Introduction
Obesity is increasing globally and is a potential
risk factor for metabolic syndrome, which is associated with
insulin resistance, blood lipid disorders and hypertension
(1,2). Furthermore, the epidemic of obesity,
independently of diabetes and hypertension, has resulted in an
increased incidence of obesity-associated glomerulopathy, which is
defined morphologically as glomerulomegaly with or without focal
segmental glomerulosclerosis (3–5).
Chronic-grade inflammation is markedly observed in obesity
(2), which may induce podocyte
damage, and further cause proteinuria and renal injury.
It is well-known that estrogen participates in the
regulation of the physiological processes of the human body in
women, and estrogen deficiency increases the susceptibility of
postmenopausal women to metabolic disorders including obesity,
osteoporosis, cardiovascular disease and chronic kidney disease
(CKD) (6–8). Numerous studies have suggested that
decreasing the biosynthesis of estrogen in ovariectomized animals
or menopausal women results in inflammatory cell infiltration and
the production of inflammatory cytokines, which are associated with
an increased incidence of CKD (9–11).
Natural products derived from plants have long been
used in the treatment of lifestyle-associated diseases, including
obesity (12,13). Traditional Chinese Medicine has been
used as a conventional or complementary therapy for the treatment
of renal injury (14,15). It has previously been reported that
natural products such as poricoic acid, are able to inhibit renal
fibrosis by regulating the tumor growth factor β (TGF-β)/Smad
signaling pathway (16–19). Resveratrol
(trans-3,5,4-trihydroxystilbene) is a natural polyphenol with
antioxidant and anti-inflammatory properties (20) which is produced in a myriad of plants
including grapes, berries, peanuts and other traditional Chinese
medicinal plants. It has been reported to be effective in
preventing the development of a number of diseases including
cardiovascular disease (21,22), diabetes (23), cancer (24), memory deficit (25) and functional gastrointestinal
dyspepsia (26). Several studies
have revealed that resveratrol has anti-obesity activity (13,27,28).
Although resveratrol protects against the development of
obesity-associated renal damage, the underlying mechanisms are not
fully clear. The present study established an obese rat model via
an ovariectomy and a high-fat diet (HFD), and investigated the
function of resveratrol in the early stages of podocyte injury in
this model.
Materials and methods
Chemicals and reagents
Resveratrol (purity >98%; cat. no. 0810018-22)
was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
High-fat feed (1% cholesterol, 10% lard, 0.1% cholate and 88.9%
basic feeding product, cat. no. SCXK 2009-0012) was purchased from
Keaoxieli Feed Co., Ltd. (Beijing, China). The primary antibodies
used in the present study were: Rabbit anti-tumor necrosis factor α
(TNF-α; cat. no. ab9755), rabbit anti-interleukin 6 (IL-6; cat. no.
ab25107), rabbit anti-monocyte chemotactic protein-1 (MCP-1; cat.
no. ab25124), rabbit anti-nephrin (cat. no. ab136894) and rabbit
anti-Wilms' tumor-1 (WT-1; cat. no. ab180840) (all from Abcam,
Cambridge, MA, USA). GAPDH polyclonal antibody (cat. no.
10494-1-AP) was purchased from ProteinTech Group, Inc. (Chicago,
IL, USA). Horseradish peroxidase-conjugated Affinipure goat
anti-rabbit immunoglobulin G (IgG) secondary antibodies (cat. no.
ZB2301) were obtained from OriGene Technologies, Inc. (Rockville,
MD, USA).
Animals and different treatments
A total of 40 female 3-month-old Wistar rats,
weighing 200±12 g, were provided by Shandong University Laboratory
Animal Center (Jinan, China). Rats were housed at standard
conditions of temperature (20±2°C) and humidity (50–70%) under a
12-h light/dark cycle (lights on at 7:00 a.m.) with ad
libitum access to food and water. A rat model of kidney
function and structure changes was intervened by ovariectomy
combined with a HFD (11). Following
one week of acclimatization, the rats were randomly divided into
four groups (n=10 per group): Sham operation with a standard diet
(S+N); sham operation with a HFD (S+H); ovariectomy with a HFD
(O+H); ovariectomy plus a HFD treated with resveratrol (O+H+R).
Rats in the aforementioned four groups were anesthetized with
sodium pentobarbital (50 mg/kg body weight; intraperitoneal). They
all underwent either a sham surgery or bilateral ovariectomy.
Following the surgery, the rats were fed using a standard diet or
HFD. Rats were administered resveratrol or sodium carboxymethyl
cellulose in their food with a daily dosage of 40 mg/kg/day for 12
weeks (29). Body weights were
measured once a week. All animal procedures were ethically approved
by the Animal Ethics Committee of Shandong University (Shandong,
China). Symptoms such as unwieldy body affecting their daily
activities were set as the humane endpoints for the present study.
However, no animal was sacrificed prior to the completion of the
experiment as a result of displaying similar symptoms. All efforts
were made to minimize animal suffering and stress during the
experiments.
Blood and kidney sample
preparation
Three months later, owing to surgery or infection, 7
to 9 rats survived in each group. At the end of the experiment, the
remaining rats were reserved for overnight fasting with water
available. The rats were then anesthetized using sodium
pentobarbital (50 mg/kg body weight) by an intraperitoneal
injection, and blood was collected from the femoral artery. Serum
was collected by centrifugation at 1,048 × g at 4°C for 15 min, and
stored at −80°C until assayed. The rats were then sacrificed by
decapitation under deep anesthesia. The bilateral kidneys were
rapidly removed and dissected on ice. Portions of the kidneys were
snap-frozen in liquid nitrogen and stored at −80°C for protein
isolation. An additional portion was fixed in 4% paraformaldehyde
at 4°C for 24 h before histopathologic observation.
Biochemical assays of serum
The serum levels of 17β-estradiol (E2) were detected
using an iodine (125I) estradiol radioimmunoassay kit
(Tianjin Jiuding Medical Bio-Engineering Co., Ltd.) according to
the manufacturer's protocol. The serum total cholesterol (TC),
triglycerides (TG), low-density lipoprotein cholesterol (LDL-C),
serum creatinine (SCr) and fasting blood glucose (FBG) levels were
measured using a DVI-1650 automatic biochemistry and analysis
instrument (Bayer, Pittsburgh, PA, USA). Serum insulin levels were
measured using insulin enzyme-linked immunosorbent assay kits (cat.
no. DY8056-05, R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's protocol. The homeostasis model
assessment of insulin resistance (HOMA-IR) was calculated via the
following formula: FBG × insulin/22.5.
Histological analysis
The kidneys were excised, and sections were fixed in
10% formaldehyde at room temperature for 24 h and embedded in
paraffin and cut into 3–5 µm-thick sections for light microscopy.
Prior to dyeing, following heating at 60°C for 20 min, paraffin in
the section was removed using xylene before rehydration with
absolute ethanol and subsequently 95, 85 and 75% ethanol gradients
for 5 min each and finally added to distilled water. At room
temperature, the sections which had been placed into distilled
water were stained using hematoxylin-eosin (H&E; dyed with
hematoxylin aqueous solution for 7 min and eosin for 2–3 min),
periodic acid-Schiff (PAS; oxygenation with 1% periodic acid for
10–15 min and colored in Schiff's solution for 10–30 min) and
Masson's trichrome (immersed in Masson complex solution for ~5 min,
2.5% phosphomolybdic acid for ~5 min and aniline blue for ~5 min).
Subsequent to dehydration in 75, 85 and 95% ethanol then absolute
ethanol gradients for 2 min before washing using xylene and sealing
by gum, the sections were observed under a light microscope at a
×400 magnification.
Immunohistochemical analysis
At room temperature, the kidney tissue sections (3–5
µm) were deparaffinized by soaking in xylene for 20 min after
heating at 60°C for 20 min, rehydrated with 100, 95, 90 and 80%
ethanol gradients for 5 min and then washed three times with
phosphate buffered saline (PBS). Antigen retrieval was performed
through incubation in 0.01 M sodium citrate buffer (pH 6.0) at 95°C
for 15 min. Subsequent to cooling to room temperature, the slides
were washed three times with PBS and then immersed in 0.1% Triton
X-100 for another 15 min. Following that, the slides were incubated
in 3% hydrogen peroxide at room temperature for 10 min and
subsequently placed in blocking buffer (10% goat serum) for 1 h at
37°C. The primary antibodies anti-nephrin (1:150) and anti-WT-1
(1:100), were applied overnight at 4°C, while the negative control
sections were incubated with PBS instead of the primary antibody
overnight at 4°C. All sections were washed three times with PBS and
incubated with horseradish peroxidase-conjugated goat anti-rabbit
antibodies (1:500) for 1 h at room temperature, and stained with
3,3′-diaminobenzidine and hematoxylin for 60 min at 37°C. The
stained slides were observed by light microscopy (magnification,
×400), and brown areas were regarded as positive. The colored
sections were graded semi-quantitatively (Leica QWin V3 image
analysis software; Leica Microsystems GmbH, Wetzlar, Germany) Two
specimens were randomly selected in each group and 10 high-power
fields per specimen were observed. Each score primarily reflected
the extent of staining, rather than the intensity, and depended on
the percentage of positive areas: No staining or <5%=0; 5–25%=1;
26–50%=2; 51–75%=3; and >75%=4.
Western blotting
Tissues were homogenized in a lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China; cat.
no. R0020) and were centrifuged at 12,000 × g for 20 min at 4°C to
collect the supernatant for protein quantification. The
concentration of protein was determined using the Pierce
Bicinchoninic Acid protein assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). A total of 20 µg proteins of each
sample were resolved on 10% SDS-PAGE gels, and the proteins were
transferred to nitrocellulose membranes. Subsequent to blocking
with 5% non-fat milk at room temperature for 1 h, the membranes
were incubated with the primary antibodies anti-TNF-α (1:2,000),
anti-IL-6 (1:1,000), anti-MCP-1 (1:2,000) and anti-GAPDH (1:5,000)
overnight at 4°C. Then, the membranes were incubated with the
secondary antibodies (goat anti-rabbit IgG; 1:10,000) at room
temperature for 60 min. Finally, proteins were visualized with
enhanced chemiluminescence substrate (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and exposed to film in a dark
room. The quantification of protein density was performed using
Image J software (version 1.45; National Institutes of Health,
Bethesda, MD, USA). GAPDH was used as a loading control and the
relative quantities were obtained by the ratio of protein quantity
to GAPDH, and the mean value was obtained.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). Single comparisons were
performed using a Student's t-test and multiple comparisons by a
two-way analysis of variance with post-hoc least significant
difference tests for comparisons where appropriate. P<0.05 was
considered to indicate a statistically significant difference.
Results
Resveratrol affects body weight and
serum E2, TC and LDL-C levels in ovariectomized rats with a
HFD
Body weight was measured at the beginning of the
experiment, and exhibited no significant difference among all
groups (S+N, 201±10 g; S+H, 201±8 g; O+H, 200±13 g; O+H+R, 198±11
g; P>0.05). At the end of the experiment, the body weight in the
O+H group was significantly higher compared with those of the S+N
and S+H groups (P<0.05). However, the final body weight in the
O+H+R group was significantly decreased compared with that in the
O+H group (P<0.05; Fig. 1).
To assess whether the ovariectomy operation and
hyperlipidemic rat model establishment were successful, serum E2,
TC, TG, LDL-C and SCr levels were monitored. As presented in
Table I, serum E2 levels in the O+H
group were significantly lower compared with those in the S+N and
S+H groups (P<0.05). Subsequent to 3 months of treatment with
resveratrol, the E2 levels in the O+H+R group were significantly
higher compared with that in the O+H group (P<0.05). Serum TC
and LDL-C levels in the S+H and O+H groups were significantly
higher compared with those in the S+N group (P<0.05). However,
serum TC and LDL-C levels significantly reduced in the O+H+R group
compared with the O+H group (P<0.05). There was no significant
difference in serum TG and SCr levels among all groups
(P>0.05).
 | Table I.Effects of resveratrol on serum E2,
TC, TG, LDL-C and SCr in all rats. |
Table I.
Effects of resveratrol on serum E2,
TC, TG, LDL-C and SCr in all rats.
|
| Animal groups |
|---|
|
| S+N (n=9) | S+H (n=9) | O+H (n=7) | O+H+R (n=8) |
|---|
| E2 (pg/ml) | 15.04±1.74 | 14.36±2.31 |
8.00±1.89a,b |
11.38±2.34c |
| TG (mmol/l) | 1.54±0.43 | 1.56±0.36 | 1.63±0.35 | 1.44±0.39 |
| TC (mmol/l) | 1.62±0.28 |
2.25±0.49a |
2.93±0.56a,b |
2.28±0.14c |
| LDL-C (mmol/l) | 0.17±0.05 |
0.25±0.05a |
0.27±0.03a |
0.25±0.06c |
| SCr (umol/l) | 46.8±4.66 | 48.6±2.86 | 50.3±5.63 | 52±3.55 |
Resveratrol improves insulin
sensitivity in ovariectomized rats with an HFD
The effect of resveratrol on metabolic parameters in
obese rats was evaluated by testing serum FBG and insulin, and
calculating the HOMA-IR. As Fig. 2
reveals, FBG, serum insulin and HOMA-IR were significantly
increased in the O+H group compared with the S+N and S+H groups
(P<0.05). Notably, FBG, serum insulin and HOMA-IR were
significantly decreased in the O+H+R group compared with the O+H
group (P<0.05).
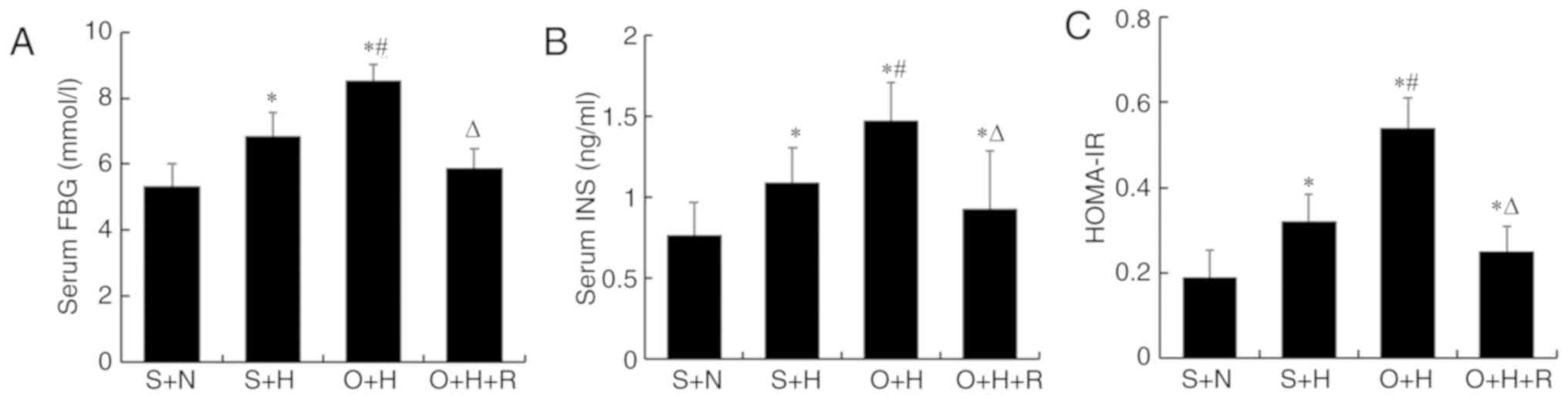 | Figure 2.Effect of resveratrol on metabolic
parameters in obese rats, evaluated by testing serum FBG and
insulin and calculating the HOMA-IR. (A) Serum FBG, (B) insulin
levels and (C) HOMA-IR in different treatment groups of rats. Data
were presented as the mean ± standard deviation (n=7–9 per group).
S+N, sham-operation with standard diet, S+H, sham-operation with
high-fat diet, O+H, ovariectomy with high-fat diet, O+H+R,
ovariectomy plus high-fat diet treated with resveratrol; FBG,
fasting blood glucose; HOMA-IR, homeostasis model assessment of
insulin resistance; INS, insulin. *P<0.05 vs. S+N group,
#P<0.05 vs. S+H group, ∆P<0.05 vs. O+H
group. |
Effects of resveratrol on podocyte
injury and renal pathology
In order to investigate whether resveratrol protects
against podocyte injury, WT-1 and nephrin expression levels were
detected using immunohistochemical analysis as they are specific
markers of podocytes (30,31). Nephrin and WT-1 were primarily
expressed in the podocytes of the S+N group. The expression of
nephrin and WT-1 were significantly decreased in the O+H group
compared with that in the S+N group (P<0.05; Fig. 3). However, subsequent to treatment
with resveratrol for 12 weeks, these expression changes were
significantly reversed in the O+H+R group compared with the O+H
group (P<0.05). No significant pathological changes in any group
were observed by PAS staining (Fig.
4), H&E staining or Masson staining (data not shown).
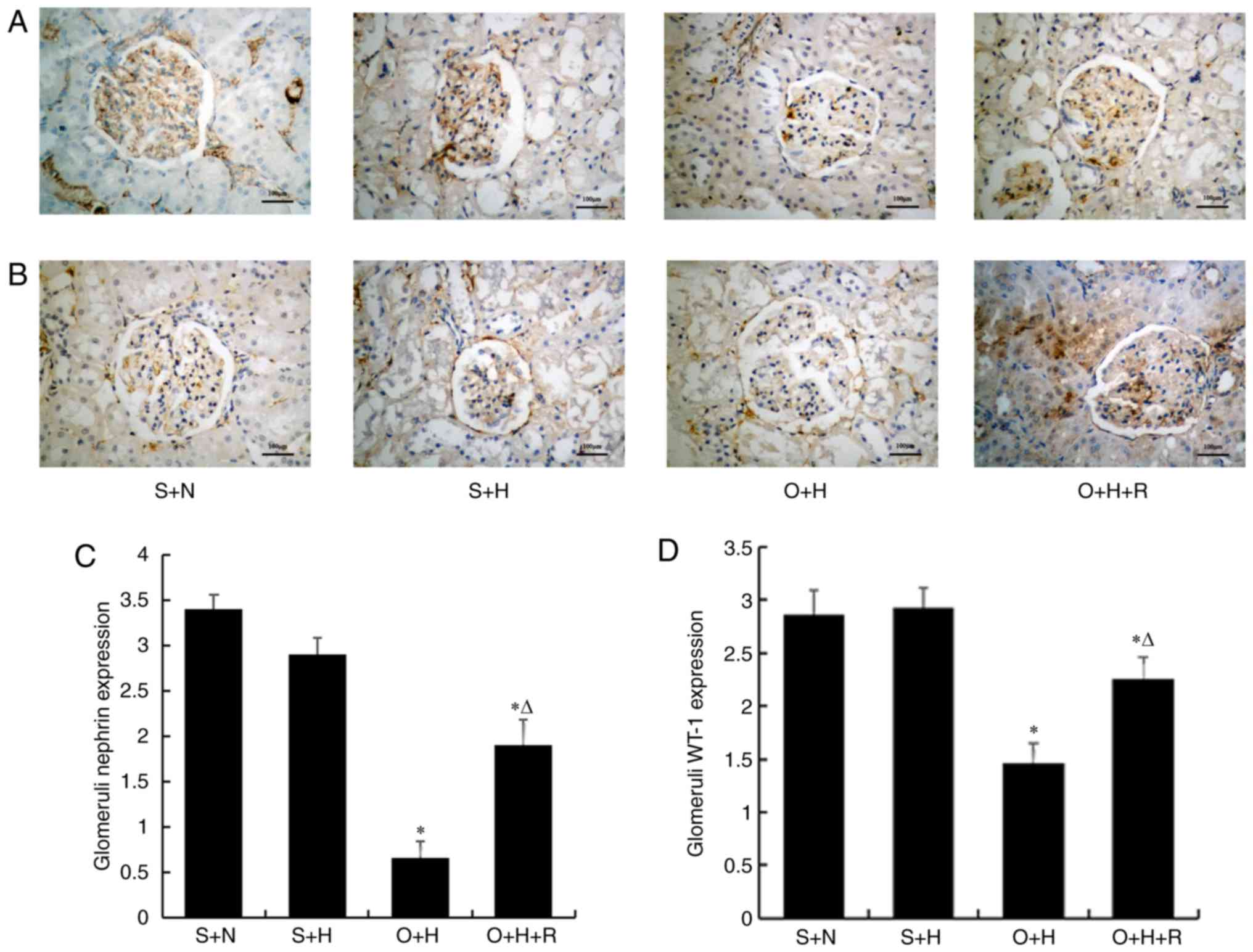 | Figure 3.Immunohistochemical staining for (A)
nephrin and (B) WT-1 in glomeruli (magnification, ×400; scale bar:
100 µm). Semiquantitative analysis for (C) nephrin and (D) WT-1.
Data were presented as the mean ± standard deviation (n=7–9 per
group). S+N, sham-operation with standard diet, S+H, sham-operation
with high-fat diet, O+H, ovariectomy with high-fat diet, O+H+R,
ovariectomy plus high-fat diet treated with resveratrol; WT-1,
Wilms' tumor-1. *P<0.05 vs. S+N group, ∆P<0.05 vs.
O+H group. |
Effects of resveratrol on renal
inflammatory cytokine levels
To investigate the effect of resveratrol on renal
inflammation, the present study investigated the expression of
inflammation-associated cytokines, including TNF-α, IL-6 and MCP-1,
by western blotting. The expression levels of these cytokines were
significantly increased in the O+H group compared with that in the
S+N group and S+H group (P<0.05; Fig.
5). As expected, resveratrol treatment significantly reduced
the expression of these inflammatory cytokines in the O+H+R group
compared with the O+H group (P<0.05).
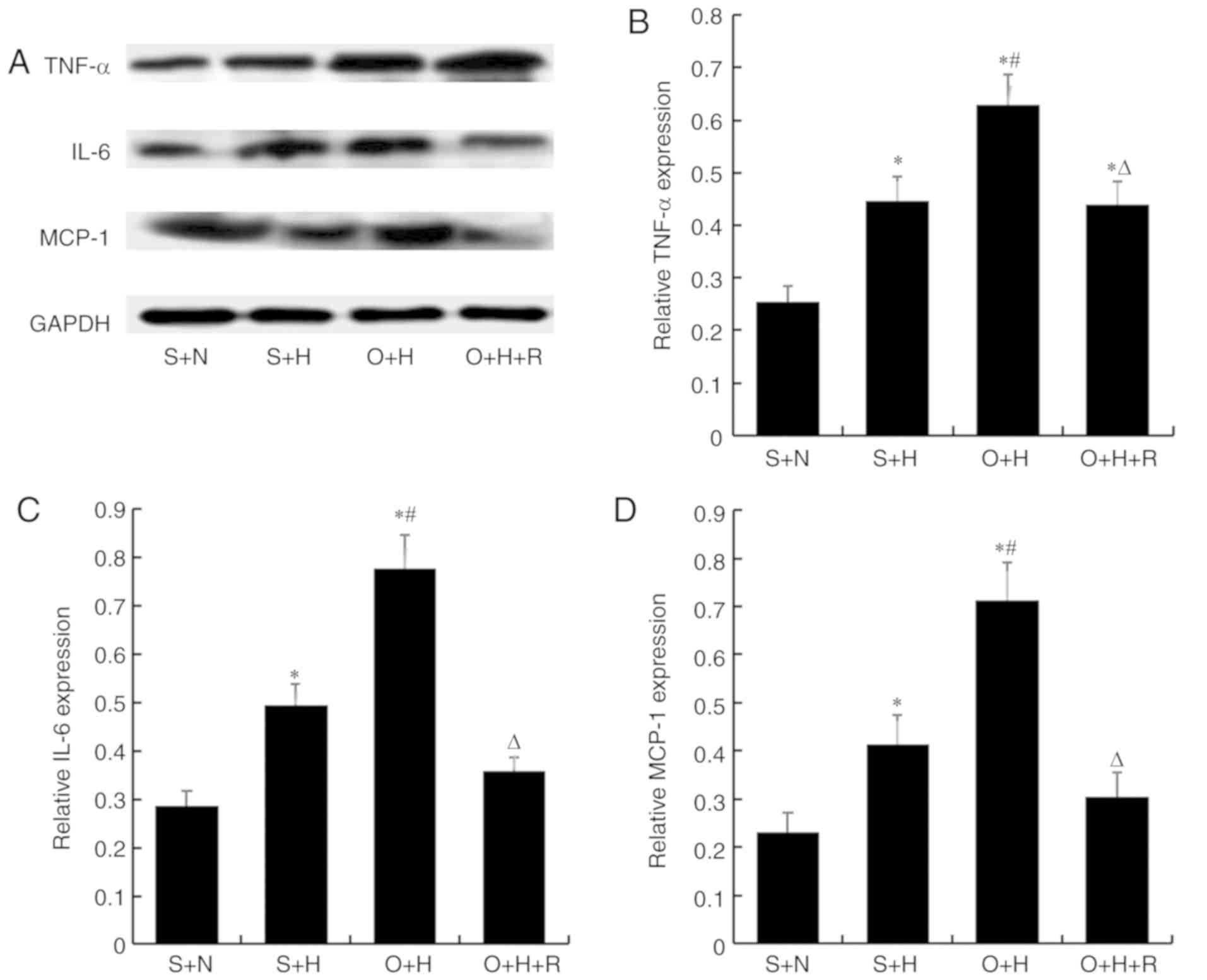 | Figure 5.TNF-α, IL-6 and MCP-1 expression in
groups of rats with varying treatment types. (A) Representative
TNF-α, IL-6 and MCP-1 expression assessed using western blotting.
Quantified protein values were calculated as a ratio of (B) TNF-α,
(C) IL-6 and (D) MCP-1 protein to GAPDH. Data were presented as the
means ± standard deviation (n=7–9 per group). S+N, sham-operation
with standard diet, S+H, sham-operation with high-fat diet, O+H,
ovariectomy with high-fat diet, O+H+R, ovariectomy plus high-fat
diet treated with resveratrol; TNF-α, tumor necrosis factor α;
IL-6, interleukin 6; MCP-1, monocyte chemotactic protein-1.
*P<0.05 vs. S+N group, #P<0.05 vs. S+H group,
∆P<0.05 vs. O+H group. |
Discussion
The present study aimed to investigate the function
of resveratrol in podocyte injury induced by ovariectomy and a HFD.
The results revealed that resveratrol largely ameliorated obesity,
hyperlipemia and insulin resistance in ovariectomized rats with an
HFD. Notably, resveratrol significantly relieved podocyte injury
(P<0.05), with a parallel decrease in inflammation-associated
cytokines in these rats.
Obesity is mainly caused by white adipose tissue
accumulation, and it affects the whole body through the release of
pro-inflammatory cytokines, chemokines and adipokines (32). Obesity is also considered to be a
chronic low-grade inflammatory state. It has been confirmed that
inflammatory responses and oxidative stress are able to be
activated in obese rats induced by ovariectomy (33) or a HFD, which results in proteinuria,
glomerular hypertrophy and renal dysfunction (30). In the present study, it was
demonstrated that the level of serum E2 was significantly decreased
in ovariectomized rats compared with those not treated with an
ovariectomy (P<0.05), while an ovariectomy combined with a HFD
induced marked obesity characterized by weight gain,
hypercholesterolemia, hyperinsulinemia and insulin resistance. This
indicated that the obese rat model had been successfully developed,
though there were no differences in terms of serum TG and SCr
levels in the four groups. It was also revealed that an ovariectomy
in addition to a HFD promoted an increase in the levels of
proinflammatory cytokines in the kidney tissue, including TNF-α,
IL-6 and MCP-1, which are generally produced by macrophages
infiltrating adipose tissue. This result suggested that a renal
inflammatory response was activated in the rats treated with
ovariectomy and an HFD, which may further aggravate podocyte
injury. This result was consistent with a previous study by Tang
et al (34).
It has been demonstrated that the body initiates
abnormal inflammatory responses during the menopause (35), and the primary inflammation is
amplified in postmenopausal women with obesity (9). All these changes may affect renal
function and structure, in particular podocyte injury. Furthermore,
an increase in body weight and abdominal fat, and altered renal
function and structure, have been observed in rats fed with a HFD
for 24-weeks in addition to an ovariectomy (11). However, no significant renal
functional, structural or pathological changes were observed in the
experimental groups in the present study, potentially due to the
short observation period. In the present study, the animals were
observed for 12 weeks to investigate very early-stage
obesity-associated kidney disease prior to the occurrence of renal
functional and structural change. Regretfully, the present study
did not set an ovariectomy combined standard diet group as another
control, which is a limitation of the present study.
Nephrin, as a specific marker of podocytes, is
crucial for slit diaphragm and foot process formation. A decline in
nephrin expression and molecular damage will result in
morphological changes in podocytes, the widening and fusion of the
foot process, and subsequently leakage of plasma proteins (36,37).
WT-1, a transcription factor for nephrin, serves an important
function in maintaining the phenotypic and functional status of
glomerular podocytes (38).
Meanwhile, changes in WT-1 expression are consistent with changes
in nephrin (31). Therefore, the
present study detected early podocyte injury by examining the
expression of nephrin and WT-1. The present results demonstrated
that nephrin and WT-1 were significantly reduced in ovariectomized
rats with HFD compared with the sham operation and standard diet
group (P<0.05), suggesting that podocyte injury had occurred in
ovariectomized rats with HFD.
Resveratrol may be regarded as a phytoestrogen,
owing to its similarity to diethylstilbestrol (a synthetic
estrogen). It is able to regulate lipid metabolism, and alleviate
renal damage through antioxidation and the inhibition of
inflammatory factors (30). Previous
evidence supports the theory that resveratrol not only improves
insulin sensitivity and metabolic parameters, but also exhibits
potential anti-inflammatory activity in renal mesangial cells by
influencing the p38 mitogen-activated protein kinase signaling
pathway (39). In the present study,
it was revealed that resveratrol treatment for 12 weeks effectively
upregulated the level of E2, decreased body weight, lowered serum
cholesterol and FBG, and enhanced insulin sensitivity. However, one
study identified that estrogen synthesis increased following 9
weeks of a high cholesterol diet in ovariectomized mice (40). In the report, Li et al
(40) considered that the high
cholesterol intake may facilitate the synthesis of sex steroid
hormones in the non-gonadal organs/tissues including the liver,
skeletal muscle and adrenal gland in addition to the brain, and in
turn, partially reverse serum sex hormone declines following a
bilateral ovariectomy. Therefore, it was hypothesized that
resveratrol may be have a similar effect in rats with ovariectomy
and HFD. The specific underlying mechanism requires further study.
The results of the present study suggest a functional role for
resveratrol in the control of body weight and glycolipid metabolism
in ovariectomized rats fed an HFD, consistent with previous report
(33). The overexpression of the
inflammatory factors TNF-α, IL-6 and MCP-1 in the kidney tissue was
also reversed significantly after resveratrol treatment, suggesting
that resveratrol exerts certain renoprotective effects in
postmenopausal obese model rats. Resveratrol was shown to have
marked protective effects on podocytes in db/db mice and on
cultured human podocytes through the stimulation of autophagy in a
previous study (41). Another recent
study also proved that resveratrol ameliorated podocyte injury and
proteinuria in obese rats through suppression of the NF-κB
signaling pathway, which initiates transcription and protein
expression, including TNF-α, IL-6 and MCP-1, resulting in
substantial inflammatory responses (30). Notably, resveratrol treatment
significantly enhanced nephrin and WT-1 expression levels in
ovariectomized rats with HFD compared with the sham operation and
standard diet group (P<0.05) in the present study. These results
indicated that resveratrol may alleviate podocyte injury and exert
a renoprotective effect.
In conclusion, resveratrol improves lipid
metabolism, improves insulin sensitivity and regulates renal
inflammatory responses, and thereby modulates nephrin and WT-1
protein expression in obese rats. Resveratrol serves a critical and
novel function in the amelioration of podocyte injury in obese rats
induced by ovariectomy and an HFD. The present study suggested that
resveratrol may be used as a promising agent for obesity-associated
early renal damage.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no. ZR2014HM037),
the Science & Technology Development Program of Jinan (grant
no. 201401241) and the Key Research and Development Project of
Shandong Province (grant no. 2017GSF21116).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BL, XYX, BJ and ZH participated in the conception
and design of the study, data analysis interpretation and drafting
of the manuscript. BL, YLM, LG and XHL contributed to the data
acquisition and analysis. JHZ collected and analyzed the
pathological data. BL and XYX participated in the drafting of the
manuscript and substantive revisions of the important content of
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was ethically approved by the
Animal Ethics Committee of Shandong University (Shandong,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
E2
|
17β-estradiol
|
|
SCr
|
serum creatinine
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
LDL-C
|
low-density lipoprotein
cholesterol
|
|
FBG
|
fasting blood glucose
|
|
HOMA-IR
|
homeostasis model assessment of
insulin resistance
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-6
|
interleukin 6
|
|
MCP-1
|
monocyte chemotactic protein-1
|
|
WT-1
|
Wilms' tumor-1
|
|
HFD
|
high-fat diet
|
|
PAS
|
periodic acidschiff
|
References
|
1
|
Finucane MM, Stevens GA, Cowan MJ, Danaei
G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN,
et al: National, regional, and global trends in body-mass index
since 1980: Systematic analysis of health examination surveys and
epidemiological studies with 960 country-years and 9·1 million
participants. Lancet. 377:578–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu H: Obesity and metabolic inflammation.
Drug Discov Today Dis Mech. 10:e21–e25. 2013. View Article : Google Scholar
|
|
3
|
Hsu CY, Iribarren C, McCulloch CE,
Darbinian J and Go AS: Risk factors for end-stage renal disease:
25-year follow-up. Arch Intern Med. 169:342–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morandi A and Maffeis C: Urogenital
complications of obesity. Best Pract Res Clin Endocrinol Metab.
27:209–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kastarinen M, Juutilainen A, Kastarinen H,
Salomaa V, Karhapaa P, Tuomilehto J, Gronhagen-Riska C, Jousilahti
P and Finne P: Risk factors for end-stage renal disease in a
community-based population: 26-year follow-up of 25,821 men and
women in eastern Finland. J Intern Med. 267:612–620. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gambacciani M, Ciaponi M, Cappagli B,
Benussi C, De Simone L and Genazzani AR: Climacteric modifications
in body weight and fat tissue distribution. Climacteric. 2:37–44.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burns KA and Korach KS: Estrogen receptors
and human disease: An update. Arch Toxicol. 86:1491–1504. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sayakhot P, Vincent A, Deeks A and Teede
H: Potential adverse impact of ovariectomy on physical and
psychological function of younger women with breast cancer.
Menopause. 18:786–793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chedraui P, Escobar GS, Ramírez C,
Pérez-López FR, Hidalgo L, Mannella P, Genazzani A and Simoncini T:
Nitric oxide and pro-inflammatory cytokine serum levels in
postmenopausal women with the metabolic syndrome. Gynecol
Endocrinol. 28:787–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mercantepe T, Unal D, Selli J, Mercantepe
F, Unal B and Karabiyik TN: Protective effects of estrogen and
bortezomib in kidney tissue of post-menopausal rats: An
ultrastructural study. Ren Fail. 38:1129–1135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amaral LS, Silva JA, Trindade TM, Ribas
WB, Macedo CL, Coimbra TM, Belo NO, Magalhaes AC and Soares TJ:
Renal changes in the early stages of diet-induced obesity in
ovariectomized rats. Physiol Res. 63:723–732. 2014.PubMed/NCBI
|
|
12
|
Meydani M and Hasan HS: Dietary
polyphenols and obesity. Nutrients. 2:737–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagao K, Jinnouchi T, Kai S and Yanagita
T: Effect of dietary resveratrol on the metabolic profile of
nutrients in obese OLETF rats. Lipids Health Dis. 12:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen DQ, Hu HH, Wang YN, Feng YL, Cao G
and Zhao YY: Natural products for the prevention and treatment of
kidney disease. Phytomedicine. 50:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen DQ, Feng YL, Cao G and Zhao YY:
Natural products as a source for antifibrosis therapy. Trends
Pharmacol Sci. 39:937–952. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang M, Chen DQ, Chen L, Cao G, Zhao H,
Liu D, Vaziri ND, Guo Y and Zhao YY: Novel inhibitors of the
cellular renin-angiotensin system components, poricoic acids,
target Smad3 phosphorylation and Wnt/β-catenin pathway against
renal fibrosis. Br J Pharmacol. 175:2689–2708. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng YL, Chen DQ, Vaziri ND, Guo Y and
Zhao YY: Small molecule inhibitors of epithelial-mesenchymal
transition for the treatment of cancer and fibrosis. Med Res Rev.
2019.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Chen L, Yang T, Lu DW, Zhao H, Feng YL,
Chen H, Chen DQ, Vaziri ND and Zhao YY: Central role of
dysregulation of TGF-β/Smad in CKD progression and potential
targets of its treatment. Biomed Pharmacother. 101:670–681. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de la Lastra CA and Villegas I:
Resveratrol as an anti-inflammatory and anti-aging agent:
Mechanisms and clinical implications. Mol Nutr Food Res.
49:405–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia N, Daiber A, Forstermann U and Li H:
Antioxidant effects of resveratrol in the cardiovascular system. Br
J Pharmacol. 174:1633–1646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Y, Feng B, He S, Su Z and Zheng G:
Resveratrol combined with total flavones of hawthorn alleviate the
endothelial cells injury after coronary bypass graft surgery.
Phytomedicine. 40:20–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huo X, Zhang T, Meng Q, Li C and You B:
Resveratrol effects on a diabetic rat model with coronary heart
disease. Med Sci Monit. 25:540–546. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fulda S: Resveratrol and derivatives for
the prevention and treatment of cancer. Drug Discov Today.
15:757–765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeon BT, Jeong EA, Shin HJ, Lee Y, Lee DH,
Kim HJ, Kang SS, Cho GJ, Choi WS and Roh GS: Resveratrol attenuates
obesity-associated peripheral and central inflammation and improves
memory deficit in mice fed a high-fat diet. Diabetes. 61:1444–1454.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai CC, Tey SL, Lee MC, Liu CW, Su YT and
Huang SC: Mechanism of resveratrol-induced relaxation of the guinea
pig fundus. Phytomedicine. 43:55–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim S, Jin Y, Choi Y and Park T:
Resveratrol exerts anti-obesity effects via mechanisms involving
down-regulation of adipogenic and inflammatory processes in mice.
Biochem Pharmacol. 81:1343–1351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tou JC: Resveratrol supplementation
affects bone acquisition and osteoporosis: Pre-clinical evidence
toward translational diet therapy. Biochim Biophys Acta.
1852:1186–1194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alberdi G, Rodríguez VM, Miranda J,
Macarulla MT, Arias N, Andrés-Lacueva C and Portillo MP: Changes in
white adipose tissue metabolism induced by resveratrol in rats.
Nutr Metab (Lond). 8:292011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pan QR, Ren YL, Zhu JJ, Hu YJ, Zheng JS,
Fan H, Xu Y, Wang G and Liu WX: Resveratrol increases nephrin and
podocin expression and alleviates renal damage in rats fed a
high-fat diet. Nutrients. 6:2619–2631. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato T and Mizuno S: Nephron, Wilms'
tumor-1 (WT1), and synaptopodin expression in developingpodocytes
of mice. Exp Anim. 66:183–189. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zagotta I, Dimova EY, Debatin KM, Wabitsch
M, Kietzmann T and Fischer-Posovszky P: Obesity and inflammation:
Reduced cytokine expression due to resveratrol in a human in vitro
model of inflamed adipose tissue. Front Pharmacol. 6:792015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sharma R, Sharma NK and Thungapathra M:
Resveratrol regulates body weight in healthy and ovariectomized
rats. Nutr Metab (Lond). 14:302017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang J, Yan H and Zhuang S: Inflammation
and oxidative stress in obesity-related glomerulopathy. Int J
Nephrol. 2012:6083972012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abu-Taha M, Rius C, Hermenegildo C,
Noguera I, Cerda-Nicolas JM, Issekutz AC, Jose PJ, Cortijo J,
Morcillo EJ and Sanz MJ: Menopause and ovariectomy cause a low
grade of systemic inflammation that may be prevented by chronic
treatment with low doses of estrogen or losartan. J Immunol.
183:1393–1402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brunskill EW and Potter SS: Changes in the
gene expression programs of renal mesangial cells during diabetic
nephropathy. BMC Nephrol. 13:702012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Batlle D, Wysocki J, Soler MJ and
Ranganath K: Angiotensin-converting enzyme 2: Enhancing the
degradation of angiotensin II as a potential therapy for diabetic
nephropathy. Kidney Int. 81:520–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kalani A, Mohan A, Godbole MM, Bhatia E,
Gupta A, Sharma RK and Tiwari S: Wilm's tumor-1 protein levels in
urinary exosomes from diabetic patients with or without
proteinuria. PLoS One. 8:e601772013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matoba K, Kawanami D, Ishizawa S, Kanazawa
Y, Yokota T and Utsunomiya K: Rho-kinase mediates TNF-α-induced
MCP-1 expression via p38 MAPK signaling pathway in mesangial cells.
Biochem Biophys Res Commun. 402:725–730. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li L, Xiao N, Yang X, Gao J, Ding J, Wang
T, Hu G and Xiao M: A high cholesterol diet ameliorates
hippocampus-related cognitive and pathological deficits in
ovariectomized mice. Behav Brain Res. 230:251–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang SS, Ding DF, Chen S, Dong CL, Ye XL,
Yuan YG, Feng YM, You N, Xu JR, Miao H, et al: Resveratrol protects
podocytes against apoptosis via stimulation of autophagy in a mouse
model of diabetic nephropathy. Sci Rep. 7:456922017. View Article : Google Scholar : PubMed/NCBI
|



















