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sung H, Kim SW, Hong M and Suk KT:
Microbiota-based treatments in alcoholic liver disease. World J
Gastroenterol. 22:6673–6682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
White JS, Hoper M, Parks RW, Clements WD
and Diamond T: Glutamine improves intestinal barrier function in
experimental biliary obstruction. Eur Surg Res. 37:342–347. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
dos Santos Rd, Viana ML, Generoso SV,
Arantes RE, Davisson Correia MI and Cardoso VN: Glutamine
supplementation decreases intestinal permeability and preserves gut
mucosa integrity in an experimental mouse model. JPEN J Parenter
Enteral Nutr. 34:408–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li N, Lewis P, Samuelson D, Liboni K and
Neu J: Glutamine regulates Caco-2 cell tight junction proteins. Am
J Physiol Gastrointest Liver Physiol. 287:G726–G733. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li N and Neu J: Glutamine deprivation
alters intestinal tight junctions via a PI3-K/Akt mediated pathway
in Caco-2 cells. J Nutr. 139:710–714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sellmann C, Baumann A, Brandt A, Jin CJ,
Nier A and Bergheim I: Oral supplementation of glutamine attenuates
the progression of nonalcoholic steatohepatitis in C57BL/6J mice. J
Nutr. 147:2041–2049. 2017.PubMed/NCBI
|
|
15
|
Huang H, Zeng Y, Lin H, Lin XY and Lin ZH:
Effects of fecal microbiota transplantation and joint application
of probiotics on rats with alcoholic liver disease. Int J Clin Exp
Med. 11:12368–12374. 2018.
|
|
16
|
Hartmann P, Chen WC and Schnabl B: The
intestinal microbiome and the leaky gut as therapeutic targets in
alcoholic liver disease. Front Physiol. 3:4022012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Addolorato G, Montalto M, Capristo E,
Certo M, Fedeli G, Gentiloni N, Stefanini GF and Gasbarrini G:
Influence of alcohol on gastrointestinal motility: Lactulose breath
hydrogen testing in orocecal transit time in chronic alcoholics,
social drinkers and teetotaler subjects. Hepatogastroenterology.
44:1076–1081. 1997.PubMed/NCBI
|
|
18
|
Wang L, Llorente C, Hartmann P, Yang AM,
Chen P and Schnabl B: Methods to determine intestinal permeability
and bacterial translocation during liver disease. J Immunol
Methods. 421:44–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lieber CS: Hepatic, metabolic and toxic
effects of ethanol: 1991 update. Alcohol Clin Exp Res. 15:573–592.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao B, Xu MJ, Bertola A, Wang H, Zhou Z
and Liangpunsakul S: Animal models of alcoholic liver disease:
Pathogenesis and clinical relevance. Gene Expr. 17:173–186. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian F, Chi F, Wang G, Liu X, Zhang Q,
Chen Y, Zhang H and Chen W: Lactobacillus rhamnosus CCFM1107
treatment ameliorates alcohol-induced liver injury in a mouse model
of chronic alcohol feeding. J Microbiol. 53:856–863. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Furuse M, Hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S and Tsukita S: Occludin: A novel integral
membrane protein localizing at tight junctions. J Cell Biol.
123:1777–1788. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chaudhry KK, Shukla PK, Mir H, Manda B,
Gangwar R, Yadav N, McMullen M, Nagy LE and Rao R: Glutamine
supplementation attenuates ethanol-induced disruption of apical
junctional complexes in colonic epithelium and ameliorates gut
barrier dysfunction and fatty liver in mice. J Nutr Biochem.
27:16–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Beek JH, de Moor MH, de Geus EJ, Lubke
GH, Vink JM, Willemsen G and Boomsma DI: The genetic architecture
of liver enzyme levels: GGT, ALT and AST. Behav Genet. 43:329–339.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruan P, Gong ZJ and Zhang QR: Changes of
plasma D(−)-lactate, diamine oxidase and endotoxin in patients with
liver cirrhosis. Hepatobiliary Pancreat Dis Int. 3:58–61.
2004.PubMed/NCBI
|
|
26
|
Li H, Qiu P, Wang J, Niu C and Pan S:
Effects of compound Ginkgo biloba on intestinal permeability in
rats with alcohol-induced liver injury. Food Funct. 6:470–478.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mcclain C, Hill D, Schmidt J and Diehl AM:
Cytokines and alcoholic liver disease. Semin Liver Dis. 13:170–182.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciećko-Michalska I, Szczepanek M, Cibor D,
Owczarek D, Skulina D, Szczepański W and Michalski M: Serum
cytokine concentration as prognostic factor in patients with
alcoholic liver disease. Przegl Lek. 63:249–252. 2006.(In Polish).
PubMed/NCBI
|
|
29
|
Gong ZY, Yuan ZQ, Dong ZW and Peng YZ:
Glutamine with probiotics attenuates intestinal inflammation and
oxidative stress in a rat burn injury model through altered iNOS
gene aberrant methylation. Am J Transl Res. 9:2535–2547.
2017.PubMed/NCBI
|
|
30
|
Hastings CN, Sheridan H, Pariante CM and
Mondelli V: Does diet matter? The use of polyunsaturated fatty
acids (PUFAs) and other dietary supplements in
inflammation-associated depression. Curr Top Behav Neurosci.
31:321–338. 2016. View Article : Google Scholar
|
|
31
|
Cresci GA: The gut microbiome: A new
frontier for alcohol investigation. Alcohol Clin Exp Res.
39:947–949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mutlu EA, Gillevet PM, Rangwala H,
Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK and Keshavarzian
A: Colonic microbiome is altered in alcoholism. Am J Physiol
Gastrointest Liver Physiol. 302:G966–G978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bullotterson L, Feng W, Kirpich I, Wang Y,
Xiang Q, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino
J, et al: Metagenomic analyses of alcohol induced pathogenic
alterations in the intestinal microbiome and the effect of
lactobacillus rhamnosus GG treatment. PLoS One. 8:e530282013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bajaj JS, Ridlon JM, Hylemon PB, Thacker
LR, Heuman DM, Smith S, Sikaroodi M and Gillevet PM: Linkage of gut
microbiome with cognition in hepatic encephalopathy. Am J Physiol
Gastrointest Liver Physiol. 302:G168–G175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vassallo G, Mirijello A, Ferrulli A,
Antonelli M, Landolfi R, Gasbarrini A and Addolorato G: Review
article: Alcohol and gut microbiota-the possible role of gut
microbiota modulation in the treatment of alcoholic liver disease.
Aliment Pharmacol Ther. 41:917–927. 2015. View Article : Google Scholar : PubMed/NCBI
|