Introduction
Hepatitis B virus (HBV) is a noncytopathic
hepadnavirus that can lead to a wide spectrum of human liver
diseases, ranging from acute to chronic hepatitis, cirrhosis and
hepatocarcinoma (1). In total, ~10%
of adults infected with HBV will develop chronic liver infection
and it is estimated that 240 million people are chronic HBV
carriers worldwide; ~650,000 people die each year due to
complications of chronic hepatitis B (CHB) (2,3).
Understanding of the immunological events that take
place in controlling HBV infection during its early phases has
accelerated over recent years. Upon entering the body, the binding
of the HBV pre-S1 region to the sodium taurocholate cotransporting
polypeptide on hepatocytes elicits immediate HBV infection of the
liver (4). After an incubation
period of 4–10 weeks, hepatitis B surface antigen (HBsAg),
hepatitis B e-antigen (HBeAg) or HBV DNA become detectable in the
serum (1–3). The immune system can be activated in
response to viral antigen expression or viral replication in
infected hepatocytes. During the latter stages of infection,
specific protective anti-HBV antibodies are produced, and memory T
cells begin to develop, followed by the clearance of HBV infection.
However, if the HBV infection is not adequately controlled during
the acute stage of infection, chronic HBV infection can develop.
Therefore, the immune response during this early stage is critical
in determining the outcome of infection. However, the exact
mechanism associated with this process remains unclear (5,6).
Understanding of the immunological mechanisms that
occur during the early stages of HBV infection in the liver is
limited due to the lack of a suitable model research. Nevertheless,
some researchers have investigated these very early events using
woodchuck (7), mouse (8) or chimpanzee (9) models of acute HBV infection, with mouse
models being the most widely used. The mouse model of acute HBV
infection by hydrodynamic injection (HI) with an HBV supergenomic
DNA construct was first developed by Yang et al (10). This immunocompetent model can be used
to examine the hepatic immunological effectors required for HBV
clearance. Previous studies using this model have suggested that
cells or mediators associated with the innate immune response,
including NK cells (11), toll-like
receptors 2 (12) and iNOS (13), participate in the early response to
HBV infection.
The innate immune system can respond very rapidly
during the early or acute stages of infection to exert functions
and boost the subsequent specific immunity. Compared with the
extensively studied HBV-specific immunity, mechanisms of innate
immune responses during the early stages of HBV infection remain to
be defined (14–16).
γδ T cells, unlike conventional αβ T cells, express
the γ and δ chains in their T cell receptors (TCRs). γδ T cells are
a class of innate immune cells that share some functions with NK
cells, including surface molecules (CD56 and killer cell lectin
like receptor K1), production of cytokines [interferon (IFN)-γ and
tumor necrosis factor-α (TNF-α)] and cytotoxic activity against
infected or transformed cells (17).
Indeed, the potential role of γδ T cells is garnering attention due
to their reported participation in a plethora of immunological
functions, including immune cytotoxicity, cytokine production,
antigen presentation and immunological cross-talk with other cells
(18,19). In murine cytomegalovirus or
Plasmodium falciparum infection, γδ T cells are activated
rapidly and initiate the secondary immune response (20,21). In
HBV infection, previous studies have demonstrated reduced
percentages of peripheral Vδ2 T cells in patients with CHB
(22), whilst patients with
asymptomatic, persistent HBV infection exhibit increased
IFN-γ-producing γδ T cells (23). In
a mouse model carrying HBV, γδ T cells have been shown to mobilize
myeloid-derived suppressor cell (MDSC) infiltration into the liver,
leading to MDSC-mediated CD8+ T cell exhaustion
(24).
However, at present, the role of γδ T cells during
acute HBV infection remains unclear. Therefore, the present study
focused on assessing the changes that occur in the population of γδ
T cells during acute HBV infection, especially in the liver, and
whether they participate in the innate immune response during the
early stages of HBV clearance. A mouse model of acute HBV infection
was constructed using a hydrodynamics-based HBV plasmid
transfection method reported previously (25,26).
Using this immunocompetent mouse model, which mimics acute HBV
infection, liver γδ T cells and innate immune responses in the
liver tissue were dynamically observed. The results suggested that
during the early stages of acute HBV infection, the percentage and
function of liver γδ T cells was enhanced, which occurred
concurrently with increased IFN-β expression and other innate
immune responses in the liver.
Materials and methods
Mice, plasmids and HI
Female C57BL/6J mice (age, 4–6 weeks; weight range,
16–22 g) were purchased from the Animal Center of Chongqing Medical
University (Chongqing, China). All animals were housed under
specific pathogen-free conditions in which the ambient temperature
(23±1°C) and humidity (~35–45%) were controlled with a 12-h
light/dark cycle and food and water ad libitum and treated
according to the guidelines of the animal facility at the Chongqing
Medical University. All experiments were approved by Chongqing
Medical University and were conducted in accordance with the
Guidelines for the Care and Use of Laboratory Animals in China
(27).
An HBV replication-competent plasmid encoding the
1.3-fold overlength HBV genome [pcDNA3.1-HBV 1.3 (ayw subtype)] was
a kind gift from Professor Ni Tang (Key Laboratory of Molecular
Biology for Infectious Diseases, Institute for Viral Hepatitis,
Chongqing Medical University, Chongqing, China). Corresponding
control pcDNA3.1 vector was purchased from Invitrogen (Thermo
Fisher Scientific, Inc.). All plasmids were reserved at −20°C.
A total of 55 female mice were randomly divided into
11 groups, including 0 (normal mice), 1, 3, 5, 7 or 15 days after
pHBV plasmid injection and 1, 3, 5, 7 or 15 days after control
plasmid injection. Mice were then hydrodynamically injected with 15
µg plasmid dissolved in 1.5 ml saline solution through their tail
veins within 5 sec. There were 5 mice per group in each
experiment.
Peripheral blood, spleen and liver samples were
collected for analysis at different timepoints following plasmid
transfection. Mice were anesthetized by exposure to ether presented
on a cotton ball inside a conical tube. A conical tube containing
diethyl ether-soaked cotton balls was placed near the nose of each
mouse without contact. The mice were fully anesthetized several
minutes later, but remained alive with their hearts beating and
body temperature kept constant at 37°C. The mice were then fixed
and placed in a supine position, the abdominal and thoracic
cavities were subsequently opened and 0.5–0.8 ml blood samples were
obtained from the heart, which were collected into a tube
containing the anticoagulant EDTA. The portal vein was then
perfused with 5 ml saline and the liver and spleen were collected
in a plate filled with iced RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) for cell and lymphocyte isolation. At the
completion of the procedure, all mice were sacrificed by cervical
dislocation prior to awakening from anesthesia. At the time of
sacrifice, the weights of the mice had decreased to 15–19 g due to
blood and tissue collection. No fixatives were applied during any
of the aforementioned procedures.
Detection of serum HBV antigens, HBV
DNA and liver function
On days 0, 1, 3, 5, 7 and 15 following transfection,
levels of HBsAg and HBeAg in the serum were measured using
cobas® HBsAg detection kit and cobas® HBeAg
detection kit by electrochemiluminescence immunoassay (Roche
Diagnostics) according to the manufacturer's protocols, with the
results represented as cut-off index (COI) values. To avoid plasmid
contamination, mouse serum was treated with 20 U DNAase I for ≥12
h, following which HBV DNA was extracted using a Viral DNA
extraction kit (Da An Gene Co., Ltd., China), according to the
manufacturer's protocol, and detected by reverse
transcription-quantitative PCR (RT-qPCR) using a Roche Thermocycler
(Roche Diagnostics) according to the manufacturer's protocol.
Within the same timeframe, alanine aminotransferase
(ALT) levels were also measured in serum collected from the mice
using a Hitachi 7600 Automatic Biochemical Analyzer (Hitachi,
Ltd.).
Histology and immunohistochemical
(IHC) staining for HBsAg and HBcAg expression in liver tissues
Liver histology was determined using
hematoxylin-eosin (H&E) staining. Liver tissues (6 µM sections)
from pHBV-transfected mice on days 0, 1, 3, 5, 7 and 15 were fixed
in 10% neutral formalin for 24 h at room temperature (RT),
dehydrated using an ethanol gradient (70, 80, 90 and 100%) and
embedded in paraffin. The slides were subsequently stained using
H&E for 10 min at RT for histological examination.
IHC staining procedures were conducted according to
the manufacturer's protocols. The main steps were as follows: Liver
specimens (6 µM sections) from pHBV-transfected mice on days 0, 1
and 5 were paraffin-embedded. Following de-paraffinization,
rehydration using an ethanol gradient (95 and 80%) and antigen
retrieval in a 0.1% trypsin solution at 37°C for 20 min, endogenous
peroxidase was quenched using 3% H2O2 and
unspecific binding was blocked using 2.5% goat serum (Cell
Signaling Technology, Inc.) for 20 min at RT. Mouse anti-HBsAg
primary monoclonal antibody (1:100, dilution; cat. no. ZM-0122) or
mouse anti-HBcAg primary antibody (1:100, dilution; cat. no.
ZM-0421; both ZSGB-BIO) was subsequently added, followed by
incubation overnight at 4°C. The samples were then incubated with
horseradish peroxidase-conjugated goat anti-mouse polymer
(Elivision™ plus Polyer HRP (Mouse/Rabbit) IHC Kit; cat. no.
KIT-9902) for 30 min at room temperature (Fuzhou Maixin Biotech
Co., Ltd., China), followed by treatment with 3,3′-diaminobenzidine
(DAB; Elivision Super; Fuzhou Maixin Biotech Co., Ltd., China).
Yellow or brown dye in hepatocytes indicated positive staining. The
percentage of positively stained cells were determined by counting
in five random high-power fields using an Olympus optical light
microscope (Olympus Corporation), where there were ≥100 cells in
each field (magnification, ×400).
Preparation of lymphocytes from the
livers and spleens
Liver lymphocytes were isolated as previously
reported (28). Briefly, mice were
anaesthetized with diethyl ether and the portal vein was perfused
with 5 ml saline until the liver became pale in color. The liver
was then cut into small pieces, and incubated in RPMI 1640 solution
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 0.05%
collagenase IV (cat. no. C5138; Sigma-Aldrich; Merck KGaA) and
0.01% DNAase I (cat. no. D5025; Sigma-Aldrich; Merck KGaA) at 37°C
for 30 min, after which the pieces were pressed through a 200-gauge
stainless steel mesh. Following centrifugation at 50 × g (4°C, 10
min, the precipitate was discarded) and again at 500 × g (4°C, 10
min, the supernatant was discarded), the remaining cell pellet was
resuspended in 3 ml RPMI-1640 solution (Gibco; Thermo Fisher
Scientific, Inc.) and overlaid onto a 33% Percoll solution (cat.
no. 17-0891-01; Pharmacia Biotech; GE Healthcare, USA), followed by
centrifugation at 800 × g for 30 min at room temperature. The
supernatant was then aspirated, and the red blood cells (RBC) were
lysed using a 0.75% NH4Cl solution. After subsequent
washing with PBS, the liver lymphocytes were prepared for immediate
FACS analysis.
Mouse spleen tissues were smashed and dissociated
thoroughly using two glass slides with rough surfaces smeared
beforehand with a RBC lysis buffer (0.75% NH4Cl
solution). After RBC lysis for 10 min, the cell suspension was
filtered through a 70 µM filter (BD Biosciences) to obtain a
single-cell suspension. The suspension was then washed with PBS and
the lymphocyte-enriched spleen cells were prepared for later
use.
Fluorescence-activated cell sorting
(FACS) analysis of lymphocytes for cell surface markers and
intracellular cytokine production
The following fluorochrome-conjugated mAbs were used
according to the manufacturer's protocol: Purified anti-mouse
CD16/CD32 (1:100 dilution; cat. no. 14-0161-81; eBioscience; Thermo
Fisher Scientific, Inc.); peridinin-chlorophyll-protein
complex-conjugated hamster anti-mouse CD3e (1:50 dilution; cat. no.
553067; BD Biosciences); phycoerythrin (PE)-conjugated hamster
anti-mouse γδ TCR (1:50 dilution; cat. no. 553178; BD Biosciences);
PE-Cy™7-conjugated anti-mouse CD69 (1:50 dilution; cat. no.
25-0691-81; eBioscience; Thermo Fisher Scientific, Inc.);
allophycocyanin (APC)-conjugated rat anti-mouse CD25 (1:50
dilution; cat. no. 558643; BD Biosciences); fluorescein
isothiocyanate (FITC)-conjugated hamster anti-mouse γδ TCR (1:100
dilution; cat. no. 553177; BD Biosciences); PE-conjugated
anti-mouse IFN-γ (1:100 dilution; cat. no. 12-7311-81; eBioscience;
Thermo Fisher Scientific, Inc.); PE-Cy™7-conjugated rat anti-mouse
TNF-α (1:100 dilution; cat. no. 557644; BD Biosciences); PE-Cy™7
anti-mouse NK1.1 (1:50 dilution; cat. no. 552878; BD Biosciences);
FITC-conjugated anti-mouse CD4 (1:100 dilution; cat. no. 553047; BD
Biosciences); and APC-Cy™7-conjugated anti-mouse CD8 (1:50
dilution; cat. no. 557654; BD Biosciences).
For surface staining, liver lymphocytes
(~5×105), splenic cells (~5×105) or 100 µl of
fresh peripheral anticoagulated blood samples were used for
staining. Cells were blocked using 0.5 µg anti-CD16/32 antibody for
10 min at 4°C, after which an appropriate volume of each specific
antibody was added, and the samples were incubated for 30 min in
the dark at 4°C. For whole-blood staining, erythrocytes were lysed
using BD™ FACS™ lysing solution (BD Biosciences) and cells were
washed using PBS supplemented with 1% fetal calf serum (FCS; Gibco;
Thermo Fisher Scientific, Inc.).
Intracellular cytokine staining was performed as
follows: Liver lymphocytes were adjusted to ~5×106
cells/ml in RPMI 1640 culture medium supplemented with 10% FCS and
stimulated with 100 ng/ml phorbol myristate acetate plus 1 µg/ml
ionomycin at 37°C for 4 h in the presence of the secretion
inhibitor monensin (0.16 µg/ml; BD Biosciences) (29). Cells were blocked using 0.5 µg
anti-CD16/32 antibody for 10 min at 4°C and then stained with
anti-TCR γδ mAb for 30 min at 4°C, followed by washing with PBS and
fixing in 4% paraformaldehyde. Stained cells were permeabilized
using 0.1% saponin (Sigma-Aldrich; Merck KGaA) and incubated with
anti-IFN-γ and anti-TNF-α for 30 min at 4°C.
Stained cells were immediately analyzed using the
FACSCanto™ II flow cytometer (BD Immunocytometry Systems; BD
Biosciences). Data were analyzed using FACSDiva™ 2.0 software (BD
Immunocytometry Systems; BD Biosciences). Cell gating strategies
were as follows: The population of cells double positive for γδ TCR
and CD3 was defined as the γδ T cell subtype, which was
subsequently subdivided into several subsets, including
CD25+, CD69+, IFN-γ+ or
TNF-α+ γδ T cells, according to their positivity in the
FACS dot plots. CD3 and NK1.1 were used to measure the presence of
mouse NK and NKT cells; CD3- NK1.1+ cells were defined as NK cells
(left upper quadrant) and CD3+ NK1.1+ cells were defined as NKT
cells (right upper quadrant) (30).
RT-qPCR analysis for gene expression
in the liver tissue
Liver tissue (~40 mg) in 1 ml TRIzol®
solution (Invitrogen; Thermo Fisher Scientific, Inc.) was
homogenized using a power homogenizer and the total RNA was
extracted according to the manufacturer's protocols. RNA quality
was evaluated by electrophoresis and spectral analysis. Only RNA
without degradation or contamination with DNA or protein was used
for subsequent RT-qPCR analyses.
RNA (~1 µg) was reverse transcribed by 2 min at
70°C, 15 min at 37°C, and then 1 min at 95°C with oligo (dT)
primers using the PrimeScript™ RT Reagent kit with gDNA eraser,
according to the manufacturer's protocol (Takara Bio, Inc.). qPCR
was then performed using SYBR® Green quantitative PCR
dye with SYBR® Premix Ex Taq™ II, according to the
manufacturer's protocol (Takara Bio, Inc.). All samples were
detected in triplicate using an Applied Biosystems 7300 Real-Time
PCR Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocols. GAPDH was used as
an internal control and the data were analyzed using the
2−ΔΔCq method (31).
Primer sequences for IFN-α, IFN-β, IFN-γ, TNF-α and GAPDH used for
RT-qPCR are listed in Table I.
 | Table I.Sequences of primers used for reverse
transcription-quantitative PCR. |
Table I.
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Primer
sequence |
|---|
| IFN-α | F:
5′-GGATGTGACCTTCCTCAGACTC-3′ |
| (NM_010502) | R:
5′-ACCTTCTCCTGCGGGAATCCAA-3′ |
| IFN-β | F:
5′-GCCTTTGCCATCCAAGAGATGC-3′ |
| (NM_010510) | R:
5′-ACACTGTCTGCTGGTGGAGTTC-3′ |
| IFN-γ | F:
5′-CAGCAACAGCAAGGCGAAAAAGG-3′ |
| (NM_008337) |
|
|
| R:
5′-TTTCCGCTTCCTGAGGCTGGAT-3′ |
| TNF-α | F:
5′-GGTGCCTATGTCTCAGCCTCTT-3′ |
| (NM_013693) | R:
5′-GCCATAGAACTGATGAGAGGGAG-3′ |
| HBsAg | F:
5′-GTGTCTGCGGCGTTTTATCA −3′ |
|
| R:
5′-GACAAACGGGCAACATACCTT-3′ |
| HBcAg | F:
5′-TAGCTACCTGGGTGGGTGTT-3′ |
|
| R:
5′-AAGCTGGAGGAGTGCGAATC-3′ |
| GAPDH | F:
5′-CATCACTGCCACCCAGAAGACTG-3′ |
|
| R:
5′-ATGCCAGTGAGCTTCCCGTTCAG-3′ |
Statistical analysis
SPSS software (version 15.0; SPSS Inc.) was used to
analyze all data. Experimental data are expressed as the mean ± SD,
from five experimental repeats. Following one-way ANOVA,
differences between every two groups were calculated using the
Least Significant Difference method. For comparison of RNA
expression, significant changes were assessed as at least a 2-fold
increase or a 0.5-fold decrease. Pearson correlation analysis was
performed to evaluate the correlation in the percentage of γδT
cells with the relative fold changes in HBsAg or HBcAg RNA
expression. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of HBV markers in serum and
livers from mice with acute HBV infection
To verify that the mouse model of acute HBV
infection was constructed successfully in the present study, serum
HBsAg, HBeAg and HBV DNA levels and the intrahepatic expression of
HBsAg and HBcAg were measured.
On day 1 after hydrodynamic-based pHBV plasmid
injection, serum tested positive for HBsAg, HBeAg, and HBV DNA
(Fig. 1A). HBV markers were
undetectable in mice after control plasmid transfection (data not
shown). After pHBV plasmid injection, HBsAg serum levels increased
from 113.9±31.1 (COI) on day 1 to a peak value of 255.3±47.6 (COI)
on day 3, followed by a decrease to 5.3±1.5 (COI) on day 15. Serum
HBeAg levels were the highest on day 1 at 31.1±6.9 (COI) and then
decreased gradually to 1.5±0.7 (COI) on day 15 (Fig. 1A). Additionally, the serum HBV DNA
load declined from an average of 3.2×104 copies/ml on
day 1 to virtually undetectable levels on days 7 and 15 (Fig. 1A). In terms of liver function, serum
ALT levels in pHBV-transfected groups increased on day 1 and then
decreased back to normal levels (<30 U/l).
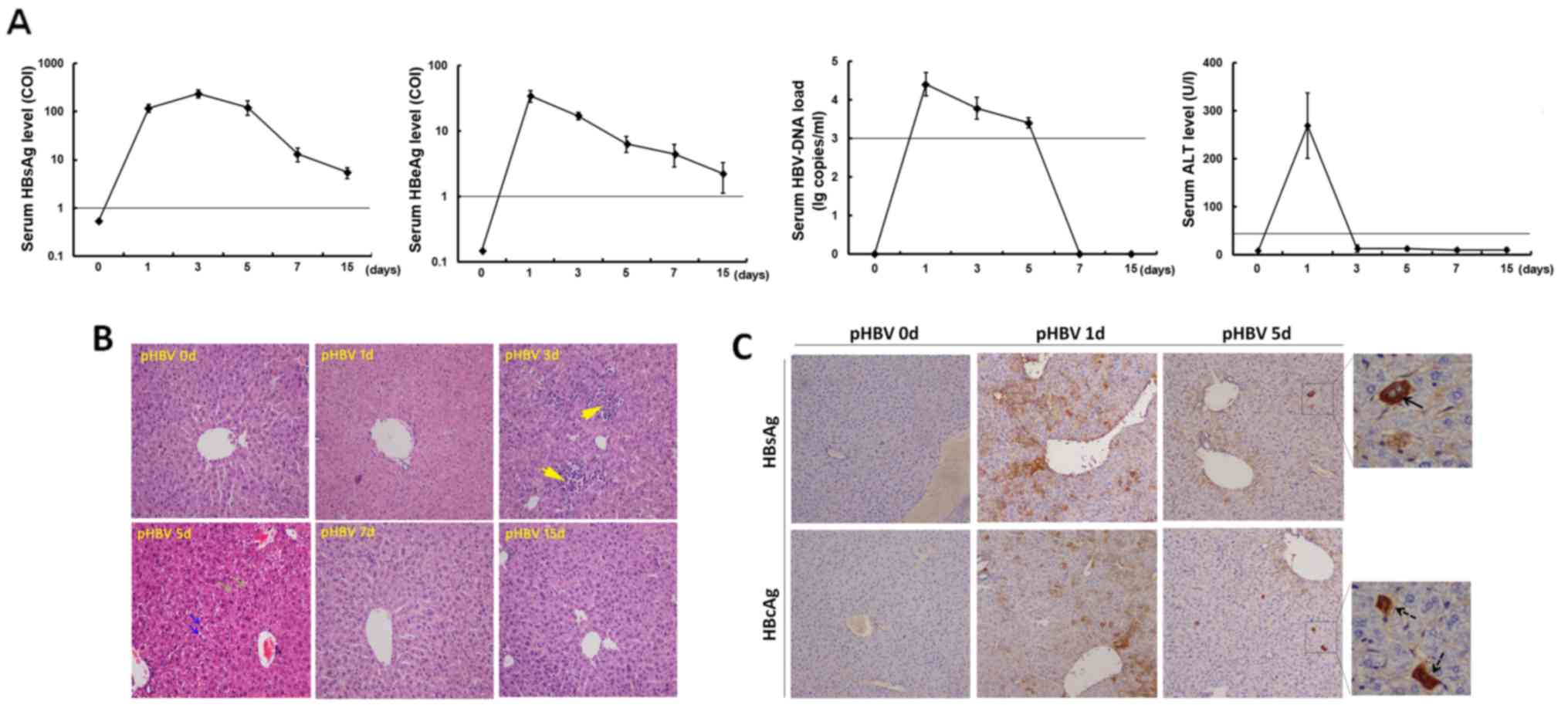 | Figure 1.Assessment of acute HBV infection in
mice following hydrodynamic transfection. Female C57BL/6J mice were
transfected with the pcDNA3.1-HBV1.3 plasmid using the hydrodynamic
method. (A) Serum levels of HBsAg, HBeAg, HBV DNA and ALT were
detected on days 0, 1, 3, 5, 7 and 15 after pHBV plasmid injection.
The straight lines in the graphs indicate the cut-off values. The
HBV DNA load was displayed in a log 10 scale format. A total of
five mice were used for each time point. Data at each time point
are expressed as the mean ± SD. (B) Hematoxylin-eosin staining of
liver specimens from pHBV-transfected mice on days 0, 1, 3, 5, 7
and 15. Yellow arrows indicated infiltrating mononuclear cells, and
green or blue arrows indicated necrotic lesions or degeneration of
hepatocytes, respectively. Magnification, ×100. (C) HBsAg and HBcAg
expression in murine liver tissue samples detected using
immunohistochemistry on days 0, 1 and 5 in pHBV-transfected mice.
Arrows with solid or dashed lines show HBsAg- or HBcAg-positive
hepatocytes, respectively. Magnification, ×100 or ×400. HBV,
hepatitis B virus; HBeAg, hepatitis B virus e-antigen; HBsAg,
hepatitis B surface antigen; pHBV, pcDNA3.1-HBV1.3 plasmid; ALT,
alanine aminotransferase; COI, cut-off index. |
Liver histopathology was evaluated on days 0, 1, 3,
5, 7 and 15 after injection (Fig.
1B). An apparent accumulation of mononuclear cells was observed
in the mouse livers on day 3. On day 5, the liver structure changed
with the appearance of some necrotic lesions and hepatocyte
degeneration. By day 7, the liver tissue recovered back to a normal
architecture, which was also maintained on day 15 (Fig. 1B).
HBsAg and HBcAg expression was subsequently examined
by immunohistochemistry with DAB staining in the liver specimens
isolated from pHBV-transfected mice (Fig. 1C). The average percentages of
positively stained cells were determined by counting in five random
high-power fields. On day 1, an average of 13.8 or 11.2%
hepatocytes were staining positive for HBsAg or HBcAg,
respectively. HBsAg was mainly expressed in the cytoplasm, whilst
HBcAg expression was observed in both the cytoplasm and the
nucleus. However, HBsAg and HBcAg expression decreased sharply on
day 5 post-injection, with only 0.6 and 1.2% of hepatocytes on
average displaying positive expression of HBsAg and HBcAg,
respectively (Fig. 1C).
Aside from HBsAg+ or HBcAg+
hepatocytes, HBsAg and HBcAg mRNA expression were also measured in
the liver tissues using RT-qPCR, where the results also showed that
HBV expression was increased at day 1 (Fig. S1).
Subsequent changes in liver γδ T cell
percentages in mice following acute HBV infection
To investigate changes in γδ T cell numbers, the
percentages of γδ T cells were first measured in the liver, spleen
and peripheral blood samples of mice using FACS analysis, to
dynamically monitor any changes following HBV plasmid injection
(Fig. 2). Representative FACS dot
plots for γδ T cells are shown in Fig.
2A. On day 0, the percentage of γδ T cells in the liver was
4.5±0.6% (percentage of total T cells), which was significantly
higher compared with that observed in the spleen (2.1±0.3%;
P<0.05) or the peripheral blood (1.2±0.6%; P<0.05). On day 1
post-injection, the percentage of liver γδ T cells increased to
8.3±2.9%, which was significantly higher compared with that on day
0 (4.5±0.6%; P<0.05) and that found in the liver samples of mice
transfected with the control plasmid on day 1 (pcDNA; 3.8±0.7%;
Fig. 2B). The percentage of liver γδ
T cells in the pHBV-infected group gradually decreased to a level
similar to that observed on day 0, while there were no significant
changes in the percentage of liver γδ T cells after control plasmid
transfection. Additionally, the percentages of spleen and blood γδ
T cells from either the pHBV or pcDNA control groups displayed no
significant changes compared with those on day 0 (P>0.05;
Fig. 2C and D). In addition, the
percentage of liver γδ T cells increased dramatically on day 1,
which was in accordance with the highest percentage of
HBsAg+ or HBcAg+ hepatocytes and the
increased mRNA expression of HBsAg and HBcAg in the mouse livers
(Fig. S1)
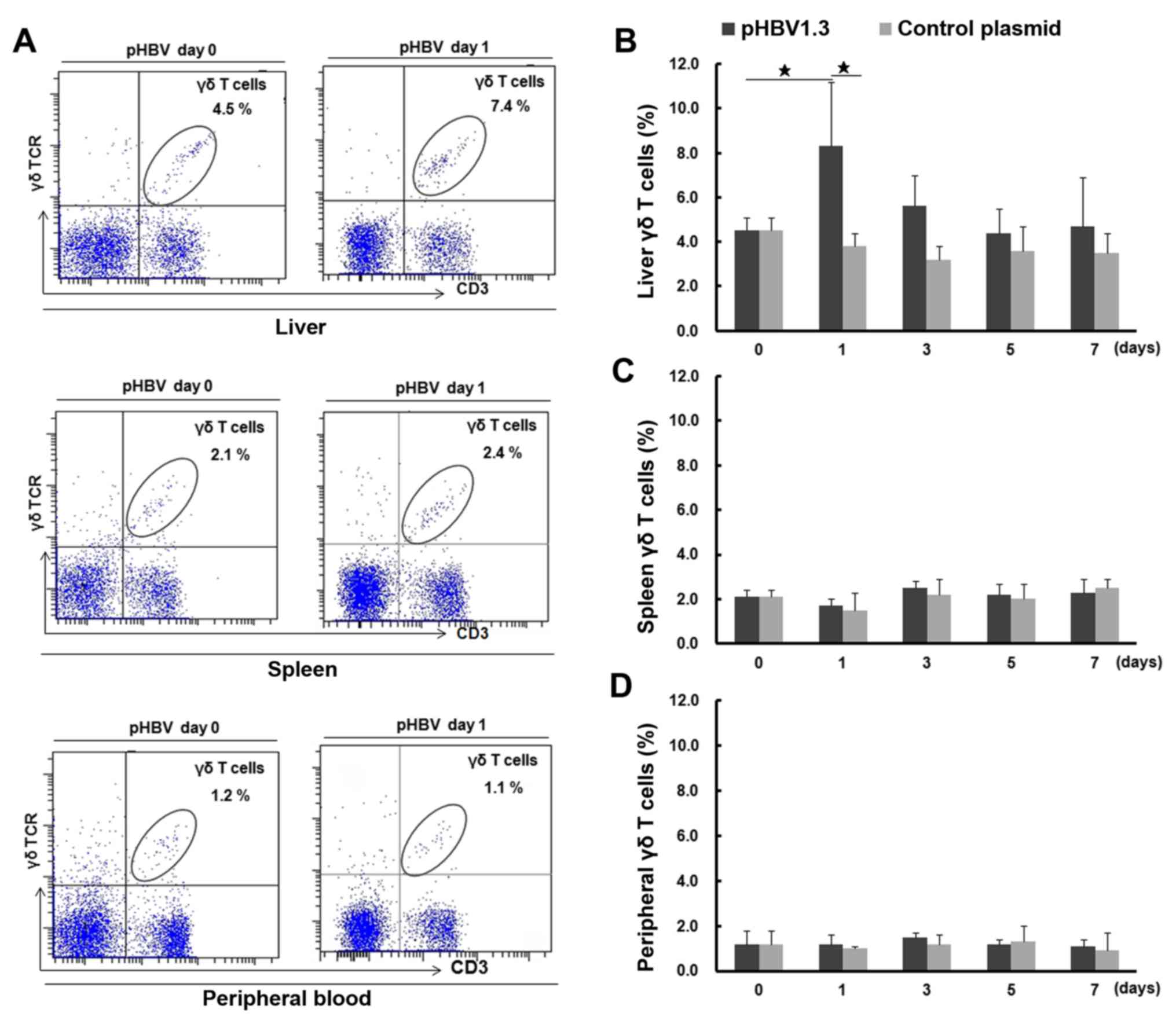 | Figure 2.Percentage of γδ T cells in the
liver, spleen and peripheral blood of pHBV- or control
plasmid-transfected mice on days 0, 1, 3, 5 and 7. (A) The
representative fluorescence-activated cell sorting plots show
lymphocyte and γδ T cell populations in the liver, spleen and
peripheral blood from pHBV mice at days 0 and 1 after plasmid
transfection. The cell population in the right upper quadrant shows
γδ T cells, and their percentages among the CD3+ T cell
populations are indicated in each panel. (B) The percentage of γδ T
cells (% of total T cells) in the liver, (C) spleen and (D)
peripheral blood at different time points after pHBV or control
plasmid transfection. A total of five mice were used at each time
point. Data at each time point are expressed as the mean ± SD.
⋆P<0.05. γδTCR, γδ T cell receptor; HBV, hepatitis B
virus; pHBV, pcDNA3.1-HBV1.3 plasmid. |
Activation of liver γδ T cells in
pHBV-transfected mice
Following the observation that there was an
increased percentage of total liver γδ T cells following pHBV
transfection, the activation and function of these liver γδ T cells
were investigated further by assessing the expression of CD25 and
CD69, surface markers for activation (32) and intracellular cytokines IFN-γ and
TNF-α (Fig. 3A). The percentages of
CD25+ and CD69+ liver γδ T cells on day 0
were found to be 2.5±0.5 and 6.5±0.9% (of total γδ T cells),
respectively (Fig. 3B and C). The
percentage of CD69+ liver γδ T cells in the
pHBV-transfected group increased to 18.9±4.7% on day 1
post-injection, which was significantly higher compared with that
in the day 0 group (6.5±0.9%) or the pcDNA control group (7.7±1.8%)
on day 1. CD69 expression declined to 8.7±1.6% on day 3 and settled
to 9.6±1.5% on day 7. The percentage of CD25+ liver γδ T
cells was found to be gradually elevated in the pHBV-transfected
group, with an increase from 3.9±1.8% on day 1 to 7.1±2.1% on day
5, followed by a drop to 1.9±0.4% on day 7. The percentage of
CD25+ γδ T cells on day 5 in pHBV-transfected group was
significantly different when compared with the percentages on day 0
(2.5±0.5%) and in the pcDNA control on day 5 (3.2±0.9%; P<0.05).
For the pcDNA control group, no significant differences were found
in the percentages of either CD25+ or CD69+
γδ T cells across the different time points tested (P>0.05).
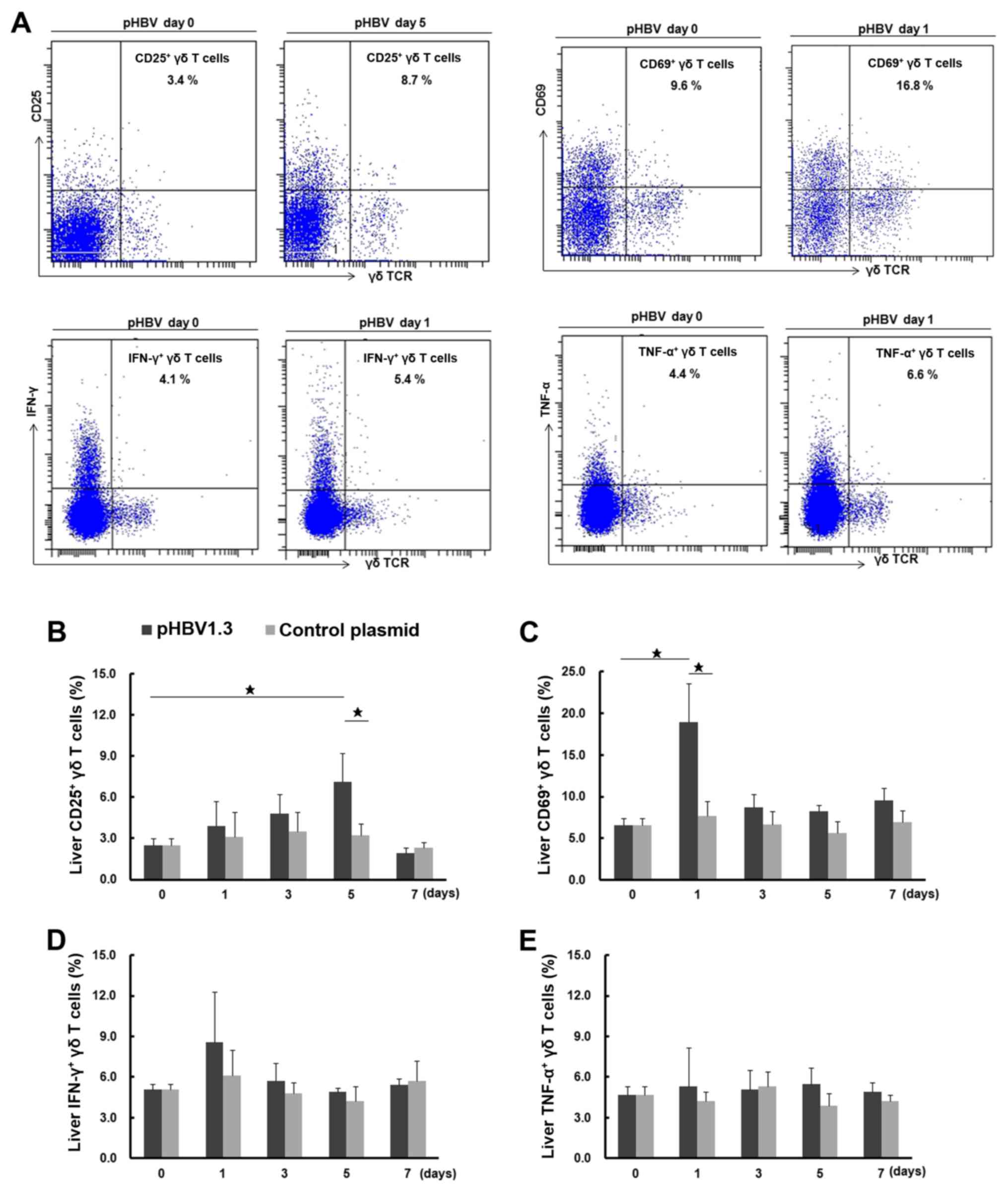 | Figure 3.Activation of liver γδ T cells after
pHBV plasmid transfection. (A) Representative FACS dot plots
showing the expression of CD25, CD69, IFN-γ and TNF-α in liver γδ T
cells from mice transfected with pHBV on days 0 and 1 or 5 after
plasmid transfection. The cell population in the right upper
quadrant showed CD25+, CD69+,
IFN-γ+ or TNF-α+ γδ T cells and their
respective percentages among total γδ T cells as indicated in the
figures. (B) Dynamic changes in the percentage of CD25+,
(C) CD69+, (D) IFN-γ+ and (E)
TNF-α+ liver γδ T cells on days 0, 1, 3, 5 and 7 after
transfection with the pHBV1.3 or control plasmid. A total of five
mice were used at each time point. Data at each time point are
expressed as the mean ± SD. ⋆P<0.05. γδTCR, γδ T cell
receptor; HBV, hepatitis B virus; pHBV, pcDNA3.1-HBV1.3 plasmid;
IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α. |
As shown in Fig. 3D and
E, the percentages of IFN-γ- and TNF-α-producing liver γδ T
cells were 5.1±0.4% and 4.7±0.6%, respectively, on day 0. After
pHBV plasmid injection, the percentage of IFN-γ+ γδ T
cells increased on day 1 (8.6±3.7%), but this difference was not
significant when compared to the day 0 group (5.1±0.4%) or the
pcDNA control group on day 1 (6.1±1.9%; P>0.05). The percentage
then dropped to 5.4±0.5% on day 7. As for TNF-α-producing γδ T
cells in the pHBV-transfected group, no significant changes were
observed across all time points (P>0.05). There were also no
significant differences in the percentages of IFN-γ+ or
TNF-α+ γδ T cells compared with the pcDNA control group
among all these time points (P>0.05).
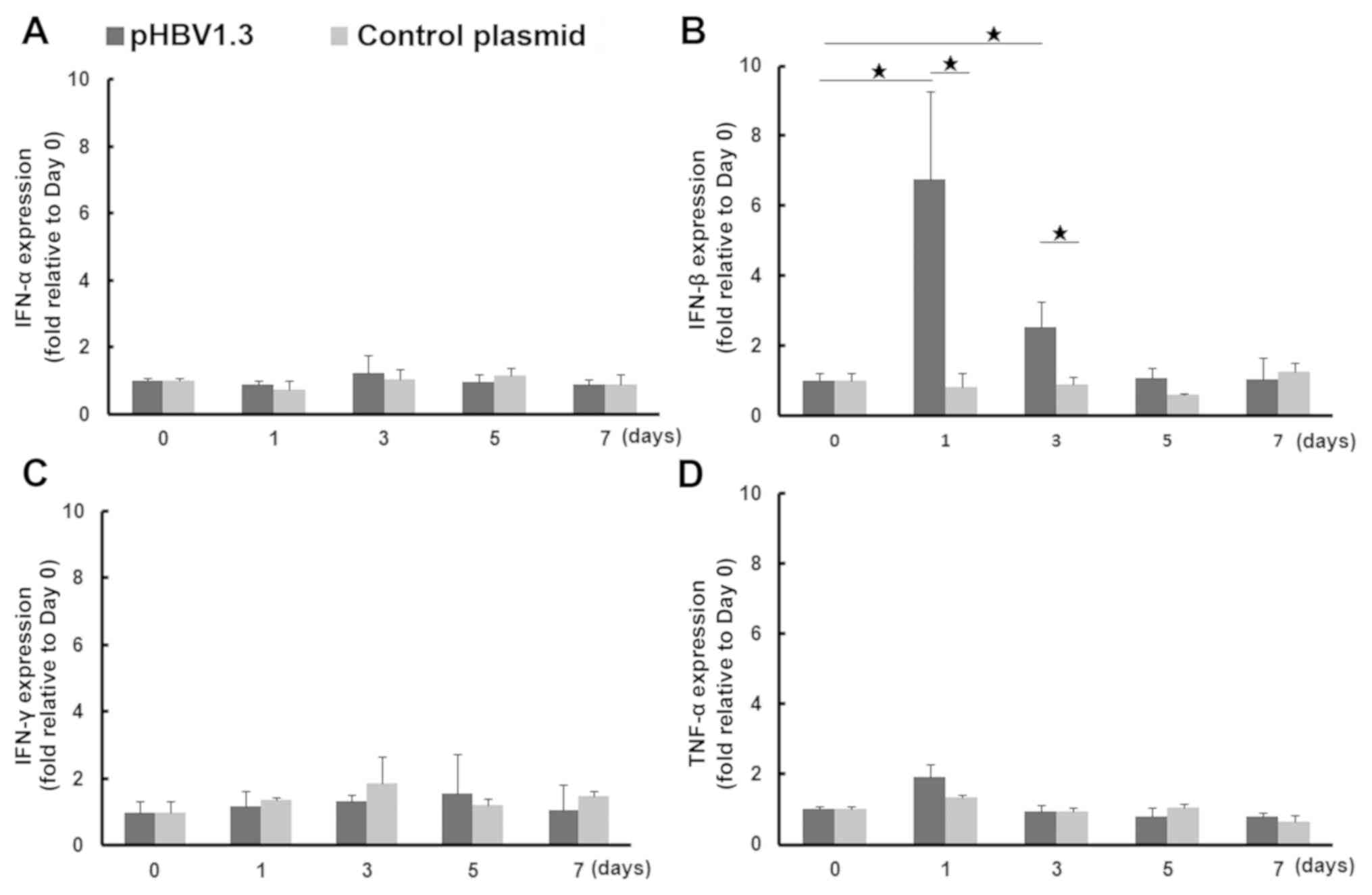
Increased expression of IFN-β during
the early phases of acute HBV infection
Since it was discovered that the number of liver γδ
T cells from mice with acute HBV infection was increased, the
possibility that other innate immune responses were activated in
the liver was next explored. Early cytokine production is the most
important activity associated with the antiviral innate immune
response (33). Therefore, the mRNA
expression of cytokine markers associated with the activation of
the innate immune response, IFN-α and IFN-β, in addition to IFN-γ
and TNF-α, were measured in liver tissues on days 0, 1, 3, 5 and 7
following injection with pHBV or the control plasmid. After pHBV
injection, IFN-β mRNA expression was significantly upregulated, by
an average of 6.8-fold on day 1 and by 2.5-fold on day 3 compared
with that on day 0. It was also significantly higher compared with
that in mice injected with the control pcDNA plasmid on days 1
(8.4-fold) and 3 (2.8-fold). IFN-β expression subsequently
decreased to normal levels on days 5 and 7. No significant changes
in IFN-β expression were observed in the pcDNA control group. TNF-α
expression in tissues from the pHBV-transfected group was increased
slightly on day 1 (1.9-fold) compared with day 0, but no
significant difference was observed in IFN-α or IFN-γ mRNA
expression between pHBV-transfected or control groups across all
time points examined (Fig. 4).
Changes in CD4+ T,
CD8+ T, NK and NK T cell populations from mice with
acute HBV infection
FACS dot plots of liver CD4+ T,
CD8+ T, NK and NK T cells are shown in Fig. 5A. The percentage of liver NK cells
from the pHBV-transfected group on day 1 was significantly higher
compared with that in the control group (Fig. 5D). No significant differences were
found in the percentages of CD4+ T, CD8+ T or
NK T cells between the pHBV-transfected and control groups
(Fig. 5B, C and E). Notably, the
percentage of liver NK T cells decreased on day 3 compared with day
0 after plasmid injection in either the pHBV or pcDNA control
groups, but the observed differences were not significant (Fig. 5E).
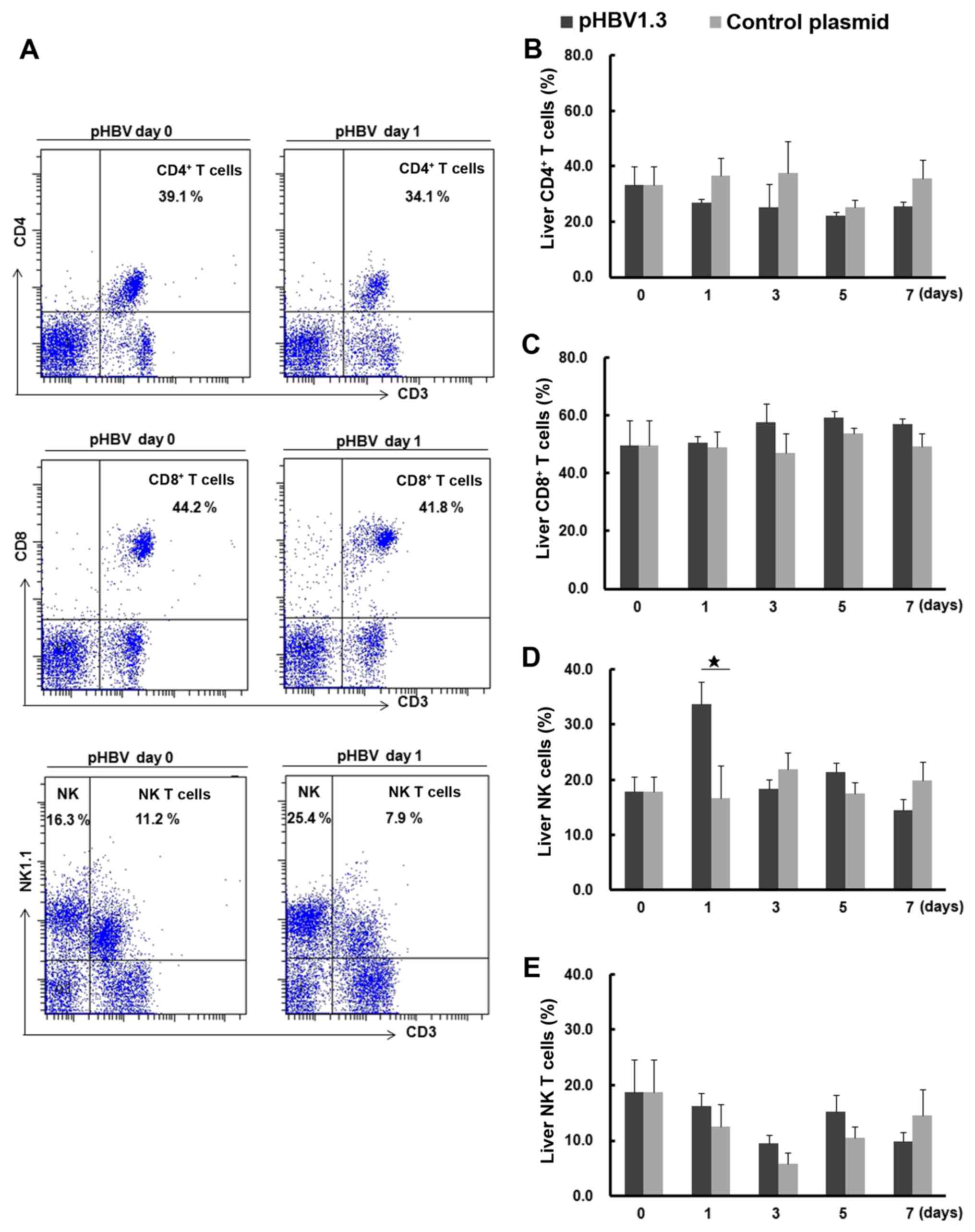 | Figure 5.Percentages of liver CD4+
T, CD8+ T, NK or NKT cells in mice following acute HBV
infection. (A) Representative fluorescence-activated cell sorting
dot plots of CD4+ T, CD8+ T, NK and NKT cells
in mouse liver samples on days 0 and 1 after pHBV or control
plasmid transfection. The percentages of CD4+ T and
CD8+ T cells among CD3+ T cells, or NK and
NKT cells among all lymphocytes gated by FSC and side scatter SSC
are indicated. (B) Changes in the percentages of CD4+
and (C) CD8+ T cells, (D) NK and (E) NKT cells on days
0, 1, 3, 5 and 7 after pHBV1.3 or control plasmid transfection. A
total of five mice were used at each time point. Data at each time
point are expressed as the mean ± SD. ⋆P<0.05. NK,
natural killer; HBV, hepatitis B virus; pHBV, pcDNA3.1-HBV1.3
plasmid; FSC, forward scatter; SSC, side scatter. |
Discussion
It has been unclear what the exact mechanism of HBV
clearance is in the early phases of infection (34,35). In
early previous studies, CD8+ T cells were reported as
the key cellular mediator of HBV clearance from the liver (3,5,6,11).
Activated and polyclonal HBV-specific CD8+ T cells can
always be detected during the recovery of HBV-infected patients,
whereas weakly activated and oligoclonal T cells are generally
associated with persistent HBV infections (36). However, there were some recent
reports demonstrating that the innate immune response serves
important roles in the early stages of HBV infection (37,38).
Although γδ T cells are innate immune cells with multifaceted
functions, including antigen presentation, cytotoxicity, and
cytokine production (39–41), the role of this cell type in the
mechanism of HBV clearance during the early stages of infection
remains unclear.
In the present study, to observe changes in innate
immune responses and γδ T cells during the acute stage of HBV
infection, specifically in the liver, a mouse model of acute HBV
infection was successfully constructed, evidenced by the mouse
serum and liver samples testing positive for HBV markers on day 1
and then mostly eliminated 15 days after pHBV plasmid injection.
Previous studies have also shown that HBV can replicate in the
liver of this mouse model (10,13,25).
Differences in mouse strains, plasmids/vectors or plasmid quality
can result in different levels of HBV marker expression in
different mouse models (25). Using
the mouse model established in the present study, the apparent
infiltration of inflammatory cells into the liver was observed, in
addition to increased ALT levels in the pHBV-transfected group
compared with the pcDNA control group. This suggested that this
mouse model of HBV infection may involve liver inflammation and
injury.
The observation of positive correlation between the
percentage of γδ T cells and expression of HBsAg or HBcAg in liver
on day 1, suggesting that γδ T cells responded rapidly to HBV
expression in the early stages of infection. In contrast, during
the course of HBV expression, different expression patterns of CD69
and CD25 or inflammatory cytokines IFN-γ and TNF-α were found on γδ
T cells, suggesting that these subtypes may carry different
functions. Indeed, in previous studies where comparisons in the
function of IFN-γ- or TNF-α- producing T cells were conducted,
HBV-specific TNF-α producing CD4 T cells was found to be associated
with liver damage, whereas HBV-specific IFN-γ producing CD4 T cells
was more associated with viral clearance in chronic HBV infection
patients (42). This is in contrast
with another study, where TNF-α production by γδ T cells was not as
crucial as IFN-γ in the pathogenesis of experimental autoimmune
encephalomyelitis (43).
The initiation of immune responses was next assessed
in the mouse model of acute HBV infection from the present study.
IFN-β is the most important cytokine associated with antiviral
innate responses in host cells (44). When viruses enter a host cell, viral
DNA or RNA is recognized by intracellular receptors or sensors
which can induce the expression of type I IFNs, especially that of
IFN-β (45). Meanwhile, the NF-κB
pathway is activated, which is followed by an increase in the
expression of inflammatory cytokines such as TNF-α (44,45). In
the present study, the expression of IFN-β and TNF-α were elevated
on day 1 in pHBV-transfected mice, which may be associated with the
immune response to HBV infection. Both IFN-β and IFN-α are type I
IFNs; however, unlike IFN-β, IFN-α expression was not significantly
increased in the model from the present study. This difference may
be the result of differential mechanisms in IFN-α and IFN-β
production. IFN-γ has been previously reported to be the key immune
factor for HBV clearance in chimpanzee models of acute HBV
infection (46). However, in the
present study, IFN-γ mRNA expression did not show any significant
changes in the pHBV-transfected group.
Changes in the numbers of γδ T cells of patients
with other viral hepatitis infections have been previously
observed. A study by Tseng et al (47) showed that liver biopsy specimens of
hepatitis C virus (HCV)-infected patients contained high numbers of
γδ T cells, with high levels of non-major histocompatibility
complex-restricted cytotoxic activity, and IFN-γ and TNF-α
production, demonstrating that γδ T cells may serve a role in the
pathology of HCV infections. In another study by Abravanel et
al (48), increased CD69
expression was exhibited by γδ cells during the acute phase of
hepatitis E virus (HEV) infection in patients with a solid-organ
transplant, but this change was not associated with HEV clearance.
In another study by Wu et al (49), activated liver γδT cells were found
to be cytotoxic towards hepatocytes infected with murine hepatitis
virus strain 3 (MHV-3), which may have contributed to the
pathogenesis of MHV-3-induced murine fulminant viral hepatitis. In
the present study, increased liver γδ T cells were found to be
associated with the elimination of acute HBV infection. The
aforementioned data suggest that changes in γδ T cell levels appear
to be common in infections with hepatitis viruses, including HBV,
HCV and HEV. However, this change in γδ T cell number may serve
different roles in a manner that is dependent on the type of
infection.
In a previous study by Kong et al (24), an HBV immunotolerant (HBV carrier)
mouse model was constructed by injection of a relatively low amount
(6 µg) of AAV/HBV1.2 plasmid. This mouse model was characterized by
persistent HBV expression in the liver for >6 months, which was
used to mimic the process of chronic HBV infection with
immunotolerant status. Using this model, Kong et al found
that γδT cells served a regulatory role in liver tolerance by
inducing MDSC-mediated CD8+ T cell exhaustion. In another study, Li
et al (26) observed HBsAg
expression and immune responses in a C57/BL6 or BALB/c HBV
infection mouse model mediated by pAAV-HBV HI of different doses of
plasmids. In this model, high plasmid doses (10 or 100 µg) resulted
in rapid HBV clearance and stronger immune responses, and HBV
persistence for >6 months tended to occur at higher frequencies
in C57/BL6 mice. In the present study, an acute HBV infection mouse
model was constructed by injecting a relatively high amount (15 µg)
of pcDNA3.1/HBV1.3 plasmid. Serum HBV DNA was negative at day 7
after plasmid injection, which suggests that the clearance of HBV
occurred in this mouse model. In addition, the present study showed
that γδ T cells may contribute to HBV clearance in this model.
Therefore, γδT cells may serve different roles at different stages
of HBV infection.
The roles of NK cells in acute hepatitis B infection
have been extensively studied in mice and humans. Early and rapid
activation of NK cells has been reported to contribute to HBV
clearance, where their dysfunction was associated with the
persistence of HBV infection (14).
γδT cells are innate immune cells that are not dissimilar to NK
cells, which share some common characteristics with regards to
virus clearance, including the expression of cytotoxic molecules,
including granzyme and perforin, or the production of inflammatory
cytokines, including IFN-γ and TNF-α (17). In the present study, γδ T and NK
cells were found to be enhanced at 1 day after pHBV injection,
implying that these two populations of immune cells exert some
common functions during the process of acute HBV infection and/or
subsequent HBV clearance.
Although the contribution of the innate immune
response during the early stages of HBV infection remains to be
clearly defined (6), it has been
previously shown to be involved in HBV clearance in a mouse model
(11), a woodchuck model (7) and human hepatocyte cell lines (50). These aforementioned studies found
that expression of type I IFN, IL-6 and chemokine CXCL10 are
enhanced shortly after HBV infection and are involved in HBV
clearance. Similar to these studies, the present study also showed
that the levels of the intrahepatic cytokine IFN-β increased during
the early stages of infection, which coincided with the increased
expression of HBV DNA and antigens. Compared with the peripheral
blood or spleen, NK, NKT or γδT cells are more abundant in liver,
where they may rapidly respond to stimuli (51). Therefore, immediately after HBV
expression and type I IFN production, the intrahepatic γδ T cells
are activated very quickly; no significant changes in the
percentage of γδ T cells were found in peripheral blood or spleen
in the present study.
The lack of HCV or HAV inclusion is a possible
limitation to the present study. In addition, changes in γδT cell
populations at timepoints earlier than 1 day, and analysis of the
correlation between γδT cells and liver HBsAg and HBcAg mRNA
expression at each timepoint beyond 1 day, are required in further
experiments.
In conclusion, taking all of these results into
consideration, data from the present study indicated that
immediately after HBV expression in the mouse liver, the percentage
of γδ T cells increased along with their enhanced function. These
observations were accompanied by the activation of immune
responses, including increased IFN-β expression. Therefore, liver
γδ T cells may be involved in the early innate immune response to
HBV infection, which may provide a new clue to elucidating the
mechanism of viral clearance during the acute stages of HBV
infection.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Wenwei Yin
(Institute for Viral Hepatitis of Chongqing Medical University,
Chongqing, China) for assistance with the animal experiments.
Funding
This work was supported by National Natural Science
Foundation of China (grant nos. 81772198 and 30901264), National
Science and Technology Major Project of China (grant nos.
2017ZX10202203 and 2018ZX10302206), Chongqing Research Program of
Basic Research and Frontier Technology (grant no.
cstc2015jcyjA10016) and Program for Excellent Young talents of
Chongqing Kuanren Hospital (2015).
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC designed and supervised this research. LC, LW, NL
and HP performed the animal experiments, detected all indices,
analyzed the data and wrote manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Chongqing Medical University (Chongqing, China)
Patient consent for publication
Not applicable.
Competing interests
The author declare that they have no competing
interests.
References
|
1
|
Seto WK, Lo YR, Pawlotsky JM and Yuen MF:
Chronic hepatitis B virus infection. Lancet. 392:2313–2324. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang LSY, Covert E, Wilson E and Kottilil
S: Chronic hepatitis B infection: A review. JAMA. 319:1802–1813.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsai KN, Kuo CF and Ou JJ: Mechanisms of
hepatitis B virus persistence. Trends Microbiol. 26:33–42. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z,
Huang Y, Qi Y, Peng B, Wang H, et al: Sodium taurocholate
cotransporting polypeptide is a functional receptor for human
hepatitis B and D virus. Elife. 1:e000492012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang JJ and Lewin SR: Immunopathogenesis
of hepatitis B virus infection. Immunol Cell Biol. 85:16–23. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gehring AJ and Protzer U: Targeting innate
and adaptive immune responses to cure chronic HBV infection.
Gastroenterology. 156:325–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guy CS, Mulrooney-Cousins PM, Churchill ND
and Michalak TI: Intrahepatic expression of genes affiliated with
innate and adaptive immune responses immediately after invasion and
during acute infection with woodchuck hepadnavirus. J Virol.
82:8579–8591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stevens KE, Thio CL and Osburn WO: CCR5
deficiency enhances hepatic innate immune cell recruitment and
inflammation in a murine model of acute hepatitis B infection.
Immunol Cell Biol. 97:317–325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murray JM, Wieland SF, Purcell RH and
Chisari FV: Dynamics of hepatitis B virus clearance in chimpanzees.
Proc Natl Acad Sci USA. 102:17780–17785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang PL, Althage A, Chung J and Chisari
FV: Hydrodynamic injection of viral DNA: A mouse model of acute
hepatitis B virus infection. Proc Natl Acad Sci USA.
99:13825–13830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang PL, Althage A, Chung J, Maier H,
Wieland S, Isogawa M and Chisari FV: Immune effectors required for
hepatitis B virus clearance. Proc Natl Acad Sci USA. 107:798–802.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Y, Huang X, Wu J, Liu J, Chen M, Ma Z,
Zhang E, Liu Y, Huang S, Li Q, et al: Pre-activation of toll-like
receptor 2 enhances CD8+ T-cell responses and
accelerates hepatitis B virus clearance in the mouse models. Front
Immunol. 9:14952018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang WW, Su IJ, Lai MD, Chang WT, Huang W
and Lei HY: The role of inducible nitric oxide synthase in a murine
acute hepatitis B virus (HBV) infection model induced by
hydrodynamics-based in vivo transfection of HBV-DNA. J Hepatol.
39:834–842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Golsaz-Shirazi F, Amiri MM and Shokri F:
Immune function of plasmacytoid dendritic cells, natural killer
cells, and their crosstalk in HBV infection. Rev Med Virol.
28:e20072018. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peeridogaheh H, Meshkat Z, Habibzadeh S,
Arzanlou M, Shahi JM, Rostami S, Gerayli S and Teimourpour R:
Current concepts on immunopathogenesis of hepatitis B virus
infection. Virus Res. 245:29–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fisicaro P, Valdatta C, Boni C, Massari M,
Mori C, Zerbini A, Orlandini A, Sacchelli L, Missale G and Ferrari
C: Early kinetics of innate and adaptive immune responses during
hepatitis B virus infection. Gut. 58:974–982. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lawand M, Déchanet-Merville J and
Dieu-Nosjean MC: Key features of gamma-delta T-cell subsets in
human diseases and their immunotherapeutic implications. Front
Immunol. 8:7612017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kabelitz D and Déchanet-Merville J:
Editorial: ‘Recent advances in gamma/delta T cell biology: New
ligands, new functions, and new translational perspectives’. Front
Immunol. 6:3712015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rajoriya N, Fergusson JR, Leithead JA and
Klenerman P: Gamma delta T-lymphocytes in hepatitis C and chronic
liver disease. Front Immunol. 5:4002014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khairallah C, Netzer S, Villacreces A,
Juzan M, Rousseau B, Dulanto S, Giese A, Costet P, Praloran V,
Moreau JF, et al: γδ T cells confer protection against murine
cytomegalovirus (MCMV). PLoS Pathog. 11:e10047022015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Howard J, Zaidi I, Loizon S,
Mercereau-Puijalon O, Déchanet-Merville J and Mamani-Matsuda M:
Human Vγ9Vδ2 T Lymphocytes in the Immune Response to P.
falciparum Infection. Front Immunol. 9:27602018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen M, Zhang D, Zhen W, Shi Q, Liu Y,
Ling N, Peng M, Tang K, Hu P, Hu H and Ren H: Characteristics of
circulating T cell receptor gamma-delta T cells from individuals
chronically infected with hepatitis B virus (HBV): An association
between V(delta)2 subtype and chronic HBV infection. J Infect Dis.
198:1643–1650. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conroy MJ, Mac Nicholas R, Taylor M, O'Dea
S, Mulcahy F, Norris S and Doherty DG: Increased frequencies of
circulating IFN-γ-producing Vδ1(+) and Vδ2(+) γδ T cells in
patients with asymptomatic persistent hepatitis B virus infection.
Viral Immunol. 28:201–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong X, Sun R, Chen Y, Wei H and Tian Z:
γδT cells drive myeloid-derived suppressor cell-mediated CD8+ T
cell exhaustion in hepatitis B virus-induced immunotolerance. J
Immunol. 193:1645–1653. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang M, Sun R, Huang Q and Tian Z:
Technical improvement and application of hydrodynamic gene delivery
in study of liver diseases. Front Pharmacol. 8:5912017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Li S, Zhou Y, Yang L, Zhou D, Yang
Y, Lu M, Yang D and Song J: The dose of HBV genome contained
plasmid has a great impact on HBV persistence in hydrodynamic
injection mouse model. Virol J. 14:2052017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
China National Standardization
Administration, . Guidelines for the Care and Use of Laboratory
Animals in China. http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=9BA619057D5C13103622A10FF4BA5D14February
6–2018
|
|
28
|
Duwaerts CC, Sun EP, Cheng CW, van Rooijen
N and Gregory SH: Cross-activating invariant NKT cells and kupffer
cells suppress cholestatic liver injury in a mouse model of biliary
obstruction. PLoS One. 8:e797022013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Fu R, Guo C, Huang Y, Wang H,
Wang S, Zhao J and Yang N: Anti-dsDNA antibodies bind to TLR4 and
activate NLRP3 inflammasome in lupus monocytes/macrophages. J
Transl Med. 14:1562016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li L, Cha H, Yu X, Xie H, Wu C, Dong N and
Huang J: The characteristics of NK cells in Schistosoma
japonicum-infected mouse spleens. Parasitol Res. 114:4371–4379.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inatsuka C, Yang Y, Gad E, Rastetter L,
Disis ML and Lu H: Gamma delta T cells are activated by
polysaccharide K (PSK) and contribute to the anti-tumor effect of
PSK. Cancer Immunol Immunother. 62:1335–1345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Melchjorsen J: Learning from the
messengers: Innate sensing of viruses and cytokine regulation of
immunity-clues for treatments and vaccines. Viruses. 5:470–527.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nosratabadi R, Alavian SM, Zare-Bidaki M,
Shahrokhi VM and Arababadi MK: Innate immunity related pathogen
recognition receptors and chronic hepatitis B infection. Mol
Immunol. 90:64–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tzeng HT, Tsai HF, Chyuan IT, Liao HJ,
Chen CJ, Chen PJ and Hsu PN: Tumor necrosis factor-alpha induced by
hepatitis B virus core mediating the immune response for hepatitis
B viral clearance in mice model. PLoS One. 9:e1030082014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boni C, Fisicaro P, Valdatta C, Amadei B,
Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A,
Missale G, et al: Characterization of hepatitis B virus
(HBV)-specific T-cell dysfunction in chronic HBV infection. J
Virol. 81:4215–4225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bertoletti A and Ferrari C: Adaptive
immunity in HBV infection. J Hepatol. 64 (1 Suppl):S71–S83. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshio S and Kanto T: Host-virus
interactions in hepatitis B and hepatitis C infection. J
Gastroenterol. 51:409–420. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deniger DC, Moyes JS and Cooper LJ:
Clinical applications of gamma delta T cells with multivalent
immunity. Front Immunol. 5:6362014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu YL, Ding YP, Tanaka Y, Shen LW, Wei CH,
Minato N and Zhang W: γδ T cells and their potential for
immunotherapy. Int J Biol Sci. 10:119–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Paul S and Lal G: Regulatory and effector
functions of gamma-delta (γδ) T cells and their therapeutic
potential in adoptive cellular therapy for cancer. Int J Cancer.
139:976–985. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang H, Luo H, Wan X, Fu X, Mao Q, Xiang
X, Zhou Y, He W, Zhang J, Guo Y, et al: TNF-α/IFN-γ profile of
HBV-specific CD4 T cells is associated with liver damage and viral
clearance in chronic HBV infection. J Hepatol. Sep 6–2019.(Epub
ahead of print). View Article : Google Scholar
|
|
43
|
Wohler JE, Smith SS, Zinn KR, Bullard DC
and Barnum SR: Gammadelta T cells in EAE: Early trafficking events
and cytokine requirements. Eur J Immunol. 39:1516–1526. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma Z, Ni G and Damania B: Innate sensing
of DNA virus genomes. Annu Rev Virol. 5:341–362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Brubaker SW, Bonham KS, Zanoni I and Kagan
JC: Innate immune pattern recognition: A cell biological
perspective. Annu Rev Immunol. 33:257–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wieland S, Thimme R, Purcell RH and
Chisari FV: Genomic analysis of the host response to hepatitis B
virus infection. Proc Natl Acad Sci USA. 101:6669–6674. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tseng CT, Miskovsky E, Houghton M and
Klimpel GR: Characterization of liver T-cell receptor gammadelta T
cells obtained from individuals chronically infected with hepatitis
C virus (HCV): Evidence for these T cells playing a role in the
liver pathology associated with HCV infections. Hepatology.
33:1312–1320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abravanel F, Barragué H, Dörr G, Sauné K,
Péron JM, Alric L, Kamar N, Izopet J and Champagne E: Conventional
and innate lymphocytes response at the acute phase of HEV infection
in transplanted patients. J Infect. 72:723–730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu D, Yan WM, Wang HW, Huang D, Luo XP and
Ning Q: γδ T cells contribute to the outcome of murine fulminant
viral hepatitis via effector cytokines TNF-α and IFN-γ. Curr Med
Sci. 38:648–655. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yoneda M, Hyun J, Jakubski S, Saito S,
Nakajima A, Schiff ER and Thomas E: Hepatitis B virus and DNA
stimulation trigger a rapid innate immune response through NF-κB. J
Immunol. 197:630–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Heymann F and Tacke F: Immunology in the
liver-from homeostasis to disease. Nat Rev Gastroenterol Hepatol.
13:88–110. 2016. View Article : Google Scholar : PubMed/NCBI
|