Introduction
Lumbar fusion has been employed in a variety of
disorders, including degenerative intervertebral disc herniation,
lumbar spondylolisthesis, lumbar tuberculosis and spine tumors.
Lumbar fusion originally involved autologous bone grafting, usually
from the iliac crest, into the intervertebral space without the use
of any screws or cages. The utilization of autogenous bone graft
was thought to be the most reliable method for achieving solid
spinal fusion. In 1936, Mercer (1)
used tricortical iliac crest autografts for anterior interbody
fusions. At present, autologous cortico-cancellous bone grafts are
still regarded as the gold standard for spinal fusion (2). However, lumbar spinal fusion using
autografts is commonly associated with extended surgical time,
hematoma, wound healing problems, pain lasting >6 months with
sensory loss, infection and fracture at the donor site (3–5). Even
after the introduction of spinal instrumentation, including
interbody cages and transpedicular screws, which provide instant
stability to aid fusion, the procedure was still commonly
associated with complications, including instrumentation failure,
kyphosis and pseudarthrosis (6,7).
Therefore, it would be beneficial if a more reliable autogenous
bone graft for clinical therapy were to be established. In this
light, the present study reported on a newly developed cage-shaped
demineralized bone plus local bone graft (CDBLG), in which an
allograft and autograft are combined to improve the above-mentioned
issues. A comparison of the clinical outcomes of autogenous iliac
crest bone graft (ICBG) with CDBLG in single-level lumbar fusion
was provided.
Patients and methods
CDBLG design
A CDBLG (Chinese patent no. ZL2008301126534) was
developed, in which a local autograft was inserted into hollow
allogeneic bone (Fig. 1A-C). In the
present study, the allogeneic bone was not a simple graft as in
previous studies but it was demineralized and formed into a
cage-like shape. The structure of the CDBLG was designed to be wide
at the front and narrow at the back, in accordance with the
physiological characteristics of the spine. In addition, the
allogeneic bone had high strength and was of low cost to suit the
requirements of patients in the poor region of western China.
Inclusion and exclusion criteria
In this retrospective study, the inclusion criteria
were as follows: i) Patient age, 20–75 years at the time of
treatment; ii) degenerative disc disease, discogenic back pain
with/without leg pain documented on X-ray films, CT or MRI; iii)
the patient is a candidate for only one-level posterolateral lumbar
fusion; iv) unresponsive to conservative treatment for a period of
3 months and v) the patient signed an informed consent form
specific to this study that was approved by the Review Board of the
General Hospital of Xinjiang Military Region.
The following exclusion criteria were applied: i)
Previous posterior lumbar fusion at the currently involved level;
ii) presence of a hard- or soft-tissue infection at the operative
site; iii) endocrine or metabolic disorder affecting osteogenesis
(e.g., insulin-dependent diabetes, renal osteodystrophy); iv) use
of medications known to affect the skeleton, including long-term
use of glucocorticoid or non-steroidal anti-inflammatory drugs; v)
mental disorders (e.g., Alzheimer's disease or a diagnosed mental
disorder); vi) tobacco users refusing to stop smoking 6 weeks prior
to surgery until 1 year after surgery; and vii) patients with other
diseases that do not allow for surgery.
Subjects
Following approval from the Review Board of the
General Hospital of Xinjiang Military Region (approval no.
ZYLL-2018-23) and obtainment of informed consent from all patients
in accordance with the Declaration of Helsinki, 78 adult patients
who had consecutively undergone lumbar decompression with
transpedicular screw instrumented posterolateral fusion between
January 2011 and December 2013 were selected for the present study.
After exclusion of 9 patients, the cohort comprised 43 male and 26
female patients with degenerative spinal disorders. Of these, 44
received CDBLG and 25 cases were subjected to ICBG. The mean age of
the patients was 52.6±9.6 years (range, 34–75 years). The minimum
follow-up duration was 2 years and the mean follow-up duration was
53 months (range, 24–71 months). All cases were diagnosed based on
clinical symptoms, plain radiographs, MRI and electrophysiology
examination.
Graft surgery technique
Autogenous ICBG were made in a standard open fashion
(8). Freeze-dried allogeneic
cortical bone grafts from the Shanxi Aorui Bone Bank were used to
construct cage-shaped demineralized bone. Local bone grafts were
obtained from a decompression procedure of the spinous process and
lamina. All attached soft tissues were removed and the mixed
cage-shaped demineralized bone and local bone fragment graft were
used in the spinal surgery.
All patients underwent open posterior laminectomy,
nerve decompression and pedicle screw instrumented single-level
lumbar fusion, with patients placed in the prone position. After
exposure of the vertebral laminae, nerve decompression was achieved
by removal of the spinal process, vertebral lamina, attached
ligamentum flavum and partial joint facet. The nucleus pulposus was
then re-sectioned and the cartilage was removed from the endplates.
A CDBLG or ICBG was then placed in the intervertebral space
(Fig. 1D and E) and Horizon
transpedicular screws (Medtronic Sofamor Danek) were placed in the
target segments. The segments to be fused were joined using
contoured rods (8).
Determination of fusion
The first method for the determination of fusion
success was based on the Investigational Device Exemption protocol
(9). According to this method, a
fusion was considered successful when fulfilling the following
standards: The presence of bilateral, continuous trabeculated bone
connecting the transverse processes, translation of ≤3 mm and an
angulation of <5° on flexion/extension radiographs, and absence
of cracking, as evidenced by radiolucent lines through the fusion
mass. Furthermore, a second method based on CT scans was used. As
reported by Williams et al (10), the presence of continuous bone
connecting the vertebral bodies was considered to indicate
successful fusion. Bone fusion usually is near completion at 6
months with evidence of bridging of the trabecular bone. The
bridging bone is usually seen lateral to or within the implant. The
radiographs and CT scans were evaluated by two independent
radiologists who were blinded regarding the patient group 6, 12,
and 24 months after surgery. A third adjudicate reviewer was used
as required.
Clinical outcome assessments
Imaging analysis consisted of plain anteroposterior,
lateral and flexion/extension radiographs, and fine-cut axial CT
scans with sagittal and coronal reconstruction. These were
performed pre-operatively and after 6, 12 and 24 months
post-operatively.
Standard demographic data were collected for all
patients, including age, sex, body weight, smoking and drinking
history, diabetes and history of prior back surgery. Outcome
measures consisting of Oswestry Disability Index (ODI) (11), Visual Analogue Scale (VAS) for back
and leg pain (12), and Short
Form-36 general health survey physical component summary (SF-36
PCS) (13) were collected
pre-operatively and at 3, 6, 12 and 24 months post-operatively.
Statistical analysis
The data obtained from the 69 patients were compared
using SPSS software (v20.0; IBM Corp.). The two groups were
compared using the Wilcoxon rank-sum test for quantitative
variables and Fisher's exact test for categorical variables.
Outcomes were analyzed using a repeated-measures ANOVA with time as
the factor within subjects and treatment as the factor between
subjects. A post-hoc analysis using Bonferroni's adjustment was
performed for further multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Of the 78 patients initially included, 69 had
complete data regarding outcome measures and radiographic
assessments at 2 years. Diagnosis included degenerative lumbar
herniated disc in 35 cases (51%, L1 to L5), lumbosacral herniated
disc in 23 cases (33%, L5 to S1), degenerative lumbar or
lumbosacral herniated disc with spondylolisthesis in 5 cases (7%)
and degenerative scoliosis exceeding 20° in 6 cases (9%). The
demographic data and disease characteristics of the patients,
including age, sex, tobacco/alcohol use, diabetic status and fusion
level, are presented in Table I.
There is no significant difference regarding all of the
clinicopathological parameters between the two groups.
 | Table I.Demographics and characteristics of
the patients. |
Table I.
Demographics and characteristics of
the patients.
| Parameter | CDBLG (n=44) | ICBG (n=25) | P-value |
|---|
| Age (years) | 53.2±10.8 | 51.7±11.5 | 0.589 |
| Sex
(male/female) | 28/16 | 15/10 | 0.764 |
| Body weight
(kg) | 70.4±9.8 | 68.6±11.1 | 0.487 |
| Tobacco use | 11 | 6 | 0.926 |
| Alcohol use | 13 | 8 | 0.831 |
| Diabetes | 2 | 1 | 1.000 |
| Previous lumbar
surgery | 3 | 2 | 1.000 |
| Level of
fusion |
|
| 1.000 |
|
L1-L2 | 0 | 0 |
|
|
L2-L3 | 0 | 0 |
|
|
L3-L4 | 6 | 3 |
|
|
L4-L5 | 21 | 12 |
|
|
L5-S1 | 17 | 10 |
|
Radiologic outcomes
Solid fusion mass was observed at 24 months after
L4-L5 fusion using CDBLG (Representative images in Fig. 2). Evaluation with the radiographic
method indicated that 92.0% of patients in the ICBG group and 88.7%
in the CDBLG group had evidence of interbody process fusion
(Fig. 3). Thus, no significant
differences between the ICBG and CDBLG groups were observed using
the radiographic imaging and CT methods (P>0.05).
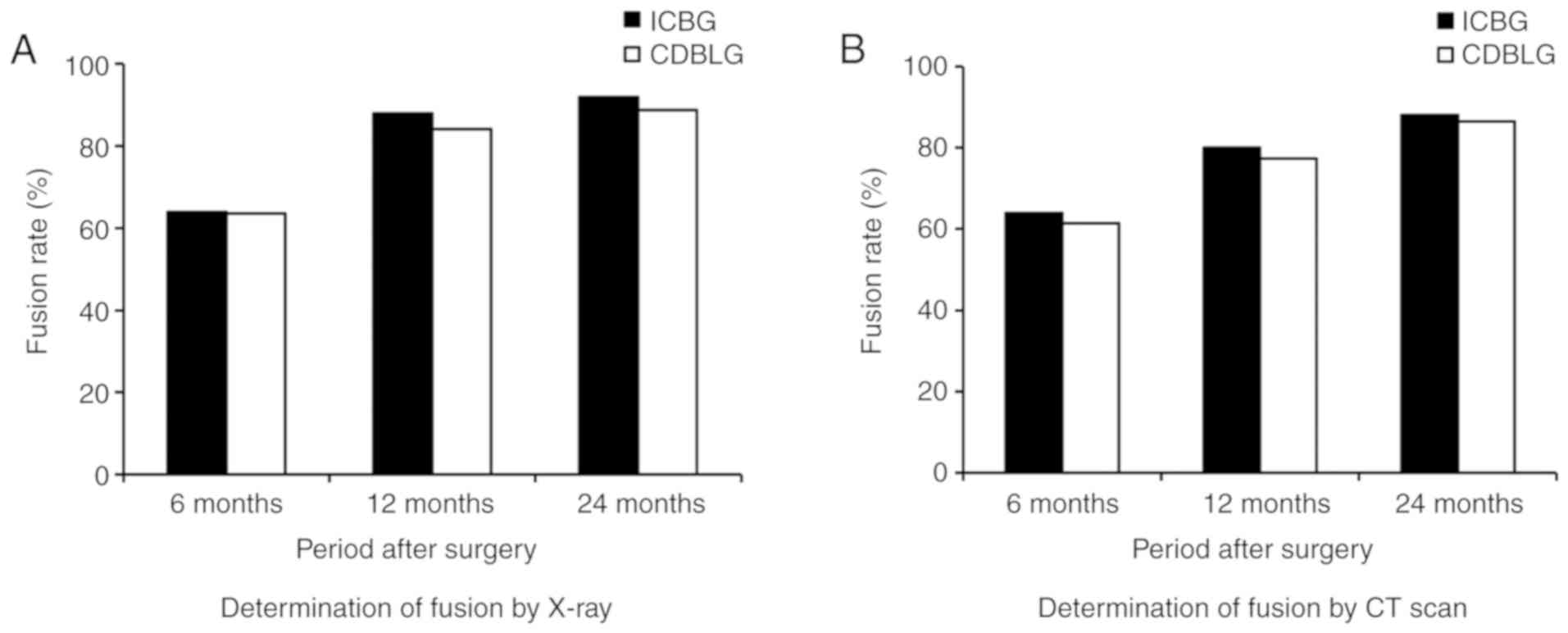 | Figure 3.Fusion rates at 6, 12 and 24 months
examined using (A) plain and flexion/extension X-ray radiographs
and (B) CT scans. There was no statistically significant difference
in the fusion rate between the CDBLG and ICBG group determined by
the first or the second detection method [first detection method
(plain and flexion/extension X-ray radiographs)], P=0.918, 0.737
and 0.967 at 6 months, 12 months and 24 months after surgery,
respectively; second detection method (CT-scans), P=0.818, 0.968
and 1.000 in 6 months, 12 months and 24 months after surgery,
respectively (n=25 in ICBG group and n=44 in CDBLG group)]. CDBLG,
cage-shaped demineralized bone plus local bone grafts; ICBG, iliac
crest bone grafts. |
Scoring
The ODI and VAS score for back and leg pain, as well
as the SF-36 PCS significantly improved in the two groups
post-operatively (P<0.05). No significant differences between
the CDBLG and ICBG groups were observed in the mean ODI score at 24
months (P>0.05). At the pre-operative and various post-operative
stages, no significant differences in ODI between the ICBG and
CDBLG groups were detected (P>0.05; Fig. 4A). In addition, no significant
differences were observed between the ICBG and CDBLG groups in VAS
score for back and leg pain, and in SF-36 PCS at any of the
time-points (P>0.05; Fig.
4B-D).
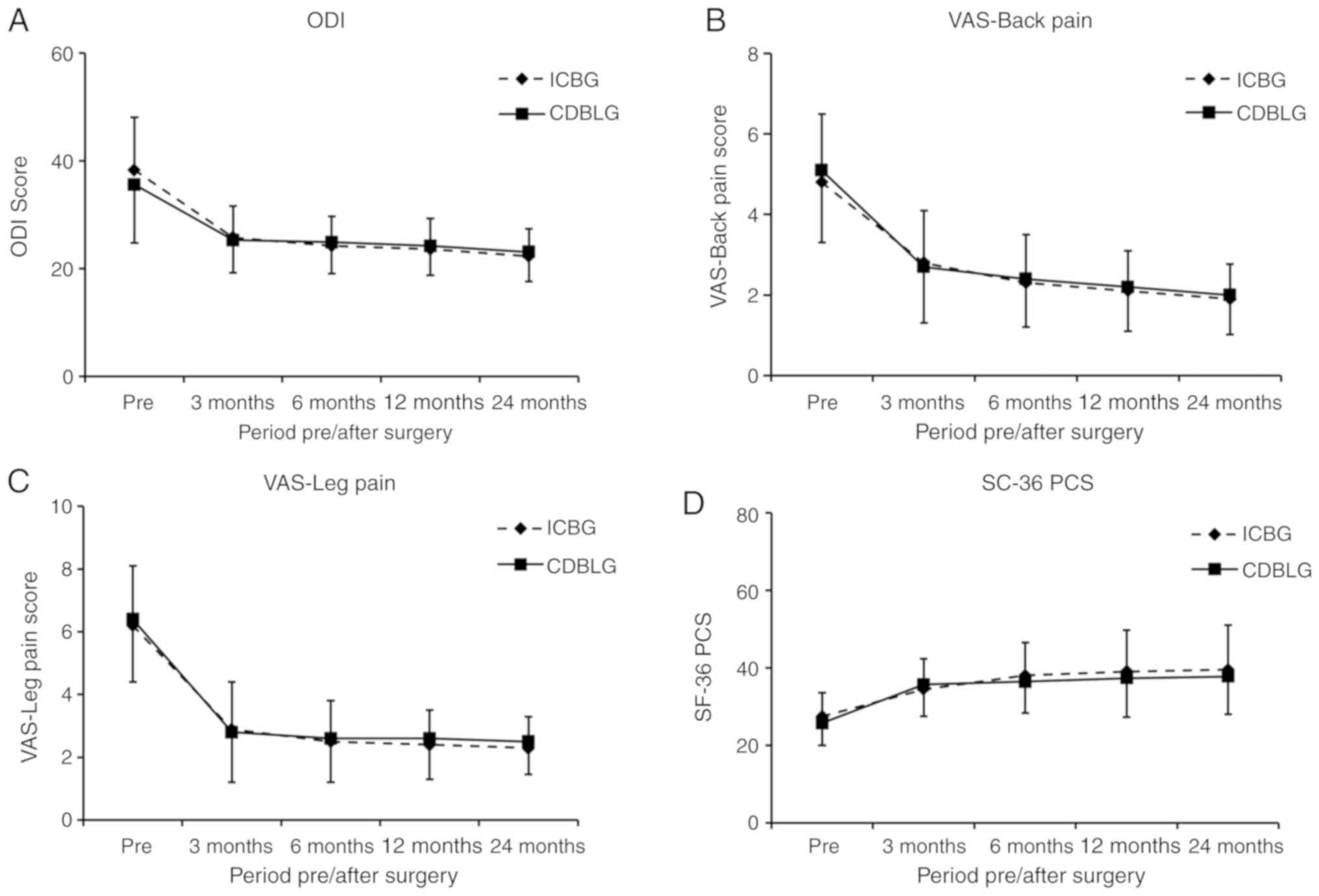 | Figure 4.ODI, VAS and SF36-PCS for the CDBLG
group and ICBG group. (A) ODI scoring; (B) VAS for back pain; (C)
VAS for leg pain; (D) SF36-PCS scoring. There was no significant
difference in ODI, VAS for back pain and leg pain, SD36-PCS scoring
prior to or at 3, 6, 12 and 24 months after surgery between the
CDBLG and ICBG groups (P>0.05; n=25 in ICBG group and n=44 in
CDBLG group). CDBLG, cage-shaped demineralized bone plus local bone
grafts; ICBG, iliac crest bone grafts; ODI, Oswestry Disability
Index; VAS, Visual Analogue Scale; Pre, prior to surgery; SF36-PCS,
Short Form-36 general health survey physical component summary. |
Surgical and clinical outcomes
Peri-operative parameters, including duration of
surgery, blood loss and length of hospital stay are listed in
Table II. The mean duration of
surgery in the CDBLG group was significantly lower than that in the
ICBG group (156±25 vs. 198±32 min; P<0.05). Furthermore, the
mean blood loss in the CDBLG group was significantly lower compared
with that in the ICBG group (385±35 vs. 589±51 ml; P<0.05).
There was no significant difference between these two groups in the
duration of hospital stay (P>0.05). There were neither
immunogenic complications nor immunosuppressive therapy after
surgery.
 | Table II.Surgical and clinical
information. |
Table II.
Surgical and clinical
information.
| Characteristic | CDBLG (n=44) | ICBG (n=25) | P-value |
|---|
| Duration of surgery
(min) | 156±25 | 198±32 | <0.001 |
| Blood loss
(ml) | 385±35 | 589±51 | <0.001 |
| Hospital stay
(days) | 8.3±2.7 | 8.6±2.9 | 0.673 |
Discussion
Spinal surgery frequently requires bone grafts for
fusion. Although numerous studies have reported successful fusion
after instrumented interbody spinal fixation using autografts,
fusion rates have large variances ranging from 40 to 98% were
obtained (14–16). Autogenous ICBG remains the gold
standard for spinal fusion; this material is easily available, and
its use has no risk of transmitting disease and is effective in
stimulating bone formation. Autogenous ICBG is the most common
source of autografts. However, ICBG has various disadvantages,
including limited resource of harvested bone and morbidity of the
donor site, including infection, hematoma and sustained pain
localized to the harvest site. Harvesting of autogenous ICBG also
increases the duration of surgery, blood loss and post-operative
pain (17). The present study
focused on comparing the efficacy of CDBLG and ICBG in single-level
lumbar instrumented fusion. The cage-shaped demineralized bone
graft provides a large surface where new bone formation occurs.
Local autogenous bone includes osteoblasts and precursor cells,
which respond to the local microenviroment, releasing stimulating
factors that accelerate new bone formation and revascularization
that has an important role in osteogenesis (18).
The cage-shaped demineralized bone graft provides
mechanical stability, while local autogenous bone is rapidly
incorporated into the surrounding lumbar vertebral bodies due to
its osteogenic properties (19). The
demineralized bone is composed of mineral and collagen, which
serves as a scaffold to stimulate revascularization and to induce
host precursor cells to form new bone. Demineralized bone is
usually derived from acid extraction of a human allograft,
resulting in a combination of properties that are osteoconductive
(organic matrix proteins) and osteoinductive (growth factors).
Demineralized bone is acellular and less osteoconductive than
autogenous bone, due to the acid extraction. The quantity and type
of growth factors and cytokines influence the osteoinductive
capacity of the demineralized bone graft (20). The extracellular matrix stimulates
new bone formation via non-collagenous proteins and growth factors
(21). Demineralized bone graft may
be used as an effective bone graft substitute and may decrease
morbidities associated with iliac bone graft harvest (22).
Flexion/extension radiographs, static radiographs,
tomograms and the older generations of CT scans differ in their
reliability and accuracy in determining the status of fusion, and
therefore, discrepancies exist among previously published studies
reporting on the rate of fusion (23–25). In
the present study, progressive radiographic films were produced at
6, 12 and 24 months' follow-up to determine the progression and
robustness of the interbody fusion mass. In the present study, the
fusion rate at 24 months determined using plain and
flexion/extension X-ray radiographs was 88.7% for CDBLG and 92.0%
in ICBG (no significant difference, P=0.967), which is comparable
to the results of previous studies that adopted a similar
methodology (14,26). The fusion rate based on CT scan
evaluation and criteria in the CDBLG group was slightly, but
insignificantly lower than that in the ICBG group (86.4 vs. 88.0%;
P=1.000).
The osteogenic, osteoconductive and osteoinductive
properties of CDBLG are similar to those of ICBG. However, the
local bone in the CDBLG has no potential for histocompatibility and
immunogenic reactions from allografts (4,9,27). The substantial morbidity associated
with the procurement of autogenous bone has been a common
complication, primarily due to the documented success of using
autografts, in addition to the lack of commercially available bone
graft substitutes that offer equal or superior rates of fusion
(28,29). In their retrospective clinical study,
Ito et al (30) reported that
the fusion results and progression from the local bone group and
the autologous iliac bone group were nearly identical.
In the present study, a novel type of bone graft,
CDBLG, which had similar clinical outcomes to those of ICBG, was
presented. The CDBLG was fabricated from osintegumentale, which has
greater mechanical strength than autologous iliac bone. The CDBLG
was effective in sustaining the height of the disc gap, better
matching its natural physiological curvature, and as previous
reported by Kang et al (31),
it is therefore believed to be able to have comparable clinical
outcomes to ICBG.
In the present study, a number of specific
complications were observed in the ICBG group that may be
attributed to the donor site. Blood loss and the duration of
surgery were greater than in the CDBLG group. Allograft bone is
available in large quantities but its osteogenic potential is
markedly reduced compared with that of autografts, and it is
associated with a risk of bacterial and viral infection (32,33).
Overall, the successful fusion rate of CDBLG is comparable to that
of an autogenous ICBG. As reported, Cage-shaped demineralized bone
is an allograft from cadaveric bone without the mineral content
which also has a low risk of disease transmission (34,35). The
remaining type I collagen contains variable concentrations of
growth factors and serves as an osteoconductive and osteoinductive
scaffold that induces new bone formation (36). Demineralized bone was not used on its
own for lumbar fusion. Combined with local autogenous bone
harvested from elements of the posterior spinal structure,
including the vertebral laminae, spinal processes and facet joint,
it provides osteogenic cells that become incorporated into the
surrounding vertebral bodies. Chen et al (37) reported that autologous laminectomy
and spinal process bone achieved high fusion but posterolateral
fusion required a greater quantity of bone. The cage-shaped
demineralized bone graft was demonstrated to be a good bone graft
extender. By combining with local bone, mainly from the spinal
process and vertebral lamina, the CDBLG provides all three bone
graft components for bone formation: Osteogenesis, osteoinduction
and osteoconduction. Its use was reported to decrease the
morbidities associated with autogenous iliac bone graft harvest for
lumbar fusion and to also have a significantly reduced cost
compared with the use of metal or polyetheretherketone (PEEK)
intervertebral cages (38). Use of
the CDBLG also decreased the duration of surgery and hospital stay
compared with those of ICBG.
The present study had certain limitations. First, it
was a retrospective study and the sample size was relatively small.
A randomized, controlled, prospective study should be performed to
compare the clinical outcomes of CDBLG and ICBG. Furthermore,
long-term follow-up should be performed. In addition, autogenous
ICBG placement remains a good candidate for successful surgical
treatment of spine instability. CDBLG was demonstrated to be a
suitable alternative without problems of limited quantity and
morbidity due to harvesting. Additional studies, including
comparison of CDBLG with metal or PEEK interbody cage, should be
performed.
In conclusion, treatment with CDBLG resulted in an
equal rate of fusion and pain relief to that obtained with ICBG.
All clinical outcome measures demonstrated significant improvement
at all time-points of post-operative follow-up and there is no risk
of rejection. Compared with ICBG, treatment with CDBLG was
associated with significantly less intra-operative blood loss and a
shorter duration of surgery. Therefore, the use of CDBLG bone graft
is recommended as an alternative option for single-level
fusion.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Research Program in ‘Science and Technology +’ projects of Xi'an
city [grant no. 201805096YX4SF30(2)] and the Shaanxi Natural
Science Basic Research Grant (grant no. 2018JQ8063). The funders
had no role in the study design, data collection and analysis or
decision to publish the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CGZ and JQ analyzed the data and wrote the
manuscript. GX, YJ and YCG carried out the surgeries. XW carried
out patient follow-up. XM and HY were the lead investigators, and
developed the design of the study, analysis and interpretations.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study has been approved by the Review Board of
the General Hospital of Xinjiang Military Region (approval no.
ZYLL-2018-23). Informed consent, in accordance with the Declaration
of Helsinki, was obtained from all patients.
Patient consent for publication
The patients have provided consent for publication
of CT images which appeared in the manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mercer W: Spondylolisthesis: With a
description of a new method of operative treatment and notes of ten
cases. Edinb Med J. 43:545–572. 1936.PubMed/NCBI
|
|
2
|
Egol KA, Nauth A, Lee M, Pape HC, Watson
JT and Borrelli J Jr: Bone grafting: Sourcing, timing, strategies,
and alternatives. J Orthop Trauma. 29 (Suppl 12):S10–S14. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bono CM and Lee CK: Critical analysis of
trends in fusion for degenerative disc disease over the past 20
years: Influence of technique on fusion rate and clinical outcome.
Spine (Phila Pa 1976). 29:455–463; discussion Z5. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sengupta DK, Truumees E, Patel CK,
Kazmierczak C, Hughes B, Elders G and Herkowitz HN: Outcome of
local bone versus autogenous iliac crest bone graft in the
instrumented posterolateral fusion of the lumbar spine. Spine
(Phila Pa 1976). 31:985–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dimitriou R, Mataliotakis GI, Angoules AG,
Kanakaris NK and Giannoudis PV: Complications following autologous
bone graft harvesting from the iliac crest and using the RIA: A
systematic review. Injury. 42 (Suppl 2):S3–S15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurien T, Pearson RG and Scammell BE: Bone
graft substitutes currently available in orthopaedic practice: The
evidence for their use. Bone Joint J. 95-B:583–597. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chau AM, Xu LL, Wong JH and Mobbs RJ:
Current status of bone graft options for anterior interbody fusion
of the cervical and lumbar spine. Neurosurg Rev. 37:23–37. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan W, Su QJ, Liu T, Yang JC, Kang N,
Guan L and Hai Y: Evaluation of Coflex interspinous stabilization
following decompression compared with decompression and posterior
lumbar interbody fusion for the treatment of lumbar degenerative
disease: A minimum 5-year follow-up study. J Clin Neurosci.
35:24–29. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gornet MF, Burkus JK, Dryer RF and Peloza
JH: Lumbar disc arthroplasty with Maverick disc versus stand-alone
interbody fusion: A prospective, randomized, controlled,
multicenter investigational device exemption trial. Spine (Phila Pa
1976). 36:E1600–E1611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams AL, Gornet MF and Burkus JK: CT
evaluation of lumbar interbody fusion: Current concepts. AJNR Am J
Neuroradiol. 26:2057–2066. 2005.PubMed/NCBI
|
|
11
|
Buttermann G, Hollmann S, Arpino JM and
Ferko N: Value of single-level circumferential fusion: A 10-year
prospective outcomes and cost-effectiveness analysis comparing
posterior facet versus pedicle screw fixation. Eur Spine J. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
AlBedah A, Khalil M, Elolemy A, Hussein
AA, AlQaed M, Al Mudaiheem A, Abutalib RA, Bazaid FM, Bafail AS,
Essa A and Bakrain MY: The use of wet cupping for persistent
nonspecific low back pain: Randomized controlled clinical trial. J
Altern Complement Med. 21:504–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qin J, Li J, Liu Y, Zhao B, Dong H, Dong
B, Zhang R, Ning N, Zhang X, Cui F, et al: Clinical comparison
between a percutaneous hydraulic pressure delivery system and
balloon tamp system using high-viscosity cement for the treatment
of osteoporotic vertebral compression fractures. Clinics (Sao
Paulo). 74:e7412019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dimar JR II, Glassman SD, Burkus JK, Pryor
PW, Hardacker JW and Carreon LY: Two-year fusion and clinical
outcomes in 224 patients treated with a single-level instrumented
posterolateral fusion with iliac crest bone graft. Spine J.
9:880–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carreon LY, Glassman SD and Djurasovic M:
Reliability and agreement between fine-cut CT scans and plain
radiography in the evaluation of posterolateral fusions. Spine J.
7:39–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carreon LY, Djurasovic M, Glassman SD and
Sailer P: Diagnostic accuracy and reliability of fine-cut CT scans
with reconstructions to determine the status of an instrumented
posterolateral fusion with surgical exploration as reference
standard. Spine (Phila Pa 1976). 32:892–895. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin SR and Tornetta P III: Donor site
morbidity after anterior iliac bone graft harvesting. J Orthop
Trauma. 30:340–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guelcher SA, Brown KV, Li B, Guda T, Lee
BH and Wenke JC: Dual-purpose bone grafts improve healing and
reduce infection. J Orthop Trauma. 25:477–482. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khan SN, Cammisa FP Jr, Sandhu HS, Diwan
AD, Girardi FP and Lane JM: The biology of bone grafting. J Am Acad
Orthop Surg. 13:77–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao Z, Wang CH and Cui WL: Comparison of
allograft and autograft in lumbar fusion for lumbar degenerative
diseases: A systematic review. J Invest Surg. 29:373–382. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rogers GF and Greene AK: Autogenous bone
graft: Basic science and clinical implications. J Craniofac Surg.
23:323–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu TS, Wang IC, Lu ML, Hsieh MK, Chen LH
and Chen WJ: The fusion rate of demineralized bone matrix compared
with autogenous iliac bone graft for long multi-segment
posterolateral spinal fusion. BMC Musculoskelet Disord. 17:32016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghiselli G, Wharton N, Hipp JA, Wong DA
and Jatana S: Prospective analysis of imaging prediction of
pseudarthrosis after anterior cervical discectomy and fusion:
Computed tomography versus flexion-extension motion analysis with
intraoperative correlation. Spine (Phila Pa 1976). 36:463–468.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Selby MD, Clark SR, Hall DJ and Freeman
BJ: Radiologic assessment of spinal fusion. J Am Acad Orthop Surg.
20:694–703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fogel GR, Toohey JS, Neidre A and
Brantigan JW: Fusion assessment of posterior lumbar interbody
fusion using radiolucent cages: X-ray films and helical computed
tomography scans compared with surgical exploration of fusion.
Spine J. 8:570–577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Larsen JM, Rimoldi RL, Capen DA, Nelson
RW, Nagelberg S and Thomas JC Jr: Assessment of pseudarthrosis in
pedicle screw fusion: A prospective study comparing plain
radiographs, flexion/extension radiographs, CT scanning, and bone
scintigraphy with operative findings. J Spinal Disord. 9:117–120.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Friedlaender GE, Strong DM and Sell KW:
Studies on the antigenicity of bone. II. Donor-specific anti-HLA
antibodies in human recipients of freeze-dried allografts. J Bone
Joint Surg Am. 66:107–112. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arrington ED, Smith WJ, Chambers HG,
Bucknell AL and Davino NA: Complications of iliac crest bone graft
harvesting. Clin Orthop Relat Res. 300–309. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cammisa FP Jr, Lowery G, Garfin SR,
Geisler FH, Klara PM, McGuire RA, Sassard WR, Stubbs H and Block
JE: Two-year fusion rate equivalency between Grafton DBM gel and
autograft in posterolateral spine fusion: A prospective controlled
trial employing a side-by-side comparison in the same patient.
Spine (Phila Pa 1976). 29:660–666. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ito Z, Matsuyama Y, Sakai Y, Imagama S,
Wakao N, Ando K, Hirano K, Tauchi R, Muramoto A, Matsui H, et al:
Bone union rate with autologous iliac bone versus local bone graft
in posterior lumbar interbody fusion. Spine (Phila Pa 1976).
35:E1101–E1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang J, An H, Hilibrand A, Yoon ST,
Kavanagh E and Boden S: Grafton and local bone have comparable
outcomes to iliac crest bone in instrumented single-level lumbar
fusions. Spine (Phila Pa 1976). 37:1083–1091. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiarello E, Cadossi M, Tedesco G, Capra
P, Calamelli C, Shehu A and Giannini S: Autograft, allograft and
bone substitutes in reconstructive orthopedic surgery. Aging Clin
Exp Res. 25 (Suppl 1):S101–S103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murphy ME, Mccutcheon BA, Grauberger J,
Shepherd D, Maloney PR, Rinaldo L, Kerezoudis P, Fogelson JL, Nassr
A and Bydon M: Allograft versus autograft in cervical and lumbar
spinal fusions: An examination of operative time, length of stay,
surgical site infection, and blood transfusions. J Neurosurg Sci.
63:11–18. 2019.PubMed/NCBI
|
|
34
|
Aghdasi B, Montgomery SR, Daubs MD and
Wang JC: A review of demineralized bone matrices for spinal fusion:
The evidence for efficacy. Surgeon. 11:39–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bouaicha S, von Rechenberg B, Osterhoff G,
Wanner GA, Simmen HP and Werner CM: Histological remodelling of
demineralised bone matrix allograft in posterolateral fusion of the
spine-an ex vivo study. BMC Surg. 13:582013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gruskin E, Doll BA, Futrell FW, Schmitz JP
and Hollinger JO: Demineralized bone matrix in bone repair: History
and use. Adv Drug Deliv Rev. 64:1063–1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen WJ, Tsai TT, Chen LH, Niu CC, Lai PL,
Fu TS and McCarthy K: The fusion rate of calcium sulfate with local
autograft bone compared with autologous iliac bone graft for
instrumented short-segment spinal fusion. Spine (Phila Pa 1976).
30:2293–2297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yson SC, Sembrano JN and Santos ER:
Comparison of allograft and polyetheretherketone (PEEK) cage
subsidence rates in anterior cervical discectomy and fusion (ACDF).
J Clin Neurosci. 38:118–121. 2017. View Article : Google Scholar : PubMed/NCBI
|


















