Introduction
Pulmonary fibrosis is a progressive, eventually
fatal disease, which is characterized by excessive accumulation of
extracellular matrix (ECM) in the alveolar parenchyma and
progressive lung scarring (1).
Recently, considerable research efforts have been devoted to its
pathogenesis (2,3); however, the complete details thereof
have remained elusive. It is well known that pulmonary fibrosis
does not only occur as a primary condition, but also secondary to
other diseases, including rheumatoid arthritis (4) and vasculitis (5). The lung is frequently affected by other
diseases, as it has abundant blood supply and connective tissues
(6), leading to secondary lung
diseases, including pulmonary fibrosis (7), pulmonary arterial hypertension
(8) and pulmonary nodules (9). Of note, pulmonary fibrosis, as an
important complication of other diseases, has a crucial role in the
progression of lung disease.
It must also be mentioned that the increment of lung
fibroblasts is the major cause of pulmonary fibrosis. In the
process, lung fibroblasts, together with a number of other immune
cells, including T and B lymphocytes, monocytes, macrophages and
neutrophils, create an inflammatory environment in the lung tissue,
which recruits an increasing number of immune cells and results in
pulmonary destruction and functional deficiency (10,11). In
addition, when lung fibroblasts are stimulated by pro-inflammatory
cytokines, including transforming growth factor (TGF)-β1 (12), the release of a number of other
pro-inflammatory cytokines (13),
including interleukin (IL)-6, may be induced, which contributes to
the further amplification of inflammatory processes.
Triptolide (TPL), a terpene compound contained in
the root, leaf, flower and fruit of Tripterygium wilfordii,
is a bioactive component used in traditional Chinese medicine known
for its anti-inflammatory and immunosuppressive effects (14,15). It
has therapeutic effects against a multitude of autoimmune diseases.
For instance, TPL was able to reduce hippocampal Aβ deposition in a
rat model of vascular dementia and exert anti-inflammatory
functions (16). Furthermore, it was
reported that TPL restrained the expression of C-X-C motif
chemokine receptor 4, thrombin, tumor necrosis factor-α and TGF-β
receptor in colon cancer cells to exert an anti-cancer effect
(17). In addition, a previous study
further demonstrated that TPL downregulates the expression of focal
adhesion kinase (FAK), which leads to the imbalance of lung cancer
cell migration and inhibits the ability of lung cancer cells to
migrate and invade in vitro (18). It was also demonstrated that TPL
inhibits the TGF-β1/extracellular signal-regulated kinase/mothers
against decapentaplegic homolog 3 signaling pathway to reduce
myofibroblast activation in the lung, thus inhibiting the
progression of radioactive pulmonary fibrosis (19). However, the molecular mechanisms
underlying the therapeutic effects of TPL, particularly regarding
the proliferation of lung fibroblasts and the molecular mechanisms
of its effects to suppress the inflammatory response have remained
elusive.
FAK is a signaling molecule that mediates the
conglutination of the cell and the ECM, and it is an intersection
of numerous signaling pathways involved in the regulation of a
variety of physiological and pathological processes, including cell
metabolism, invasion, migration, adhesion, proliferation and
cytoskeletal reorganization (20,21).
Previous studies have conveyed that FAK is closely connected with
fibrosis, including hepatic (22),
myocardial (23), vascular (24) and pulmonary fibrosis (25). Calpain is a calcium-dependent
protease and it has a critical role in adhesion disassembly in
fibroblasts (26). To date, it has
been confirmed that calpain 2-mediated proteolysis of FAK regulates
adhesion dynamics in motile cells and the calpain cleavage site of
FAK has been identified (27).
However, whether the possible involvement of the
FAK/calpain pathway in the anti-inflammatory and anti-fibrotic
properties of TPL during pulmonary fibrosis and whether this
potential mechanism is involved in the proliferation of lung
fibroblasts, has remained elusive.
Therefore, in the present study, the effects of TPL
on TGF-β1-induced proliferation and cytokine release of lung
fibroblasts were assessed with the aim of assessing the potential
functional roles of the FAK/calpain pathway in these effects.
Materials and methods
Chemicals and drugs
TPL was purchased from Sigma-Aldrich (Merck KGaA).
The compound was dissolved in dimethyl sulfoxide (DMSO) to produce
a stock solution with a concentration of 250 µM. This stock
solution was then diluted with incubation medium. The final DMSO
concentration did not exceed 0.05% (v/v).
The ELISA kit for IL-6 was purchased from Beijing Li
Ke Co., Ltd., (cat. no. XL-EH0196). Anti-FAK (cat. no. CA36131),
anti-phospho-(p)-FAK (cat. no. CN893300), anti-calpain 2 (cat. no.
BS3696) and anti-β-actin (cat. no. 17AV0303) antibodies were
obtained from Bioworld Technology, Inc. Anti-calpain 1 (cat. no.
00016377) was obtained from ProteinTech Group, Inc.
Penicillin/streptomycin solution (X100), 0.05% trypsin-EDTA and
DMSO were purchased from Sigma-Aldrich (Merck KGaA). The Cell
Counting Kit-8 (CCK-8) was obtained from Dojindo Molecular
Technologies, Inc. Ham's F12-K medium and fetal bovine serum (FBS)
were purchased from Gibco (Thermo Fisher Scientific, Inc.).
Radioimmunoprecipitation assay lysis and extraction buffer,
horseradish peroxidase (HRP)-conjugated AffiniPure goat anti-mouse
IgG, anti-rabbit IgG antibodies (cat. nos. anti-mouse 127655 and
anti-rabbit 125946) and D-glucose were purchased from OriGene
Technologies, Inc. PCR primers were obtained from Western Biotech.
Co., Ltd. Calpeptin (calpain inhibitor) and FAK inhibitor were
purchased from Sigma-Aldrich (Merck KGaA).
Cell culture and treatment
The HFL-1 human foetal lung fibroblast cell line was
obtained from the cell bank of the Chinese Academy of Sciences and
cultured in Ham's F12-K medium supplemented with 10% FBS and 1%
antibiotics (penicillin and streptomycin). Cells were cultured in
an incubator with a humidified atmosphere containing 5%
CO2 at 37°C. TGF-β1 at doses of 25, 50 and 100 ng/ml was
added to HFL-1s, which were cultured for 24, 48 and 72 h. A CCK-8
assay was used to determine the growth of the cells. Finally, the
most appropriate concentration and stimulation time of TGF-β1
regarding their effect of lung fibroblasts proliferation were
determined by the growth of the HFL-1s and applied in the
subsequent experiments.
Experimental design
The first series of experiments were designed to
establish the pulmonary fibrosis model and determine the optimal
concentration of TPL to inhibit cell proliferation. TGF-β1 at doses
of 25, 50 and 100 ng/ml was added to HFL-1s, which were cultured
for 24, 48 and 72 h. TPL at doses of 5, 10, 15 and 20 nmol/l was
added to HFL-1s, which were cultured in the presence of 50 ng/ml
TGF-β1 for 48 h.
The second series of experiments was designed to
examine the inhibitory effect of TPL and investigate the possible
mechanism. TPL, calpeptin (a calpain inhibitor) and the FAK
inhibitor were, respectively diluted in DMSO. They were then added
to the growth medium to yield the final concentrations with a DMSO
solvent concentration of <0.05% (v/v). Cells were divided into
five groups: i) Control group: Cells were treated with an equal
concentration of DMSO for 48 h, so that all cultures in the present
study had the same final concentration of DMSO. ii) Model group:
Cells were treated with 50 ng/ml TGF-β1 for 48 h. iii) TPL group:
Cells were cultured in 50 ng/ml TGF-β1 and 5 nmol/l TPL for 48 h.
iv) FAK inhibitor group: Cells were cultured in 50 ng/ml TGF-β1 and
5 nmol/l TPL for 48 h, and addition of 20 µM FAK inhibitor for 24
h. v) Calpeptin group: Cells were cultured in 50 ng/ml TGF-β1 and 5
nmol/l TPL for 48 h, and addition of 50 µm calpeptin for 24 h.
CCK-8 assay
HFL-1s were seeded into 96-well plates, the count
was adjusted to 5 104/ml, 100 µl per well and incubated
with 5% CO2 and 37°C. Subsequently, some treatments were
then performed as mentioned above. A total of 10 µl CCK-8 solution
was added to each well, followed by incubation at 37°C for 4 h.
Finally, the optical density of the resulting solution in the wells
was determined using an ELISA reader (Thermo Fisher Scientific,
Inc.) at a wavelength of 450 nm.
ELISA
The level of IL-6 in the culture supernatant was
determined using a commercial ELISA kit according to the
manufacturer's protocol and evaluated by measuring the absorption
at a 450-nm wavelength using a microplate reader (Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
To determine the expression levels of collagen type
I (ColI)α mRNA and ColIII mRNA, the total RNA extracted from the
lung fibroblasts with TRIzol reagent was reverse-transcribed, the
amplification conditions were as follows: Denaturation for 10 min
at 65°C, annealing for 10 min at 25°C, cDNA extension at 42°C for
50 min and inactivation at 85°C for 5 min, using Thermo Reverse
Transcription kits (Western Biotech. Co., Ltd.). qPCR was performed
using Quanti Nova SYBR Green PCR kit (Qiagen GmbH). The sequences
of the primers used for PCR are provided in Table I. The amplification conditions were
as follows: 4 min at 94°C, followed by 35 cycles at 94°C for 20
sec, 60°C for 30 sec and 72°C for 30 sec. The relative expression
of ColIα mRNA and ColIII mRNA was evaluated using the
2−∆∆Cq method (28).
β-actin was used as a control for normalization.
 | Table I.Primers used for quantitative
PCR. |
Table I.
Primers used for quantitative
PCR.
| Gene | Primer
sequence | Length of the
amplicon (bp) |
|---|
| β-actin | F:
5′-TGACGTGGACATCCGCAAAG-3′ | 205 |
|
| R:
5′-CTGGAAGGTGGACAGCGAGG-3′ |
|
| ColIα | F:
5′-GTGCGATGACGTGATCTGTGA-3′ | 114 |
|
| R:
5′-GTTTCTTGGTCGGTGGGTG-3′ |
|
| ColIII | F:
5′-TGCTCGGGGTAATGACGG-3′ | 138 |
|
| R:
5′-GCACCATTTGAACCAGGAGAC-3′ |
|
Western blot analysis
The protein levels of FAK, p-FAK, calpain 1 and
calpain 2 in lung fibroblasts was detected by western blot
analysis. Total protein was extracted with radioimmunoprecipitation
assay lysis buffer. Proteins from each experimental group were
quantified using the bicinchoninic acid assay (BestBio). An equal
amount of protein (30 µg) was loaded and subjected to 10% SDS-PAGE.
Total protein was separated by SDS-PAGE at 120 V and then
transferred to polyvinylidene fluoride membranes (EMD Millipore) at
200 mA for 120 min. Membranes were blocked with 5% non-fat milk
powder for 2 h at room temperature and then incubated with primary
antibodies against anti-β-actin (1:1,000 dilution), FAK (1:1,000 of
rabbit monoclonal antibody), p-FAK (1:500), calpain 1 (1:1,000) and
calpain 2 (1:500) at 4°C overnight. The membranes were then
incubated with goat anti-rabbit secondary antibody labeled with HRP
(1:15,000) at room temperature for 1.5 h. The signal was detected
using ECL hypersensitive luminescence substrate kit (GE
Healthcare). Densitometric analysis was performed using Image-Pro
Plus V software (version 7.0; Media cybernetics, Inc.).
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS Inc.).
Values are expressed as the mean ± standard error of the mean.
Multiple comparisons were achieved using one-way analysis of
variance with the Student-Newman-Keuls test. P<0.05 was
considered to indicate a statistically significant difference. Each
experiment was repeated three times.
Results
TPL inhibits TGF-β1-induced
proliferation and inflammation of lung fibroblasts
In order to observe the effect of TPL on HFL-1 cell
proliferation, cells were first treated with different doses (25,
50 and 100 ng/ml) of TGF-β1 for 48 h. The result indicated that
treatment with 50 ng/ml TGF-β1 for 48 h were the optimal conditions
for inducing lung fibroblast proliferation. Subsequently, cells
were pre-treated with 50 ng/ml TGF-β1 for 48 h and then treated
with TPL at different doses (5, 10, 15 and 20 nmol/l) for another
48 h (Fig. 1D). The proliferation of
HFL-1 cells was then determined using a CCK-8 assay. The results
indicated that relative to the control group, treatment with TGF-β1
significantly induced the proliferation of lung fibroblasts
(P<0.01; Fig. 1A). However,
compared with the TGF-β1 treatment group, subsequent treatment with
TPL significantly inhibited the proliferation of HFL-1 cells in a
dose-dependent manner (P<0.001). The minimum TPL concentration
required to achieve an significant suppressive effect on HFL-1
proliferation was 5 ng/ml (P<0.001; Fig. 1B).
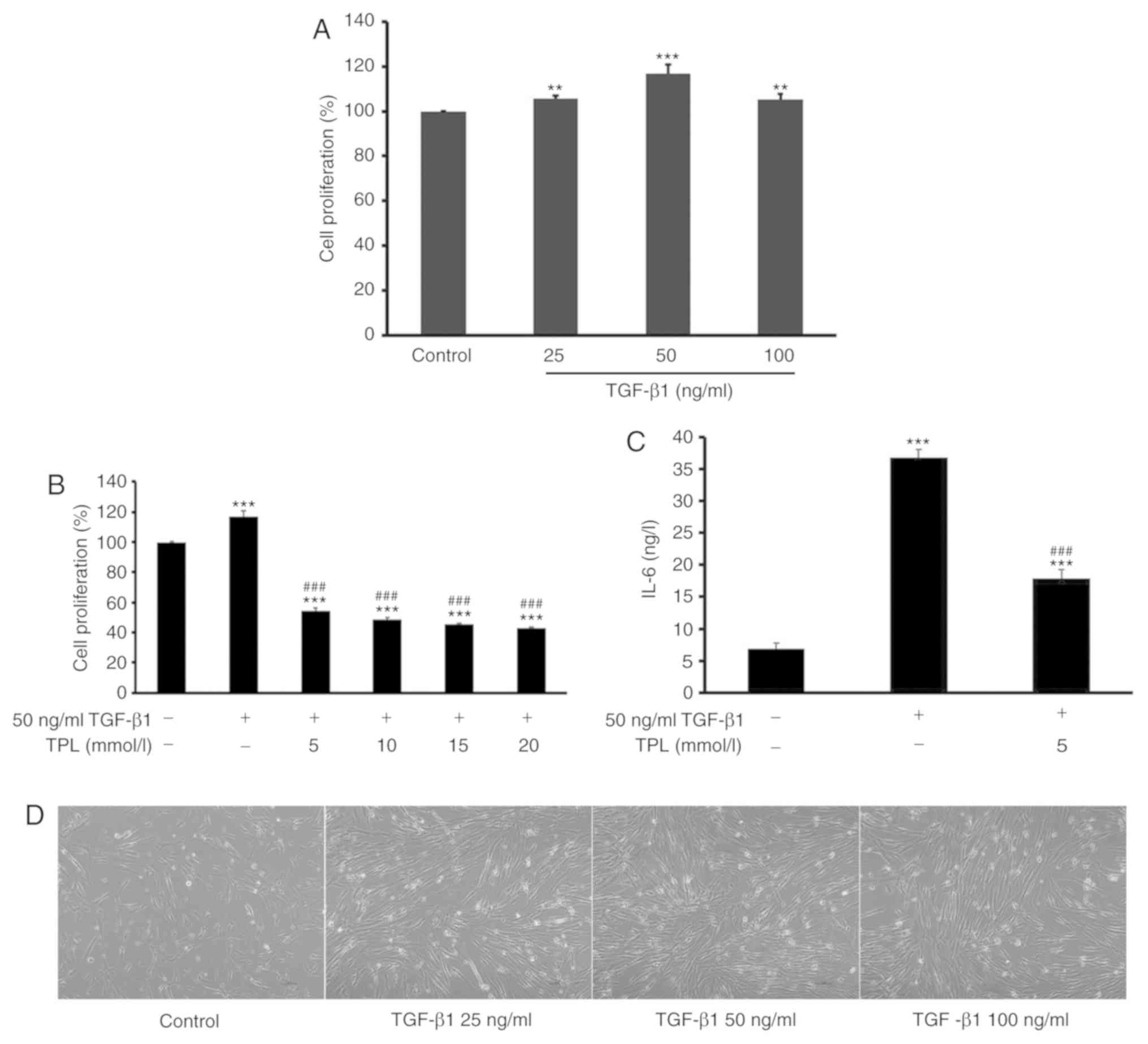 | Figure 1.TPL inhibits TGF-β1-induced
proliferation of lung fibroblasts. (A) Lung fibroblasts were
treated with different doses (25, 50 and 100 ng/ml) of TGF-β1 for
48 h and the proliferation of lung fibroblasts was detected using
the CCK-8 assay. (B) Lung fibroblasts were treated with 50 ng/ml
TGF-β1 for 48 h and addition of TPL at different doses (5, 10, 15
and 20 nmol/l) for 48 h. Untreated cells served as a control. The
proliferation of lung fibroblasts was detected using the CCK-8
assay. TGF-β1 induced the proliferation of lung fibroblasts,
whereas TPL inhibited this proliferation in a dose-dependent
manner. (C) The concentration of IL-6 in the cell culture
supernatant was detected by ELISA at 48 h after TPL treatment. TPL
markedly attenuated the level of IL-6. (D) Morphology of lung
fibroblasts treated with different doses (25, 50 and 100 ng/ml) of
TGF-β1 for 48 h (magnification, ×400). Values are expressed as the
mean ± standard error of the mean (n=5/group). **P<0.01 and
***P<0.001 vs. the control group; ###P<0.001 vs.
the model group. TGF-β1, transforming growth factor-β1; TPL,
triptolide; CCK-8, Cell Counting Kit-8; IL-6, interleukin-6. |
Previous studies have reported that HFL-1 cells may
secrete inflammatory cytokines, including IL-6 (29,30).
Therefore, the effect of TPL on the expression of IL-6 was also
investigated in the present study. HFL-1 cells were treated with 50
ng/ml TGF-β1 prior to the addition of 5 nmol/l TPL and incubation
for another 48 h. Subsequently, the concentration of IL-6 in the
cell culture supernatant was determined by ELISA. The results
demonstrated that TPL significantly inhibited TGF-β1-induced
production of IL-6 compared with TGF-β1 alone (P<0.001; Fig. 1C).
TPL regulates TGF-β1-induced
expression of FAK/calpain in lung fibroblasts
After treatment with TPL for 48 h, the protein
levels of FAK, p-FAK, calpain 1 and calpain 2 in HFL-1 cells were
assessed using western blot analysis. The results indicated that,
compared with those in the control group, the levels of FAK and
p-FAK were significantly increased in the model group, the
expression of calpain 1 and calpain 2 was significantly decreased
(P<0.001; Fig. 2). However,
compared with those in the model group, the levels of FAK and p-FAK
were significantly decreased and the expression of calpain 1 and
calpain 2 was significantly increased in the TPL group (P<0.001;
Fig. 2).
TPL inhibits TGF-β1-induced ColIα and
ColIII synthesis in lung fibroblasts
In order to determine the collagen levels, the
expression of ColIα mRNA and ColIII mRNA was determined by RT-qPCR.
The results indicated that, compared with those in the control
group, the expression levels of ColIα mRNA and ColIII mRNA were
significantly increased in the model group (P<0.001; Fig. 3). Furthermore, compared with those in
the model group, the expression levels of ColIα mRNA and ColIII
mRNA were significantly decreased in the TPL group (P<0.001;
Fig. 3).
TPL inhibits cytokine release by
TGF-β1-induced lung fibroblasts by downregulating FAK and
upregulating calpain
In order to determine the possible involvement of
FAK/calpain signaling in TPL-induced cytokine release, HFL-1 cells
were treated with 50 ng/ml TGF-β1 for 48 h followed by 5 nmol/l TPL
for 48 h and addition of 20 µM FAK inhibitor or 50 µM calpeptin for
24 h. Subsequently, IL-6 in the supernatant was determined by ELISA
and the levels of FAK, p-FAK, calpain 1 and calpain 2 in the cells
were assessed using western blot analysis. The results suggested
that in the TPL group, the level of IL-6 was significantly
decreased compared with those in the model group (P<0.001;
Fig. 4). However, compared with
those in the TPL group, the level of IL-6 was significantly
decreased in the FAK inhibitor group (P<0.001). Furthermore, the
level of IL-6 was significantly increased in the calpeptin group
(P<0.001). Western blot analysis indicated that in the TPL
group, the protein levels of FAK and p-FAK were significantly
decreased and the protein expression levels of calpain 1 and
calpain 2 were increased compared with those in the model group
(P<0.001). However, compared with those in the TPL group, the
protein expression levels of calpain 1 and calpain 2 were increased
in the FAK inhibitor group. Furthermore, the protein levels of FAK
and p-FAK were increased in the calpeptin group (Fig. 4).
 | Figure 4.TPL inhibits cytokine release of
TGF-β1-induced lung fibroblasts by downregulating FAK and
upregulating calpain. Lung fibroblasts were treated with 50 ng/ml
TGF-β1 for 48 h and addition of 5 nmol/l TPL and incubation for 48
h. In addition, cells were cultured with 50 ng/ml TGF-β1 and 5
nmol/l TPL for 48 h prior to the addition of 20 µM FAK inhibitor or
50 µM calpeptin for 24 h. Cells that were untreated served as a
control. The protein levels of FAK, p-FAK, calpain 1 and calpain 2
in lung fibroblasts were determined using western blot analysis.
The concentration of IL-6 in the cell culture supernatant was
detected by ELISA. Quantified protein levels of (A) p-FAK/FAK, (B)
calpain 1 and (C) calpain 2. Values are expressed as the mean ±
standard error of the mean (n=3/group). (D) Western blotting of
FAK, p-FAK, calpain 1 and calpain 2. (E) Concentration of IL-6 in
the cell culture supernatant. ***P<0.001 vs. the control group;
###P<0.001 vs. model group; ∆∆∆P<0.001
vs. TPL group. TGF-β1, transforming growth factor-β1; TPL,
triptolide; p-FAK, phosphorylated focal adhesion kinase; IL,
interleukin. |
TPL inhibits TGF-β1-induced pulmonary
fibrosis by downregulation of ColIα and ColIII via regulation of
FAK/calpain
In order to determine the possible involvement of
FAK/calpain signaling in TPL-induced collagen synthesis. HFL-1
cells were treated with 50 ng/ml TGF-β1 and 5 nmol/l TPL for 48 h
and addition of 20 µM FAK inhibitor or 50 µM calpeptin for 24 h.
The results suggested that in the TPL group, the expression of
ColIα mRNA and ColIII mRNA were significantly decreased compared
with those in the model group (P<0.001). However, compared with
those in the TPL group, the expression of ColIα mRNA and ColIII
mRNA was decreased in the FAK inhibitor group. Furthermore, the
expression of ColIα mRNA and ColIII mRNA were increased in the
calpeptin group (Fig. 5).
Discussion
In the present study, TGF-β1 was used to induce the
proliferation of lung fibroblasts and generate an in vitro
model of pulmonary fibrosis. In a preliminary experiment, treatment
with 50 ng/ml TGF-β1 for 48 h induced the proliferation of lung
fibroblasts, indicating that this was a suitable concentration to
establish the in vitro model. This model has also been used
in foreign and domestic studies (31–33). In
addition, as lung fibroblasts are considered to participate in
pulmonary inflammation and fibrosis in numerous autoimmune
diseases, including rheumatoid arthritis (4,34), the
model has also been used to investigate pulmonary fibrosis
secondary to numerous other diseases in vitro. The present
study suggested that TGF-β1 also induced lung fibroblasts to
secrete inflammatory cytokine IL-6 and synthesize ColIα and ColIII,
which are among the deposited ECM materials (35). It is likely that in lung tissue
affected by pulmonary fibrosis, fibroblasts were affected by
inflammation for a long time and secreted inflammatory cytokine
IL-6 and synthesized collagen. With the continuous accumulation of
collagen and inflammatory stimuli, the lung tissue became filled
with collagen and was replaced by mesenchyme tissue, which finally
led to pulmonary fibrosis and lung injury.
It is well-known that pulmonary fibrosis is an
important complication in numerous other diseases and seriously
affects the treatment of primary diseases. At present, there are no
effective therapies for pulmonary fibrosis and the development of
novel drugs is urgently required (36). In the present study, TPL was
confirmed to inhibit TGF-β1-induced proliferation of lung
fibroblasts and decrease the expression of inflammatory cytokine
IL-6, as well as ColIα and ColIII mRNA. It may therefore be implied
that TPL is an effective drug candidate for treating pulmonary
fibrosis. The results of the present study are consistent with
those provided by Chen et al (37), which reported that TPL has
anti-inflammatory and immune suppressive effects and even protects
against radiation-induced pulmonary fibrosis.
Furthermore, in the model group, the results of the
present study demonstrated that the protein levels of FAK and p-FAK
were increased and the protein expression of calpain 1 and calpain
2 in lung fibroblasts was decreased compared with the control
group. This indicated that the FAK signaling pathway was activated
and the calpain signaling pathway was inhibited in lung
fibroblasts, and it further suggested that FAK/calpain signal
disorders may promote pulmonary fibrosis. Activation of the FAK
signaling pathway has been reported to cause lung fibrosis and the
expression of FAK was overexpressed in lung tissue (38,39).
However, these results are not consistent with those of Li et
al (40) and Chan and Mattson
(41), who reported on the
activation of the calpain signaling pathway in lung fibrosis. This
may be mainly due to the lack of sufficient Ca2+ in the
in vitro experiment to activate the calpain pathway. It is
well known that calpain is a calcium-dependent intracellular
cysteine protease. The excessive inflammation may inhibit the
function of the calpain signaling pathway and the expression of
calpain1 and 2 were decreased.
In addition, the results of the present study
demonstrated that TPL inhibited activation of the FAK signaling
pathway and promoted calpain signaling to restrain pulmonary
fibrosis, indicating that inhibition of FAK signaling is a possible
mechanism of the inhibitory effect of TPL on lung fibroblast
proliferation and thereby on pulmonary fibrosis. Furthermore, in
order to investigate the possible involvement of the FAK/calpain
signaling pathways in the effects of TPL on pulmonary fibrosis, FAK
inhibitor and calpeptin were used to treat lung fibroblasts and
block the FAK and calpain signaling pathway, respectively. The
results suggested that TPL and the FAK inhibitor have a synergistic
effect on inhibiting the release of cytokine IL-6, by
TGF-β1-induced lung fibroblasts into the cell culture supernatant,
and restraining the expression of ColIα mRNA and ColIII mRNA in
lung fibroblasts. Furthermore, calpeptin reversed the effect of TPL
to inhibit the synthesis of collagen and the secretion of
inflammatory factors. The results also suggested that
phosphorylation of FAK cannot be hydrolyzed via blocking of the
calpain signaling pathway. As phosphorylation of FAK may promote
lung fibrosis, the condition may be aggravated via blocking of the
calpain signaling pathway. A previous study also confirmed that
calpain mediated the hydrolyzation of the phosphorylation of FAK
and identified a calpain cleavage site on the FAK protein (27). Additionally, these studies indicated
that TPL exerts its effects against pulmonary fibrosis and to
reduce inflammation by downregulating the FAK signaling pathway and
upregulating the calpain signaling pathway. Therefore, those
studies demonstrate that TPL has a beneficial effect for treating
pulmonary fibrosis occurring secondary to chronic diseases,
including rheumatoid arthritis.
In conclusion, to the best of the authors'
knowledge, the present study is the first to demonstrate that TPL
prevented TGF-β1-induced lung fibroblast proliferation by
downregulating the expression of Col α and Col III via inhibiting
the activation of the FAK signal pathway and promoting the
activation of the calpain signaling pathway. Furthermore, TPL may
inhibit inflammatory cytokine release by downregulating the FAK
signaling pathway and upregulating the calpain signaling pathway.
Although further investigation is required to fully unveil the
molecular mechanisms of action, the present results suggest that
TPL may be suitable as a novel therapeutic drug for pulmonary
fibrosis.
However, the study has some limitations. For
example, the study only used one lung fibroblast cell line that was
also not a primary lung fibroblast and the study about the effects
of TPL on TGFß1-induced lung fibroblasts was in vitro rather
than in vivo. Moreover, in this study, the result showed TPL
inhibits cell proliferation and at the same time induces cell
death. TPL used at 5 mmol/l may have had cytotoxic effects on the
lung fibroblast cells. In the future, the authors will further
optimize and explore a more appropriate TPL concentration for
research. On the basis of the present study, 50 ng/ml TGF-β1 was
deemed to be the most suitable model for pulmonary fibrosis where
treating cells with TGF-β1 was more appropriate. However, pulmonary
fibrosis is a slow process in nature, which was not discussed in
this study. The authors will make a detailed study of this point in
their following work. Additionally, this study mainly investigated
TPL, which is one of the active constituents in Tripterygium
wilfordii according to Chinese herbal medicine. However,
although Chinese herbal Tripterygium wilfordii is commonly
used in clinical practice, TPL has not been used alone in clinical
practice. In the future, a clinical study on TPL will need to be
conducted so that TPL can be used in the clinic.
Acknowledgements
The authors would like to thank the Key Laboratory
of Anhui Medicine of the Chinese Ministry of Education (Hefei,
China) for their technical assistance.
Funding
This study was supported by the Key Projects in the
National Natural Science Foundation of China (grant nos. 81503558
and 81403388).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
PZ, JL and RZ designed the present study and were
involved in analysis and interpretation of data. All authors
discussed the results and implications and commented on the
manuscript at all stages, as well as in the final approval of the
version to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marshall DC, Salciccioli JD, Shea BS and
Akuthota P: Trends in mortality from idiopathic pulmonary fibrosis
in the European Union: An observational study of the WHO mortality
database from 2001–2013. Eur Respir J. 51(pii): 17016032018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mikamo M, Kitagawa K, Sakai S, Uchida C,
Ohhata T, Nishimoto K, Niida H, Suzuki S, Nakayama KI, Inui N, et
al: Inhibiting skp2 e3 ligase suppresses bleomycin-induced
pulmonary fibrosis. Int J Mol Sci. 19(pii): E4742018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miao C, Xiong Y, Zhang G and Chang J:
MicroRNAs in idiopathic pulmonary fibrosis, new research progress
and their pathophysiological implication. Exp Lung Res. 44:178–190.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Redente EF, Aguilar MA, Black BP, Edelman
BL, Bahadur AN, Humphries SM, Lynch DA, Wollin L and Riches DWH:
Nintedanib reduces pulmonary fibrosis in a model of rheumatoid
arthritis-associated interstitial lung disease. Am J Physiol Lung
Cell Mol Physiol. 314:L998–L1009. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernández MC, Gonzalez A, Caputo F,
Bottinelli Y, Nastavi P and Zamboni M: Pulmonary fibrosis
associated with anti-neutrophil cytoplasmic antibody positive
vasculitis. Medicina (B Aires). 72:329–331. 2012.(In Spanish).
PubMed/NCBI
|
|
6
|
Stevenson DK, Ostrander CE and Johnson JD:
Effect of erythrocyte destruction on the pulmonary excretion rate
of carbon monoxide in adult male Wistar rats. J Lab Clin Med.
94:649–654. 1979.PubMed/NCBI
|
|
7
|
Yoshinouchi T, Ohtsuki Y, Ueda R, Sato S
and Ueda N: Myofibroblasts and S-100 protein positive cells in
idiopathic pulmonary fibrosis and rheumatoid arthritis-associated
interstitial pneumonia. Eur Respir J. 14:579–584. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadeghi S, Granton JT, Akhavan P,
Pasarikovski CR, Roos AM, Thenganatt J, Moric J and Johnson SR:
Survival in rheumatoid arthritis-associated pulmonary arterial
hypertension compared with idiopathic pulmonary arterial
hypertension. Respirology. 20:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kitamura A, Matsuno T, Narita M, Shimokata
K, Yamashita Y and Mori N: Rheumatoid arthritis with diffuse
pulmonary rheumatoid nodules. Pathol Int. 54:798–802. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grumelli S, Corry DB, Song LZ, Song L,
Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE and Kheradmand
F: An immune basis for lung parenchymal destruction in chronic
obstructive pulmonary disease and emphysema. PLoS Med. 1:e82004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taraseviciene-Stewart L, Douglas IS,
Nana-Sinkam PS, Lee JD, Tuder RM, Nicolls MR and Voelkel NF: Is
alveolar destruction and emphysema in chronic obstructive pulmonary
disease an immune disease? Proc Am Thorac Soc. 3:687–690. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arcangeli G, Cupelli V and Giuliano G:
Effects of silica on human lung fibroblast in culture. Sci Total
Environ. 270:135–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomes I, Espendshade B, Varga J and
Ackerman S: Eosinophil-derived IL-1β, TGF-β and bFGF induce lung
fibroblast secretion of the pro-fibrogenic cytokine IL-6: A
potential mechanism for subepithelial fibrosis in asthma. J Allergy
Clin Immunol. 111 (Suppl):S1872003. View Article : Google Scholar
|
|
14
|
An J, Xu R and Musser J: Methods for
isolation of triptolide compounds from tripterygium WilfordII.
Google Patents. 2007.
|
|
15
|
Fan D, He X, Bian Y, Guo Q, Zheng K, Zhao
Y, Lu C, Liu B, Xu X, Zhang G and Lu A: Triptolide modulates TREM-1
signal pathway to inhibit the inflammatory response in rheumatoid
arthritis. Int J Mol Sci. 17:4982016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lei DL, Li MB, Xiong K, Deng XH and Luo
XG: Triptolide inhibits the Aβ deposition and senile plaques
formation in the hippocampus of APP/PS1 double transgenic mice.
Acta Anatomica Sinica. 40:369–373. 2009.
|
|
17
|
Zhang C, Cui GH, Liu F, Wu QL and Chen Y:
Effects of triptolide on cell proliferation and CXCR4 expression in
Burkitt's lymphoma Raji cells in vitro. Chin J Cancer Res.
19:27–31. 2007. View Article : Google Scholar
|
|
18
|
Reno TA, Kim JY and Raz DJ: Triptolide
inhibits lung cancer cell migration, invasion, and metastasis. Ann
Thoracic Surg. 100:1817–1825. 2015. View Article : Google Scholar
|
|
19
|
Yang S, Zhang M, Chen C, Cao Y, Tian Y,
Guo Y, Zhang B, Wang X, Yin L, Zhang Z, et al: Triptolide mitigates
radiation-induced pulmonary fibrosis. Radiat Res. 184:509–517.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao X and Guan JL: Focal adhesion kinase
and its signaling pathways in cell migration and angiogenesis. Adv
Drug Del Rev. 63:610–615. 2011. View Article : Google Scholar
|
|
21
|
Zhang J, Fan G, Zhao H, Wang Z, Li F,
Zhang P, Zhang J, Wang X and Wang W: Targeted inhibition of focal
adhesion kinase attenuates cardiac fibrosis and preserves heart
function in adverse cardiac remodeling. Sci Rep. 7:431462017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao XK, Yu L, Cheng ML, Che P, Lu YY,
Zhang Q, Mu M, Li H, Zhu LL, Zhu JJ, et al: Focal adhesion kinase
regulates hepatic stellate cell activation and liver fibrosis. Sci
Rep. 7:40322017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan GP, Wang W, Zhao H, Cai L, Zhang PD,
Yang ZH, Zhang J and Wang X: Pharmacological inhibition of focal
adhesion kinase attenuates cardiac fibrosis in mice cardiac
fibroblast and post-myocardial-infarction models. Cell Physiol
Biochem. 37:515–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abedi H and Zachary I: Vascular
endothelial growth factor stimulates tyrosine phosphorylation and
recruitment to new focal adhesions of focal adhesion kinase and
paxillin in endothelial cells. J Biol Chem. 272:15442–15451. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wheaton AK, Agarwal M, Jia S and Kim KK:
Lung epithelial cell focal adhesion kinase signaling inhibits lung
injury and fibrosis. Am J Physiol Lung Cell Mol Physiol.
312:L722–L730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang HR, Lee CG, Homer RJ and Elias JA:
Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary
fibrosis. J Exp Med. 204:1083–1093. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chan KT, Bennin DA and Huttenlocher A:
Regulation of adhesion dynamics by calpain-mediated proteolysis of
focal adhesion kinase (FAK). J Biol Chem. 285:11418–11426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zitnik RJ, Kotloff RM, Latifpour J, Zheng
T, Whiting NL, Schwalb J and Elias JA: Retinoic acid inhibition of
IL-1-induced IL-6 production by human lung fibroblasts. J Immunol.
152:1419–1427. 1994.PubMed/NCBI
|
|
30
|
Zhou J, Sun X, Zhang J, Yang Y, Chen D and
Cao J: IL-34 regulates IL-6 and IL-8 production in human lung
fibroblasts via MAPK, PI3K-Akt, JAK and NF-κB signaling pathways.
Int Immunopharmacol. 61:119–125. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui Y, Robertson J, Maharaj S, Waldhauser
L, Niu J, Wang J, Farkas L, Kolb M and Gauldie J: Oxidative stress
contributes to the induction and persistence of TGF-β1 induced
pulmonary fibrosis. Int J Biochem Cell Biol. 43:1122–1133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang M, Cao SR, Zhang R, Jin JL and Zhu
YF: The inhibitory effect of salvianolic acid B on TGF-β1-induced
proliferation and differentiation in lung fibroblasts. Exp Lung
Res. 40:172–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khalil N, Xu YD, O'Connor R and Duronio V:
Proliferation of pulmonary interstitial fibroblasts is mediated by
transforming growth factor-beta1-induced release of extracellular
fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK.
J Biol Chem. 280:43000–43009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Low RB, Cutroneo KR, Davis GS and Giancola
MS: Lavage type III procollagen N-terminal peptides in human
pulmonary fibrosis and sarcoidosis. Lab Invest. 48:755–759.
1983.PubMed/NCBI
|
|
35
|
Yurovsky V: TRAIL-mediated enhancement of
collagen production by human lung fibroblasts. Arthritis Res. 4
(Spuul 1):542002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Povedano JM, Martinez P, Serrano R, Tejera
Á, Gómez-López G, Bobadilla M, Flores JM, Bosch F and Blasco MA:
Therapeutic effects of telomerase in mice with pulmonary fibrosis
induced by damage to the lungs and short telomeres. Elife. 7(pii):
e312992018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen C, Yang S, Zhang M, Zhang Z, Hong J,
Han D, Ma J, Zhang SB, Okunieff P and Zhang L: Triptolide mitigates
radiation-induced pulmonary fibrosis via inhibition of axis of
alveolar macrophages-NOXes-ROS-myofibroblasts. Cancer Biol Ther.
17:381–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tabata C, Tabata R and Nakano T: The
calpain inhibitor calpeptin prevents bleomycin-induced pulmonary
fibrosis in mice. Clin Exp Immunol. 162:560–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Giménez A, Duch P, Puig M, Gabasa M,
Xaubet A and Alcaraz J: Dysregulated collagen homeostasis by matrix
stiffening and TGF-β1 in fibroblasts from idiopathic pulmonary
fibrosis patients: Role of FAK/Akt. Int J Mol Sci. 18(pii):
E24312017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li FZ, Cai PC, Song LJ, Zhou LL, Zhang Q,
Rao SS, Xia Y, Xiang F, Xin JB, Greer PA, et al: Crosstalk between
calpain activation and TGF-β1 augments collagen-I synthesis in
pulmonary fibrosis. Biochim Biophys Acta. 1852:1796–1804. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chan SL and Mattson MP: Caspase and
calpain substrates: Roles in synaptic plasticity and cell death. J
Neurosci Res. 58:167–190. 1999. View Article : Google Scholar : PubMed/NCBI
|



















