Introduction
Currently, lung cancer exhibits the highest
incidence and mortality rates among malignant tumors in the world,
having caused an estimated 1.6 million deaths worldwide in 2012
(1). Although drug treatments for
lung cancer have been developed rapidly, and the level of clinical
multidisciplinary integrated treatment has been improved, no
breakthrough has yet been achieved in the long-term efficacy of
treatment and prognosis (2).
Immunotherapy is a novel method for tumor therapy following
surgery, radiotherapy and chemotherapy (3,4).
Immunotherapy exhibits a unique curative effect in cancer research
and clinical practice, which is expected to provide new
possibilities for the prevention and treatment of lung cancer
(5).
Interleukin (IL)-18 is a multifunctional cytokine
that exhibits antitumor, anti-infection and immunoregulatory
functions (6,7). Zheng et al (8) observed the apoptosis of melanoma ZD55
cells via adenovirus-packaged IL-18 infection. In 2016, a study by
Jia et al (9) revealed that
IL-18-607 A/C polymorphism increases the risk of non-small cell
lung cancer. In 2017, Timperi et al (10) demonstrated that IL-18R may serve as a
biomarker for a minority of functional tumor-infiltrating CD8+ T
cells in patients with non-small cell lung adenocarcinoma. In 2018,
another study demonstrated that IL-18 not only promoted the
expansion of natural killer (NK) cells, but also changed their
phenotype (11). In the current
study, an Annexin V-FITC/PI flow cytometry assay was performed, the
results of which revealed the enhanced apoptosis of A549 cells
following infection with a lentivirus carrying the hIL-18 gene.
The aim of the present study was to investigate the
effect of transfecting a lentiviral IL-18 gene expression vector
into lung adenocarcinoma A549 cells on the malignant biological
behavior, such as proliferation, apoptosis, invasion and migration,
and to explore the possible underlying mechanisms.
Materials and methods
Materials
The pGC-LV vector, pHelper 1.0 vector and pHelper
2.0 vector were purchased from Shanghai GeneChem Co., Ltd.
Lentiviral plasmids carrying enhanced green fluorescent protein
(EGFP) were purchased from Hanbio Biotechnology Co., Ltd.
Polybrene, pancreatin, MTT and DMSO were purchased from
Sigma-Aldrich; Merck KGaA. RPMI-1640 culture medium was purchased
from Gibco; Thermo Fisher Scientific, Inc. Human interferon-γ
(IFN-γ) and Human IL-4 ELISA kits were purchased from Santa Cruz
Biotechnology, Inc.
Cell culture
The human lung cancer A549 cell line was purchased
from Shanghai Cell Resource Center of the Chinese Academy of
Sciences (Shanghai, China). The A549 cells were cultured in
RPMI-1640 culture medium containing 10% FBS (HyClone; GE Healthcare
Life Sciences) at 37°C with 5% CO2. The cells were
digested and passaged every 2–3 days. Cells in the exponential
phase of growth were used for subsequent experiments.
Construction and grouping of the IL-18
gene lentiviral expression vector in A549 cells
A549 cell suspensions were seeded into six-well
plates at a density of 4×105 cells/well. The following
day, DMEM (HyClone; GE Healthcare Life Sciences) with 10% v/v FBS
(complete medium) was removed and replaced with virus concentrated
liquid (Shanghai GeneChem Co., Ltd.) containing the IL-18 gene
vector with green fluorescence protein (GFP) (Shanghai GeneChem
Co., Ltd.) to detect transfection efficacy. Polybrene reagent
(Shanghai GeneChem Co., Ltd.) was diluted to a final concentration
of 5 µg/ml. Multiplicity of infection (MOI) values (1, 2, 5, 10, 30
and 50) were then tested in A549 cells. A total of 4×106
lentivral particles were used for transfection and the optimal MOI
value was 10 in the present study. At this value, the efficiency of
infection reached 80% 3 days post-infection. After swirling the
plate gently to mix cells, the plate was placed in an incubator
with 50 ml/l CO2 at 37°C for 24 h. After 24 h, the
medium was removed and replaced by complete medium. Three days
post-transfection, GFP expression was observed as the lentivirus
was integrated into the A549 cell genome, in five randomly-selected
fields using a fluorescence microscope (magnification, ×200). Cells
with a transfection efficiency >80% on day 3 were selected for
subsequent analysis. Lentiviruses were purchased from Shanghai
GeneChem Co., Ltd. Human lung adenocarcinoma A549 cells transfected
with the hIL-18 lentiviral expression vector were deemed the IL-18
intervention group (group A). Cells transfected with the empty
lentiviral expression vector were deemed the lentiviral empty
vector group (group B) and cells without any intervention were
called the blank control group (group C).
Determination of IL-18 mRNA expression
by reverse transcription-quantitative PCR
Total RNA was extracted from A549 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA (1 µg) was reverse transcribed to cDNA using a
High Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
qPCR was performed using the SYBR Green Reverse Transcription PCR
Master mix (Takara Biotechnology Co., Ltd.) on an Applied
Biosystems 7300 plus reverse transcription PCR system (Thermo
Fisher Scientific, Inc.). The sequences were as follows: IL-18
forward, 5′-ACTGTACAACCGCAGTAATAC-3′ and reverse,
5′-AGTGAACATTACAGATTTATCCC-3′ (product size, 434 bp); β-actin
forward, 5′-CTCCATCCTGGCCTCDCTGT-3′ and reverse,
5′-GCTGTCACCTTCACCGTTCC-3′. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95°C for 30 sec; 40
cycles of 95°C for 5 sec and 60°C for 30 sec. Expression levels
were quantified using the 2−∆∆Cq method (12) and β-actin was used as an internal
reference gene.
Cell Counting Kit-8 (CCK-8) assay
At the exponential growth phase of each group, A549
cells were seeded into 96-well plates at 1×104
cells/well. Then the plates with cells were incubated at 37°C with
5% CO2 for 0, 24, 48, 72 or 96 h. CCK-8 reagent (10 µl;
Beyotime Institute of Biotechnology) was then added to each well.
Cells were incubated at 37°C for a further 2 h, after which the
optical density (OD) values were measured at 450 nm using a
microplate reader (BioTek Instruments Inc.). The mean value of 6
wells was calculated as the final optical density (OD) value. The
cell proliferation curve was plotted as OD over time. The growth
inhibition rate (IR) was calculated as: IR (%)=1-(OD value of group
A-OD background)/(OD value of group C-OD background) ×100%.
Colony-formation assay
Cells at the exponential growth phase from each
group were inoculated into 24-well plates at 200 cells/well in
triplicate. The cells were then incubated at 37°C with 5%
CO2 for 2 weeks. The incubation was terminated when
visible clones formed on the plates. The clones were washed with
PBS. Cells were fixed with 4% paraformaldehyde at room temperature
for 15 min and stained with 0.4% crystal violet at room temperature
for 10 min. The colonies with >50 cells were counted in each
well under a WTDS-1 inverted microscope (magnification, ×40;
Olympus Corporation).
Flow cytometry
A549 cells (1×106 cells/well) were
cultured at 37°C for 96 h. Cell apoptosis was then assessed using a
FITC apoptosis detection kit (Oncogene Research Products) according
to the manufacturer's protocol. Cells were subsequently harvested
and washed with ice-cold PBS. Annexin V-FITC and propidium iodide
(PI) dye (10 µl each) were then added and mixed. The mixture was
incubated for 15 min at room temperature and the stained cells were
analyzed via flow cytometry (FACSCanto; BD Biosciences). Data were
analyzed using the FlowJo software package (version 10.0.7; Tree
Star, Inc.).
Transwell invasion assay
The three groups of cells (5×105) were
resuspended in serum-free 200 µl RPMI-1640 medium and plated into
the upper chamber (8 mm; Corning Inc.) of Matrigel-coated (BD
Biosciences) 24-well transwell chambers (Corning Inc.). The lower
chambers were plated with 600 µl RPMI-1640 medium supplemented with
10% FBS. After incubating at 37°C for 48 h, non-migrated cells were
removed with cotton swabs. Migrated cells were fixed with 4%
paraformaldehyde at room temperature for 15 min and stained with
0.1% crystal violet for 10 min. Stained cells were counted from
five independent fields using an inverted fluorescent microscope
(magnification, ×400; Olympus IX53; Olympus Corporation).
Wound healing assay
The three groups of cells (5×105
cells/well) were seeded into 6-well plates. After reaching 90%
confluence, three scratches were created in each well using a 200
µl sterile pipette tip. The cells were then washed twice with PBS
and incubated in medium supplemented without FBS for at 37°C 48 h.
To observe the migration of cells, images of the wells were
obtained at 0, 24 and 48 h in three randomly selected microscopic
fields (magnification, ×400; Olympus Corporation). The distance was
measured using ImageJ software (National Institutes of Health) and
the healing rate was calculated.
Detection of T helper (Th)1 and Th2
cell cytokine expression by ELISA
Following 96 h of cell culture at 37°C, supernatants
were harvested and IFN-γ (cat. no. DIF50) or IL-4 (cat. no. D4050)
levels were measured via ELISA (R&D Systems) in accordance with
the manufacturer's protocol.
Western blot analysis
Protein from the three cell groups was isolated
following incubation at 37°C for 96 h using RIPA assay lysis buffer
(Beyotime Institute of Biotechnology) and quantified using a
bicinchoninic acid kit (Beyotime Institute of Biotechnology).
Proteins (50 µg per lane) were separated via SDS-PAGE (10% gel) and
transferred onto a PVDF membrane. The membrane was then blocked
with 5% non-fat dry milk at 4°C for 1 h. Primary antibodies against
IL-18 (1:1,000; cat. no. ab207324), NF-κBp65 (1:1,000; cat. no.
ab16502) and GAPDH (1:1,000; cat. no. ab181602) were added, and the
mixture was incubated overnight at 4°C with gentle agitation.
Following three washes with Tris-buffered saline with Tween,
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies (1:5,000; cat. no. ab97051; Abcam) was added and
incubated at room temperature for 2 h. Protein bands were detected
using the Super Signal West Pico Chemiluminescent substrate (Thermo
Fisher Scientific, Inc.). and protein expression was quantified
using ImageJ 1.43 software (National Institutes of Health).
Statistical analysis
Data were analyzed by SPSS version 19.0 (IBM Corp.)
statistical software. Quantitative data are expressed as the mean ±
standard deviation. One-way ANOVA was used to compare different
groups, followed by the least significant difference (for data with
homogeneity of variance) or Dunnett's T3 (for data with
heterogeneity of variance) post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
IL-18 protein expression in A549 cells
following lentiviral vector transfection
A hIL-18 gene lentiviral vector carrying EGFP was
transfected into human lung adenocarcinoma A549 cells, which
resulted in green fluorescence (Fig.
1A). The results of the RT-qPCR assay demonstrated that the
expression of IL-18 mRNA in group A was significantly higher
compared with that in groups B and C (P<0.05), whereas there was
no significant difference between groups B and C (Fig. 1B). Western blotting analysis revealed
that the expression and the quantification of IL-18 in group A was
significantly higher than in groups B and C (P<0.001; Fig. 1C and D), which was consistent with
the results of the RT-qPCR analysis.
Effects of IL-18 overexpression on
A549 cell proliferation
The OD values of the three groups at day 0, day 1,
day 2, day 3 and day 4 following transfection with the hIL-18
lentiviral vector were measured by CCK-8 assay (Fig. 2B). At day 1, no significant
differences in cell proliferation were observed among groups A, B
and C. At day 2, day 3 and day 4, the proliferation in group A was
significantly lower compared with that in groups B and C
(P<0.05), whereas no significant differences were observed
between groups B and C (P>0.05). According to the data, the
proliferation rate of the cells in group A was significantly slower
compared with that of the control groups. The inhibition ratios for
group A at day 1, day 2, day 3 and day 4 were 3.210±0.565,
6.640±0.398, 9.030±0.407 and 15.307±0.081%, respectively,
suggesting that IL-18 may inhibit the proliferation of A549 cells
in a time-dependent manner (Fig.
2A). However, no significant differences were observed between
groups B and C, which indicated that the empty lentivirus
expression vector had no effects on the proliferation of A549 cells
(Fig. 2B).
Colony formation in A549 cells
following hIL-18 lentiviral vector transfection
A total of 200 cells were seeded into a 24-well
plate and incubated for 2 weeks. The colony counts in groups A, B
and C were 373±24.02, 417±29.51 and 445±40.85, respectively; the
colony formation in group A was significantly decreased compared
with that in groups B and C (P<0.05), which indicated that the
cells with IL-18 intervention had lower proliferating capacity
(Fig. 3). The difference between
groups B and C was not significant.
Induction of apoptosis in A549 cells
following hIL-18 lentiviral vector transfection
Cells from each group were cultured for 96 h, and
apoptotic rates were determined by flow cytometry using the Annexin
V-FITC/PI staining method. The apoptotic rates of groups A, B and C
were 9.10±0.37, 1.57±0.21 and 1.37±0.15%, respectively, with that
of group A being significantly higher than those in the control
groups (P<0.05), whereas no significant difference was observed
between groups B and C (Fig. 4).
Invasive capacity of A549 cells
following hIL-18 lentiviral vector transfection
Cells in each group were cultured for 48 h, and the
invasive capacities were determined by a Transwell assay. The mean
number of cells permeating the membrane in groups A, B and C were
476.0±55.7, 824.3±64.6 and 988.7±44.1 respectively. The invading
cells of group A were significantly fewer than those in groups B
and C (P<0.05), whereas no significant difference was observed
between groups B and C (Fig. 5).
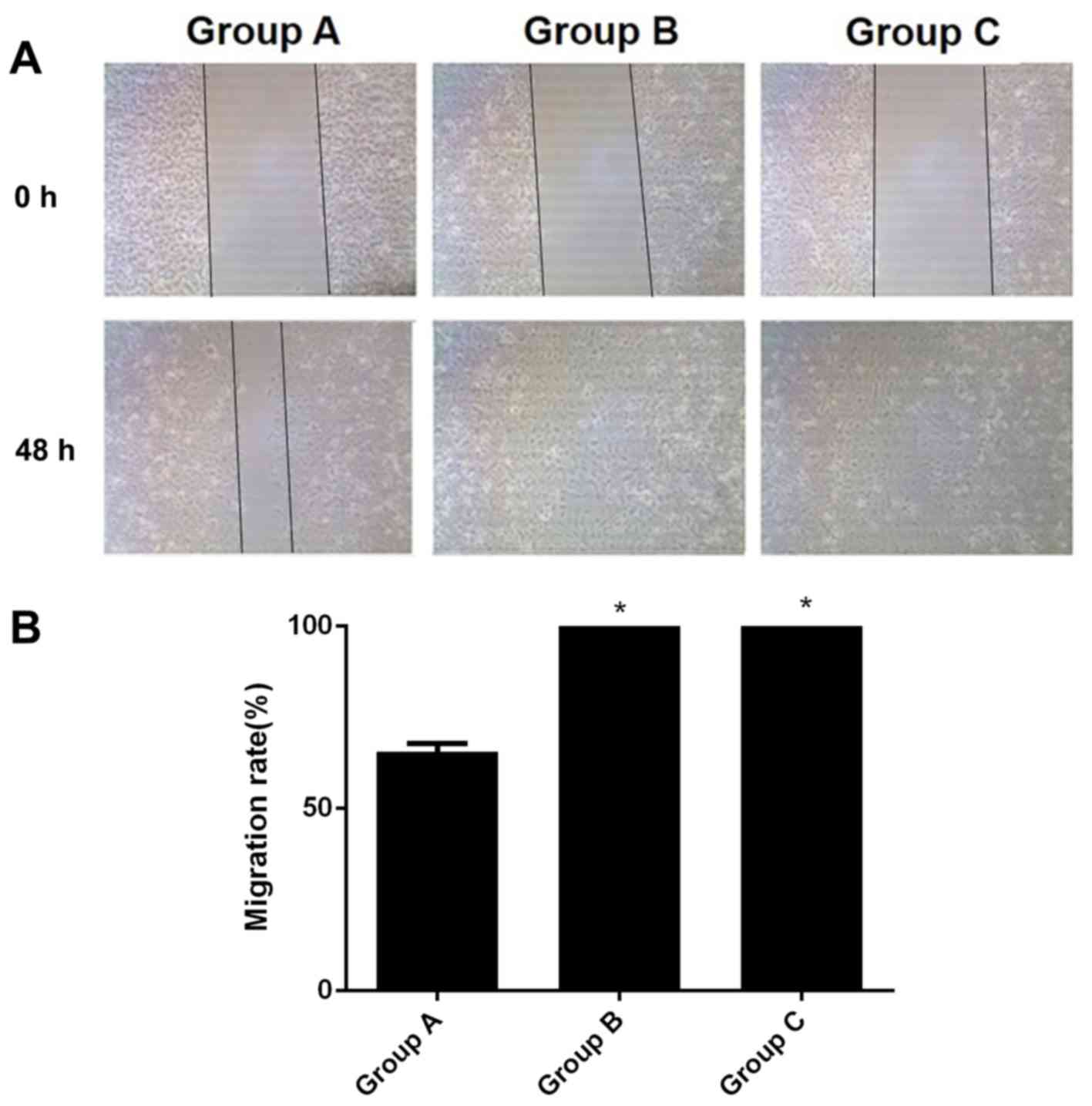
Migratory capacity of A549 cells
following hIL-18 lentiviral vector transfection
A total of 48 h after scratching, the wound-healing
rate of group A was 65.64%. The wound healing rates of B and C were
at 100.00%, which was significant when compared with the results of
group A (all P<0.05; Fig. 6).
Changes in Th cell cytokine production
by A549 cells following hIL-18 lentiviral vector transfection
The ELISA assay results (Table I) demonstrated that IFN-γ expression
was significantly higher and IL-4 expression was significantly
lower in group A than in the control groups (all P<0.05), and
the IFN-γ/IL-4 ratio in group A was significantly higher than in
groups B and C (P<0.05).
 | Table I.ELISA detection of IFN-γ and IL-4 in
the three groups of cells and the IFN-γ/IL-4 ratio (mean ± SD;
n=6). |
Table I.
ELISA detection of IFN-γ and IL-4 in
the three groups of cells and the IFN-γ/IL-4 ratio (mean ± SD;
n=6).
| Group | IFN-γ | IL-4 | IFN-γ/IL-4 |
|---|
| A |
0.52±0.02a |
0.70±0.02a |
0.736±0.028a |
| B | 0.35±0.03 | 0.78±0.02 | 0.448±0.040 |
| C | 0.33±0.02 | 0.81±0.02 | 0.403±0.031 |
Nuclear and cytoplasmic NF-κB
expression in A549 cells following hIL-18 lentiviral vector
transfection
Western blot analysis detected the expression of
NF-κB in the cytoplasm and nucleus of the cells in groups A, B and
C (Fig. 7). In group A, NF-κB
expression was decreased in the nucleus and increased in the
cytoplasm compared with groups B and C. No significant differences
were observed between groups B and C in the cytoplasm and
nucleus.
Discussion
The results of this study demonstrated that cytokine
IL-18 was stably expressed following transfection with a lentiviral
vector-packaged hIL-18 gene, which led to decreased proliferation
of human lung cancer A546 cells, increased apoptosis and impaired
invasion and migration. The hIL-18 lentivirus reverted the ratio of
Th1/Th2 subsets by enhancing IFN-γ secretion by Th1 cells and
reducing IL-4 secretion by Th2 cells to maintain the balance
between the Th1 and Th2 subsets. In addition, hIL-18 lentivirus
inhibited nuclear translocation of NF-κB to proactively exert its
anticancer activity.
Lentiviral vectors exhibit multiple merits, such as
the ability to carry a large fragment of the target gene, minimized
induction of the host immune response, the ability to infect both
dividing and non-dividing cells, and stable integration into the
genome of target cells (13).
Therefore, a lentiviral vector was selected for hIL-18 gene
delivery in the current study, to infect human lung cancer A546
cell line in order to thoroughly and systematically investigate the
anticancer function of IL-18 and its underlying mechanism.
Immunological studies have demonstrated that Th1 and
Th2 cells are dynamically balanced under healthy conditions, and
the drift of Th1 to Th2 subset may result in immune suppression,
which severely interferes with the anticancer function of immune
surveillance. The Th2 subset dominance in tumor patients was first
reported by Yamamura et al (14) in 1993 and confirmed by Asselin et
al (15) in 1998, who reported
the Th2-dominant model in lung cancer cell lines. Furthermore, a
clinical trial by Chechlińska et al (16) demonstrated a Th2 drift in patients
with lung cancer. IL-18 may therefore enhance anticancer function
via positive feedback and maintain the balance of Th1 and Th2
subsets. Yamashita et al (17) and Yamanaka and Xanthopoulos (18) demonstrated that IL-18 stimulates Th1
and NK cells to secrete IFN-γ and promote immune response mediated
by Th1 cells.
The association between IL-18 and tumor development
has been confirmed, although its antitumor function remains
controversial, particularly the effects of IL-18 on metastasis
(19,20). Xu et al (21) demonstrated that IL-18 may upregulate
the expression of Fas ligand in colorectal cancer cells to
facilitate immune surveillance evasion. Therefore, the biological
role of IL-18 requires further investigation.
The results of the present study indicated that the
hIL-18 lentiviral vector infected A549 cells to significantly alter
the invasion and migration of these cells, as revealed by the
reduced number of invading cells and decreased wound-healing rate
in the in vitro assays.
NF-κB, a dimer composed of subunits p50 and p65, is
a cytokine that regulates the expression of multiple cytokines
(22) NF-κB is shared by multiple
signal transduction pathways and is involved in various biological
processes, such as cellular immune response, stress response, cell
proliferation and apoptosis (23).
The inactive form of NF-κB is present in the cytoplasm in a complex
with the inhibitory protein IκB (24). Subsequently, the expression of the
downstream genes is regulated to exert their biological functions.
The role of NF-κB in the development of lung cancer has been
extensively investigated. Yang et al (25) revealed that activated NF-κB
dysregulates the cell cycle and suppresses apoptosis to promote
chemoresistance and tumorigenesis in lung cancer cells. Activated
NF-κB also promotes the invasion and metastasis of primary lung
adenocarcinoma by upregulating the expression of matrix
metalloproteinase (MMP)-2 and MMP9 expression (26). IL-18 has been reported to inhibit
airway inflammation of bronchial asthma through the inhibition of
NF-κB (27). The mechanism of
apoptosis in cervical cancer cells induced by mitomycin involves
not only caspase-8 and caspase-3-dependent Fas/Fas ligand pathway,
but also IL-18 expression and NF-κB activation (28). In the current study, the expression
of nuclear NF-κB was significantly decreased in A549 cells infected
with the hIL-18 lentivirus compared with the control groups,
whereas the expression of cytoplasmic NF-κB was significantly
increased, which indicated that abnormal proliferation, apoptosis,
invasion and migration of A549 cells may be associated with a
defect in the NF-κB pathway.
The results of the present study also demonstrated
that IL-18 exhibited an effect in inhibiting the proliferation and
invasion of lung cancer cells. The underlying mechanism and
potential clinical applications require further study. Clinical
trial phase I of IL-18 therapy was conducted by Robertson et
al (29) in 19 cancer patients
(including 10 myeloma and nine kidney cancer cases). Clinical trial
phase II of IL-18 therapy was conducted in non-treated metastatic
myeloma patients by Tarhini et al (30). The two trials demonstrated that IL-18
treatment exhibited high tolerance and biological efficacy in
cancer treatment. Therefore, IL-18 may be a promising option for
future clinical therapy, with increased efficacy in cancer
treatment, ideal long-term effects and prolonged survival of the
patients.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation of Fujian Province (grant no.
2011J01170), Nursery Fund of Fujian Medical University (grant no.
2010MP034) and Science Technology Innovation Joint Project
Foundation of Fujian Province (grant no. 2016Y9028).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
XC, RF, DX, SY and TL designed the experiments, and
RF and DX performed them. RF and DX analyzed and interpreted the
data. XC wrote the manuscript and SY and TL revised the manuscript.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schild SE and Vokes EE: Pathways to
improving combined modality therapy for stage III nonsmall-cell
lung cancer. Ann Oncol. 27:590–599. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fiala O, Šorejs O, Pešek M and Fínek J:
Immunotherapy in the treatment of lung cancer. Klin Onkol. 30
(Supplementum3):22–31. 2017.(In Czech). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hontscha C, Borck Y, Zhou H, Messmer D and
Schmidt-Wolf IG: Clinical trials on CIK cells: First report of the
international registry on CIK cells (IRCC). J Cancer Res Clin
Oncol. 137:305–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dougan M and Dranoff G: Immune therapy for
cancer. Annu Rev Immunol. 27:83–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li R, Wang C, Liu L, Du C, Cao S, Yu J,
Wang SE, Hao X, Ren X and Li H: Autologous cytokine-induced killer
cell immunotherapy in lung cancer: A phase II clinical study.
Cancer Immunol Immunother. 61:2125–2133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian H, Shi G, Yang G, Zhang J, Li Y, Du
T, Wang J, Xu F, Cheng L, Zhang X, et al: Cellular immunotherapy
using irradiated lung cancer cell vaccine co-expressing GM-CSF and
IL-18 can induce significant antitumor effects. BMC Cancer.
14:482014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tse BW, Russell PJ, Lochner M, Förster I
and Power CA: IL-18 inhibits growth of murine orthotopic prostate
carcinomas via both adaptive and innate immune mechanisms. PLoS
One. 6:e242412011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng JN, Pei DS, Mao LJ, Liu XY, Sun FH,
Zhang BF, Liu YQ, Liu JJ, Li W and Han D: Oncolytic adenovirus
expressing interleukin-18 induces significant antitumor effects
against melanoma in mice through inhibition of angiogenesis. Cancer
Gene Ther. 17:28–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia Y, Zang A, Jiao S, Chen S and Yan F:
The interleukin-18 gene promoter-607 A/C polymorphism contributes
to non-small-cell lung cancer risk in a Chinese population. Onco
Targets Ther. 9:1715–1719. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Timperi E, Focaccetti C, Gallerano D,
Panetta M, Spada S, Gallo E, Visca P, Venuta F, Diso D, Prelaj A,
et al: IL-18 receptor marks functional CD8+ T cells in non-small
cell lung cancer. Oncoimmunology. 6:e13283372017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Senju H, Kumagai A, Nakamura Y, Yamaguchi
H, Nakatomi K, Fukami S, Shiraishi K, Harada Y, Nakamura M, Okamura
H, et al: Effect of IL-18 on the expansion and phenotype of human
natural killer cells: Application to cancer immunotherapy. Int J
Biol Sci. 14:331–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshimitsu M, Sato T, Tao K, Walia JS,
Rasaiah VI, Sleep GT, Murray GJ, Poeppl AG, Underwood J, West L, et
al: Bioluminescent imaging of a marking transgene and correction of
Fabry mice by neonatal injection of recombinant lentiviral vectors.
Proc Natl Acad Sci USA. 101:16909–16914. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamura M, Modlin RL, Ohmen JD and Moy
RL: Local expression of antiinflammatory cytokines in cancer. J
Clin Invest. 91:1005–1010. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asselin S, Conjeaud H, Minty A, Fradelizi
D and Breban M: Stable polarization of peripheral blood T cells
towards type 1 or type 2 phenotype after polyclonal activation. Eur
J Immunol. 28:532–539. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chechlińska M, Duma A, Swierkowska K,
Kamińska J and Steffen J: Sera of lung cancer patients affect the
release of Th1, Th2 and monocyte-derived cytokines, and the
expression of IL-2Ralpha by normal, stimulated mononuclear cells.
Cell Mol Biol Lett. 9:69–81. 2004.PubMed/NCBI
|
|
17
|
Yamashita K, Iwasaki T, Tsujimura T,
Sugihara A, Yamada N, Ueda H, Okamura H, Futani H, Maruo S and
Terada N: Interleukin-18 inhibits lodging and subsequent growth of
human multiple myeloma cells in the bone marrow. Oncol Rep.
9:1237–1244. 2002.PubMed/NCBI
|
|
18
|
Yamanaka R and Xanthopoulos KG: Induction
of antigen-specific immune responses against malignant brain tumors
by intramuscular injection of sindbis DNA encoding gp100 and IL-18.
DNA Cell Biol. 24:317–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park S, Cheon S and Cho D: The dual
effects of interleukin-18 in tumor progression. Cell Mol Immunol.
4:329–335. 2007.PubMed/NCBI
|
|
20
|
Nakamura Y, Yamada N, Ohyama H, Nakasho K,
Nishizawa Y, Okamoto T, Futani H, Yoshiya S, Okamura H and Terada
N: Effect of interleukin-18 on metastasis of mouse osteosarcoma
cells. Cancer Immunol Immunother. 55:1151–1158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu T, Hao XS, Ren XB and Zhang HL: Killing
effect of interleukin-18-enhanced FasL-expressing colon cancer
cells on human hepatocytes in vitro. Zhonghua Zhong Liu Za Zhi.
32:659–662. 2010.(In Chinese). PubMed/NCBI
|
|
22
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slattery ML, Mullany LE, Sakoda L,
Samowitz WS, Wolff RK, Stevens JR and Herrick JS: The NF-κB
signalling pathway in colorectal cancer: Associations between
dysregulated gene and miRNA expression. J Cancer Res Clin Oncol.
144:269–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Zhou Y, Li Y, Zhou J, Wu Y, Cui Y,
Yang G and Hong Y: Mutations of p53 and KRAS activate NF-κB to
promote chemoresistance and tumorigenesis via dysregulation of cell
cycle and suppression of apoptosis in lung cancer cells. Cancer
Lett. 357:520–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang
L, Huang W, Huang L and Wang Q: Interleukin-32 contributes to
invasion and metastasis of primary lung adenocarcinoma via
NF-kappaB induced matrix metalloproteinases 2 and 9 expression.
Cytokine. 65:24–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Zhang Z and Xu Y: Effects of
interleukin-18 on asthmatic airway inflammation and nuclear factor
kappa-B in murine models. Chin Med J (Engl). 116:323–327.
2003.PubMed/NCBI
|
|
28
|
Kanda N, Shimizu T, Tada Y and Watanabe S:
IL-18 enhances IFN-gamma-induced production of CXCL9, CXCL10, and
CXCL11 in human keratinocytes. Eur J Immunol. 37:338–350. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Robertson MJ, Kirkwood JM, Logan TF, Koch
KM, Kathman S, Kirby LC, Bell WN, Thurmond LM, Weisenbach J and Dar
MM: A dose-escalation study of recombinant human interleukin-18
using two different schedules of administration in patients with
cancer. Clin Cancer Res. 14:3462–3469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tarhini AA, Millward M, Mainwaring P,
Kefford R, Logan T, Pavlick A, Kathman SJ, Laubscher KH, Dar MM and
Kirkwood JM: A phase 2, randomized study of SB-485232, rhIL-18, in
patients with previously untreated metastatic melanoma. Cancer.
115:859–868. 2009. View Article : Google Scholar : PubMed/NCBI
|