Introduction
Systemic-onset juvenile idiopathic arthritis (SOJIA)
is a serious type of juvenile arthritis (1). It is driven by continuous activation of
innate immune pathways producing pro-inflammatory cytokines
(2).
Treatment with tacrolimus may suppress early
activation of interleukin (IL)-2 gene transcription and inhibit the
production of tumor necrosis factor-α (TNF-α), IL-1β and IL-6
during T-cell activation (3,4). Tacrolimus treatment in patients with
SOJIA has been reported previously (5–8).
However, as an immunosuppressive agent, the therapeutic range of
tacrolimus is narrow, with considerable inter- and intra-individual
variability (9,10).
Population pharmacokinetics (PPK) may be used to
acquire PK information from sparse population data. Furthermore,
PPK may differentiate between inter-individual and intra-individual
variability and has considerable power to uncover the effects of
confounding factors on PK behavior and to ensure that a treatment
is suitable for personalized clinical therapy (11). In previous studies, PPK models of
tacrolimus were set up among different populations (12–24).
However, the PPK model of tacrolimus treatment in patients with
SOJIA has remained to be established. The aim of the present study
was to set up a tacrolimus PPK model in patients with SOJIA and to
formulate initial dosage recommendations for personalized
treatment.
Materials and methods
Patient data
Data from Chinese patients with SOJIA who attended
the Children's Hospital of Fudan University (Shanghai, China)
between January 2014 and December 2017 were retrospectively
collected. The inclusion criteria were as follows: i) Pediatric
patients with SOJIA (aged <16 years); ii) treatment with
tacrolimus; iii) tacrolimus concentrations were routinely tested by
therapeutic drug monitoring (TDM). Subjects with other concurrent
serious clinical conditions were excluded (e.g. liver, kidney or
bone marrow transplant). A schematic depicting the recruitment of
the patients is provided in Fig. 1.
Relevant clinical information and data on drug concentrations were
gathered from medical records and TDM records, respectively.
Demographic data of the patients and concomitant drugs were used as
potential covariates to be analyzed in the current PPK model. The
present study was a retrospective study and was approved by the
ethics committee of the Children's Hospital of Fudan University
(Shanghai, China) without the requirement for written informed
consent.
Drug administration and analytical
method
Oral tacrolimus dose adjustment was based on safety
and effectiveness, along with the drug trough concentration from
TDM. The whole-blood concentration of tacrolimus was analyzed using
the Emit® 2000 Tacrolimus assay (Siemens Healthcare
Diagnostics Inc.) according to the manufacturer's protocol.
PPK modeling
Patient data were analyzed using the nonlinear
mixed-effects model (NONMEM, edition 7; ICON Development
Solutions). PK parameters and their variability were estimated
using the first-order conditional estimation method with
interaction. The absorption phase was described using a first-order
absorption and elimination one-compartment model. The PK parameters
included apparent oral clearance (CL/F) and apparent volume of
distribution (V/F). F was the bioavailability. The absorption rate
constant (Ka) was set at 4.48/h (15).
Random-effects model
Inter-individual variability was analyzed using an
exponential error model, as presented in equation i:
(i)Pj=TV(P)xexp(ηj)
Pj is the value of the individual
parameter. TV(P) is the parameter of the typical value. The
individual deviation is represented by ηj, which is a
symmetrically distributed, zero-mean random variable with variance
terms.
The random residual variability was described using
equation ii:
(ii)Y=IPRED+ε
Y is the concentration observed and IPRED represents
the individual predicted concentration. The variation is
represented by ε, which is a symmetrically distributed, zero-mean
random variable with variance terms.
Covariate model
Weight and PK parameters were modeled using equation
iii:
(iii)Pj=Pnormx(Weightj/Weightnorm)COE
Pj represents the PK parameter of the
j-th individual, Weightj is the Weight of the j-th
individual and Pnorm is the parameter of an individual
with a normal Weight (Weightnorm) of 70 kg. The COE is
the allometric coefficient: 0.75 for the CL/F and 1 for the V/F
(25).
Continuous covariates and categorical covariates
were used to describe the correlation between PK parameters using
equations iv and v:
(iv)Pj=TV(P)x(Covj/Covmed)θ(v)Pj=TV(P)x(1+θxCovj)
Pj and TV(P) are the individual parameter
value and typical parameter value, respectively. Covj is
the covariate of the j-th individual and θ is the parameter to be
estimated. Covmed is the population median for the
covariate.
This stepwise protocol was used to build the
covariate model. The likelihood ratio test was used to compare
hierarchical models. The covariate model was established in a
stepwise manner, using the forward inclusion, backward elimination
method (11,21,24,26,27).
Changes in objective function values (OFV) were performed using
covariate inclusions and a decrease of OFV >3.84 (P<0.05) was
considered sufficient for inclusion in the base model (11,21,24,26,27).
After establishing a full regression model, the model was further
assessed by eliminating covariates from each PK parameter
one-by-one to obtain the final model. An increase in OFV >6.64
(P<0.01) was considered sufficient for significance in the final
model (11,21,24,26,27).
This statistical method and its description have been published in
numerous similar studies and may be considered as a fixed and
applicable statistical method for PPK analysis (11,21,24,26,27).
Model validation
The stability and reliability of the final parameter
estimates were evaluated using the internal validation method of
bootstrap, which was produced using repeated random sampling, with
replacement from the original data. The process was performed using
the Wings package for NONMEM software and was repeated 1,000 times
with different random draws. The median values and 2.5–97.5%
percentile parameters from the bootstrap results were compared with
the final PK parameters. Visual inspection of routine diagnostic
plots along with prediction-corrected visual predictive check (VPC)
plots were used to assess the final model.
Simulation of initial dosage
recommendations
Monte Carlo simulation is an approach used to
determine probability of target (28) and has been applied to determine the
most suitable drug administration (22,26). In
the present study, it was used to investigate the influence of
covariates on the probability to achieve the target concentrations.
A previous study reported that for safety reasons, the lower
concentration for tacrolimus treatment for SOJIA was 1.7 ng/ml and
the upper concentration was 5 ng/ml (7). Therefore, the probability to achieve
1.7 and 5 ng/ml concentration thresholds based on the established
model without the combination with other drugs was estimated.
Simulation was performed for each of the nine weight groups (5, 15,
20, 25, 30, 35, 40, 45 and 50 kg) and four dosing regimens [0.5 mg
once every 24 h (q24h), 0.5 mg q12h, 1/0.5 mg q24h and 1 mg q12h]
using 1,000 virtual patients with SOJIA.
Results
Data collection
Data from 17 Chinese patients with SOJIA (8 males
and 9 females), aged 9.50 (3.20–14.60) years, were collected to
build the population model. A total of 86 concentrations in the
range of 1.3 to 9.2 ng/ml were used. Patient information and drug
combinations are presented in Tables
I and II.
 | Table I.Demographic data of the patients. |
Table I.
Demographic data of the patients.
| Characteristic | Mean ± SD | Median (range) |
|---|
| Age (years) | 8.23±3.30 | 9.50
(3.20–14.60) |
| Weight (kg) | 29.83±10.66 | 33.60
(13.50–46.00) |
| Duration of
treatment with tacrolimus (days) | 190.59±169.57 | 67.00
(5.00–535.00) |
| Daily dose of
tacrolimus (mg) | 1.66±0.71 | 1.50
(1.00–4.00) |
| Alanine
transaminase (IU/l) | 28.56±60.24 | 12.00
(2.00–458.00) |
| Aspartate
transaminase (IU/l) | 21.01±26.82 | 15.00
(7.00–235.00) |
| Creatinine
(µmol/l) | 35.36±10.32 | 35.50
(19.00–59.00) |
| Hematocrit (%) | 37.35±3.43 | 37.45
(30.30–44.40) |
| Hemoglobin
(g/l) | 121.59±13.23 | 124.00
(90.20–152.00) |
| Mean corpuscular
hemoglobin (pg) | 27.13±2.01 | 27.00
(21.00–31.00) |
| Mean corpuscular
hemoglobin concentration (g/l) | 324.58±15.22 | 323.00
(285.00–358.00) |
 | Table II.Drug combinations with
tacrolimus. |
Table II.
Drug combinations with
tacrolimus.
| Drug/category | N |
|---|
| Ranitidine |
|
| 0 | 16 |
| 1 | 1 |
|
Hydroxychloroquine |
|
| 0 | 16 |
| 1 | 1 |
| Ceftazidime |
|
| 0 | 16 |
| 1 | 1 |
| Cefmetazole |
|
| 0 | 16 |
| 1 | 1 |
| Ceftriaxone |
|
| 0 | 16 |
| 1 | 1 |
| Cefprozil |
|
| 0 | 13 |
| 1 | 4 |
| Cefixime |
|
| 0 | 14 |
| 1 | 3 |
| Cefdinir |
|
| 0 | 11 |
| 1 | 6 |
| Azithromycin |
|
| 0 | 15 |
| 1 | 2 |
|
Methylprednisolone |
|
| 0 | 12 |
| 1 | 5 |
| Mycophenolate
mofetil |
|
| 0 | 16 |
| 1 | 1 |
| Prednisone |
|
| 0 | 2 |
| 1 | 15 |
| Oxcarbazepine |
|
| 0 | 16 |
| 1 | 1 |
| Levetiracetam |
|
| 0 | 16 |
| 1 | 1 |
| Methotrexate |
|
| 0 | 9 |
| 1 | 8 |
| Omeprazole |
|
| 0 | 11 |
| 1 | 6 |
| Diltiazem |
|
| 0 | 14 |
| 1 | 3 |
| Felodipine |
|
| 0 | 16 |
| 1 | 1 |
| Montelukast |
|
| 0 | 16 |
| 1 | 1 |
| Aspirin |
|
| 0 | 15 |
| 1 | 2 |
| Loratadine |
|
| 0 | 12 |
| 1 | 5 |
Modeling
The first-order absorption and elimination
one-compartment model was identified to fit the dataset. PK
parameters from the final covariate models were as follows in
equations vi and vii:
vi)CL/F=29.7x(weight/70)0.75x(1-0.362xomeprazole)x(1-0.322xloratadine)x(1-0.307xdiltiazem)vii)V/F=1,120x(weight/70)
When patients were co-administered omeprazole,
loratadine or diltiazem, the value of each was 1; otherwise, the
value was 0. All weights in equations vi and vii were measured in
kg.
Validation
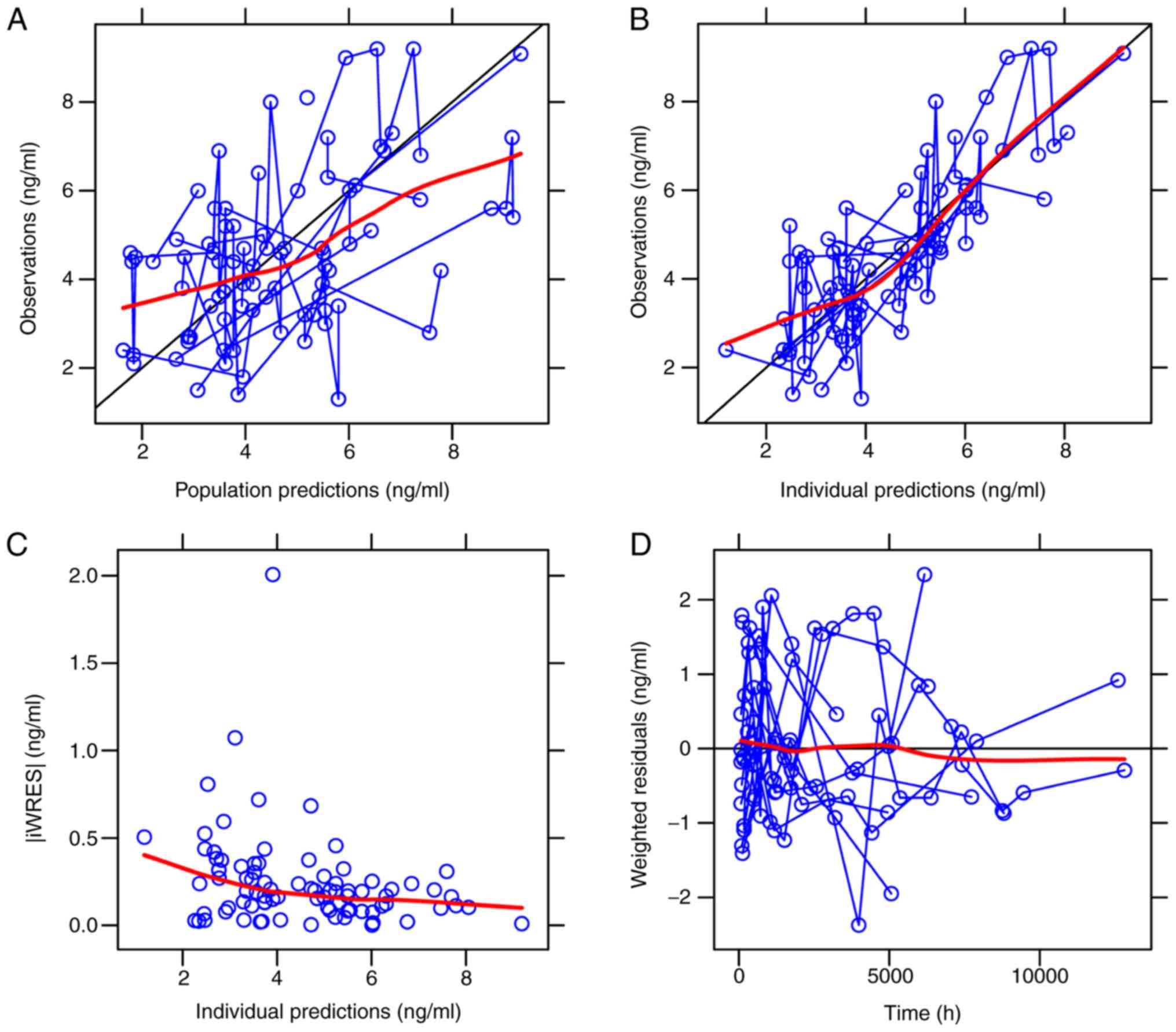
The visual inspection of routine diagnostic plots is
presented in Fig. 2 and the
parameter estimates from the final model and bootstrap validation
are presented in Table III. From
1,000 bootstrap runs, 988 runs were successfully minimized. Using
Table III, the parameter estimate
median values of bootstraps were found to be similar to the
respective values determined with the final model, indicating that
the final PPK model was accurate and reliable. The VPC plots for
the final model (Fig. 3) demonstrate
that most of the measured concentration data were included in the
95% prediction intervals of the simulation data, suggesting that
the final PPK model is able to predict concentrations
effectively.
 | Table III.Parameter estimates of final model
and bootstrap validation. |
Table III.
Parameter estimates of final model
and bootstrap validation.
|
|
|
| Bootstrap |
|
|---|
|
|
|
|
|
|
|---|
| Parameter | Estimate | SE (%) | Median | 95% CI | Bias (%) |
|---|
| CL/F (l/h) | 29.700 | 9.300 | 29.800 | (24.300,
36.400) | 0.340 |
| V/F (l) | 1120.000 | 27.900 | 1120.000 | (604.000,
2188.000) | 0 |
| Ka (1/h) | 4.480 (fixed) | – | – | – | – |
|
θomeprazole | −0.362 | 16.800 | −0.371 | (−0.499,
−0.192) | 2.490 |
|
θloratadine | −0.322 | 23.800 | −0.326 | (−0.462,
−0.081) | 1.240 |
|
θdiltiazem | −0.307 | 34.200 | −0.307 | (−0.454,
−0.006) | 0 |
|
ωCL/F | 0.265 | 18.400 | 0.243 | (0.129, 0.352) | −8.300 |
| σ1 | 1.229 | 5.100 | 1.200 | (1.040, 1.326) | −2.360 |
Simulation
In the present study, the initial tacrolimus dose
without drug combination was predicted. In clinical practice,
combination therapy is not common at the time of initial
administration. Therefore, the probability to achieve the target
concentrations based on the established model without any combined
drugs was estimated. The predicted median, along with the 2.5–97.5%
percentile parameters and the probability of achieving the target
concentration were presented in Table
IV. According to the simulation dataset, the 0.5 mg q24h
regimen appeared to be most suitable for pediatric patients with 5
kg body weight, the 0.5 mg q12h regimen was appropriate for
patients with 15–25 kg body weight, the 1/0.5 mg q24 h regimen was
most suitable for subjects with a body weight of 26–35 kg and the 1
mg q12h regimen was fit for the group with a body weight of 36–50
kg.
 | Table IV.Predicted median tacrolimus
concentration (ng/ml), 95% CI and probability (%) of achieving the
target concentration with respect to body weight for different
dosing regimens. |
Table IV.
Predicted median tacrolimus
concentration (ng/ml), 95% CI and probability (%) of achieving the
target concentration with respect to body weight for different
dosing regimens.
|
| Body weight
(kg) |
|---|
|
|
|
|---|
| Regimen | 5 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 |
|---|
| 0.5 mg q24h | 2.57
(1.00–5.82) | 1.33
(0.59–2.74) | 1.11
(0.51–2.23) | 0.96
(0.45–1.90) | 0.86
(0.41–1.66) | 0.77
(0.37–1.48) | 0.71
(0.34–1.34) | 0.65
(0.32–1.23) | 0.61
(0.30–1.13) |
|
| 77.2% | 22.5% | 12.0% | 5.3% | 1.9% | 0.9% | 0% | 0% | 0% |
| 0.5 mg q12h | 7.59
(3.54–14.64) | 3.57
(1.77–6.52) | 2.92
(1.47–5.24) | 2.49
(1.27–4.42) | 2.19
(1.12–3.85) | 1.96
(1.01–3.41) | 1.78
(0.93–3.08) | 1.63
(0.85–2.81) | 1.51
(0.80–2.58) |
|
| 12.2% | 82.7% | 89.7% | 88.0% | 79.4% | 67.7% | 55.9% | 45.4% | 35.3% |
| 1/0.5 mg q24h | 10.25
(4.45–20.66) | 4.94
(2.32–9.33) | 4.06
(1.94–7.54) | 3.48
(1.69–6.37) | 3.07
(1.50–5.55) | 2.75
(1.36–4.93) | 2.51
(1.25–4.45) | 2.31
(1.16–4.07) | 2.14
(1.08–3.75) |
|
| 4.9% | 50.9% | 71.6% | 83.1% | 87.9% | 89.1% | 87.5% | 83.3% | 77.1% |
| 1 mg q12h | 15.18
(7.08–29.27) | 7.13
(3.54–13.03) | 5.83
(2.94–10.49) | 4.98
(2.53–8.85) | 4.37
(2.25–7.69) | 3.92
(2.03–6.83) | 3.56
(1.85–6.15) | 3.27
(1.71–5.61) | 3.03
(1.59–5.17) |
|
| 0.1% | 13.7% | 31.7% | 50.5% | 65.3% | 77.3% | 89.5% | 89.8% | 92.1% |
Discussion
To control SOJIA disease, a number of patients
require long-term corticosteroid treatment (5). However, prolonged and repeated steroid
treatment increases the risk of adverse reactions, including
obesity, cushingoid appearance, hypertension, growth retardation,
osteoporosis, infections and psychological problems (29). Thus, a safe and effective therapeutic
method to treat patients with SOJIA remains to be explored
(30).
Recent studies revealed the beneficial impact of
suppressing IL-6 and other pathogenic pro-inflammatory cytokines
for controlling SOJIA (30–32). Tacrolimus potently suppresses the
production of TNF-α, IL-1β and IL-6 through T-cell activation
(4,33), and it has therefore been administered
to patients with SOJIA (5–7).
However, the therapeutic range of tacrolimus is
narrow, with considerable inter-individual and intra-individual
variability (9,10). Thus, it is necessary to build a
tacrolimus PPK model for patients with SOJIA and to formulate
initial dosage recommendations for personalized treatment.
To the best of our knowledge, the present study was
the first to provide a PPK model of tacrolimus for patients with
SOJIA. The PPK model was established for SOJIA patients by using a
population modeling method. The approach was necessary, as logistic
and ethical restrictions prohibit excessive blood sampling when
studying pediatric patients (34).
The tacrolimus PPK model is able to predict the PK process in
patients with SOJIA and it therefore has important clinical
value.
In the present study, the first-order absorption and
elimination one-compartment model fitted the dataset, as all of the
tacrolimus concentrations were trough concentrations and the Ka was
fixed at a value from the literature of 4.48/h (15). It was not possible to estimate the
area under the curve, minimum concentration and maximum
concentration of tacrolimus, as the drug was orally administered
and tacrolimus concentration data were insufficient. The typical
CL/F and V/F values of the final tacrolimus PPK model were 29.7 l/h
and 1,120 l. In the present PPK model, drug combinations were used
as categorical variables. The present study also tested the
influence of the following various covariates on different
parameters: Weight, omeprazole, loratadine and diltiazem on CL/F,
as well as Weight on V/F. Numerous studies have determined a
non-linear association between drug clearance and body weight in
pediatric patients, and it may be well described with allometric
scaling using a coefficient of 0.75 for clearance and 1 for volume
(25,26,35,36).
Body weight is the most important predictor of clearance and volume
in pediatric patients with maturation of elimination processes
(35), and is also considered to be
the primary factor determining clearance and volume based on the
theory explaining the link between mass, function and structure;
this theory is valid across numerous orders of magnitude of body
weight (37). Important factors that
also impacted tacrolimus clearance were omeprazole, loratadine and
diltiazem, possibly due to tacrolimus being a substrate of the
cytochrome P450 3A (CYP3A) enzyme (38), and omeprazole and diltiazem inhibit
CYP3A activity. In addition, loratadine is a CYP3A substrate that
is able to compete with tacrolimus for the binding site on the
enzyme and lead to a decrease in tacrolimus clearance. Thus,
concomitant medication with omeprazole, loratadine or diltiazem may
reduce tacrolimus clearance in patients with SOJIA.
In terms of model application, Monte Carlo
simulations based on the established model were used to investigate
the influence of covariates on the probability to achieve the
target concentration. The probability to achieve 1.7 and 5 ng/ml
concentration thresholds based on the established model without
drug combinations was estimated. In addition, it appears that IL-6
is a marker of pharmacodynamics of tacrolimus (8). In future studies by our group, a
population pharmacodynamics model will be built to analyze the
association between drug exposure and IL-6.
In conclusion, to the best of our knowledge, the
present study provided the first PPK model of tacrolimus in
patients with SOJIA, and may be used for precision therapy in
pediatric patients. A large external evaluation of this model will
be performed in future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Clinical
Pharmacy Key Specialty Construction Project of Shanghai (grant no.
YZ2017/5), Important Weak Subject Construction Project of Shanghai
(grant no. 2016ZB0305), the Scientific Research Project of Science
and Technology Commission of Shanghai Municipality (grant no.
18DZ1910604) and the Fudan University Hospital Management
Construction Project (Fudan medical administration 2018).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL and HX conceived and designed the study. DW and
XC collected and analyzed the data. DW and XC wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Research Ethics
Committee of the Children's Hospital of Fudan University (Shanghai,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schulert GS, Minoia F, Bohnsack J, Cron
RQ, Hashad S, KonÉ-Paut I, Kostik M, Lovell D, Maritsi D, Nigrovic
PA, et al: Effect of biologic therapy on clinical and laboratory
features of macrophage activation syndrome associated with systemic
juvenile idiopathic arthritis. Arthritis Care Res (Hoboken).
70:409–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cimaz R: Systemic-onset juvenile
idiopathic arthritis. Autoimmun Rev. 15:931–934. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawai S and Yamamoto K: Safety of
tacrolimus, an immunosuppressive agent, in the treatment of
rheumatoid arthritis in elderly patients. Rheumatology (Oxford).
45:441–444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo H, Abe T, Hashimoto H, Uchida S,
Irimajiri S, Hara M and Sugawara S: Efficacy and safety of
tacrolimus (FK506) in treatment of rheumatoid arthritis: A
randomized, double blind, placebo controlled dose-finding study. J
Rheumatol. 31:243–251. 2004.PubMed/NCBI
|
|
5
|
Chandrasekhara PK, Jayachandran NV, Thomas
J, Agrawal S and Narsimulu G: Successful treatment of pyoderma
gangrenosum associated with juvenile idiopathic arthritis with a
combination of topical tacrolimus and oral prednisolone. Clin
Rheumatol. 28:489–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimizu M, Ueno K, Ishikawa S, Tokuhisa Y,
Inoue N and Yachie A: Treatment of refractory polyarticular
juvenile idiopathic arthritis with tacrolimus. Rheumatology
(Oxford). 53:2120–2122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka H, Tsugawa K, Suzuki K, Oki ES,
Nonaka K, Kimura S and Ito E: Treatment of difficult cases of
systemic-onset juvenile idiopathic arthritis with tacrolimus. Eur J
Pediatr. 166:1053–1055. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang D, Chen X and Li Z: Treatment of
patients with systemic-onset juvenile idiopathic arthritis with
tacrolimus. Exp Ther Med. 17:2305–2309. 2019.PubMed/NCBI
|
|
9
|
Jusko WJ, Thomson AW, Fung J, McMaster P,
Wong SH, Zylber-Katz E, Christians U, Winkler M, Fitzsimmons WE,
Lieberman R, et al: Consensus document: Therapeutic monitoring of
tacrolimus (FK-506). Ther Drug Monit. 17:606–614. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Venkataramanan R, Swaminathan A, Prasad T,
Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G and
Starzl T: Clinical pharmacokinetics of tacrolimus. Clin
Pharmacokinet. 29:404–430. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vadcharavivad S, Praisuwan S,
Techawathanawanna N, Treyaprasert W and Avihingsanon Y: Population
pharmacokinetics of tacrolimus in Thai kidney transplant patients:
Comparison with similar data from other populations. J Clin Pharm
Ther. 41:310–328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Monchaud C, de Winter BC, Knoop C, Estenne
M, Reynaud-Gaubert M, Pison C, Stern M, Kessler R, Guillemain R,
Marquet P and Rousseau A: Population pharmacokinetic modelling and
design of a Bayesian estimator for therapeutic drug monitoring of
tacrolimus in lung transplantation. Clin Pharmacokinet. 51:175–186.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wallin JE, Friberg LE, Fasth A and Staatz
CE: Population pharmacokinetics of tacrolimus in pediatric
hematopoietic stem cell transplant recipients: New initial dosage
suggestions and a model-based dosage adjustment tool. Ther Drug
Monit. 31:457–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu L, Yang J, Zhang Y, Jing Y, Zhang Y
and Li G: Effects of CYP3A5 genotypes, ABCB1 C3435T and G2677T/A
polymorphism on pharmacokinetics of Tacrolimus in Chinese adult
liver transplant patients. Xenobiotica. 45:840–846. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang JW, Liao SS, Zhu LQ, Zhao Y, Zhang Y,
Sun XY, Rao W, Qu W, Li WZ and Sun LY: Population pharmacokinetic
analysis of tacrolimus early after Chinese pediatric liver
transplantation. Int J Clin Pharmacol Ther. 53:75–83. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Musuamba FT, Guy-Viterbo V, Reding R,
Verbeeck RK and Wallemacq P: Population pharmacokinetic analysis of
tacrolimus early after pediatric liver transplantation. Ther Drug
Monit. 36:54–61. 2014.PubMed/NCBI
|
|
17
|
Lu YX, Su QH, Wu KH, Ren YP, Li L, Zhou TY
and Lu W: A population pharmacokinetic study of tacrolimus in
healthy Chinese volunteers and liver transplant patients. Acta
Pharmacol Sin. 36:281–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andreu F, Colom H, Grinyó JM, Torras J,
Cruzado JM and Lloberas N: Development of a population PK model of
tacrolimus for adaptive dosage control in stable kidney transplant
patients. Ther Drug Monit. 37:246–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergmann TK, Hennig S, Barraclough KA,
Isbel NM and Staatz CE: Population pharmacokinetics of tacrolimus
in adult kidney transplant patients: Impact of CYP3A5 genotype on
starting dose. Ther Drug Monit. 36:62–70. 2014.PubMed/NCBI
|
|
20
|
Han N, Ha S, Yun HY, Kim MG, Min SI, Ha J,
Lee JI, Oh JM and Kim IW: Population
pharmacokinetic-pharmacogenetic model of tacrolimus in the early
period after kidney transplantation. Basic Clin Pharmacol Toxicol.
114:400–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang DD, Chen X and Li ZP: Wuzhi capsule
and haemoglobin influence tacrolimus elimination in paediatric
kidney transplantation patients in a population pharmacokinetics
analysis: A retrospective study. J Clin Pharm Ther. 44:611–617.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang D, Chen X, Xu H and Li Z: Population
pharmacokinetics and dosing regimen optimization of tacrolimus in
Chinese pediatric hematopoietic stem cell transplantation patients.
Xenobiotica. Apr 2;1–8. 2019.doi: 10.1080/00498254.2019.1601791
(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Wang DD, Chen X, Fu M, Zheng QS, Xu H and
Li ZP: Model extrapolation to a real-world dataset: Evaluation of
tacrolimus population pharmacokinetics and drug interaction in
pediatric liver transplantation patients. Xenobiotica. Jul 3;1–9.
2019.doi: 10.1080/00498254.2019.1631505 (Epub ahead of print).
View Article : Google Scholar
|
|
24
|
Wang D, Lu J, Li Q and Li Z: Population
pharmacokinetics of tacrolimus in pediatric refractory nephrotic
syndrome and a summary of other pediatric disease models. Exp Ther
Med. 17:4023–4031. 2019.PubMed/NCBI
|
|
25
|
Anderson BJ and Holford NH:
Mechanism-based concepts of size and maturity in pharmacokinetics.
Annu Rev Pharmacol Toxicol. 48:303–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang DD, Ye QF, Chen X, Xu H and Li ZP:
Population pharmacokinetics and initial dosing regimen optimization
of cyclosporin in pediatric hemophagocytic lymphohistiocytosis
patients. Xenobiotica. Aug 14;1–7. 2019.doi:
10.1080/00498254.2019.1651419 (Epub ahead of print). View Article : Google Scholar
|
|
27
|
Wang D, Chen X and Li Z: Population
pharmacokinetics of sirolimus in pediatric patients with kaposiform
hemangioendothelioma: A retrospective study. Oncol Lett.
18:2412–2419. 2019.PubMed/NCBI
|
|
28
|
Sprandel KA, Drusano GL, Hecht DW,
Rotschafer JC, Danziger LH and Rodvold KA: Population
pharmacokinetic modeling and Monte Carlo simulation of varying
doses of intravenous metronidazole. Diagn Microbiol Infect Dis.
55:303–309. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hahn D, Hodson EM, Willis NS and Craig JC:
Corticosteroid therapy for nephrotic syndrome in children. Cochrane
Database Syst Rev. CD0015332015.PubMed/NCBI
|
|
30
|
Tanaka H, Tsugawa K, Nakahata T, Suzuki K
and Ito E: Leukocytapheresis for the treatment of refractory
systemic-onset juvenile idiopathic arthritis. Clin Rheumatol.
26:1014–1016. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kimura Y, Pinho P, Walco G, Higgins G,
Hummell D, Szer I, Henrickson M, Watcher S and Reiff A: Etanercept
treatment in patients with refractory systemic onset juvenile
rheumatoid arthritis. J Rheumatol. 32:935–942. 2005.PubMed/NCBI
|
|
32
|
Quartier P, Taupin P, Bourdeaut F, Lemelle
I, Pillet P, Bost M, Sibilia J, Koné-Paut I, Gandon-Laloum S,
LeBideau M, et al: Efficacy of etanercept for the treatment of
juvenile idiopathic arthritis according to the onset type.
Arthritis Rheum. 48:1093–1101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mok CC, Tong KH, To CH, Siu YP and Au TC:
Tacrolimus for induction therapy of diffuse proliferative lupus
nephritis: An open-labeled pilot study. Kidney Int. 68:813–817.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kauffman RE and Kearns GL: Pharmacokinetic
studies in paediatric patients. Clinical and ethical
considerations. Clin Pharmacokinet. 23:10–29. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anderson BJ and Holford NH: Tips and traps
analyzing pediatric PK data. Paediatr Anaesth. 21:222–237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holford NH: A size standard for
pharmacokinetics. Clin Pharmacokinet. 30:329–332. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Savage VM, Deeds EJ and Fontana W: Sizing
up allometric scaling theory. PLoS Comput Biol. 4:e10001712008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jacobo-Cabral CO, García-Roca P,
Romero-Tejeda EM, Reyes H, Medeiros M, Castañeda-Hernández G and
Trocóniz IF: Population pharmacokinetic analysis of tacrolimus in
Mexican paediatric renal transplant patients: Role of CYP3A5
genotype and formulation. Br J Clin Pharmacol. 80:630–641. 2015.
View Article : Google Scholar : PubMed/NCBI
|