Introduction
Endometrial cancer, originating from the uterine
epithelium, is the most common type of gynecological tumor in
developed countries (1). It has been
indicated that the vast majority of cases of endometrial cancer are
not linked with familial inheritance (2). In the year 2016, ~60,050 new cases and
10,470 mortalities associated with endometrial cancer were reported
in the USA (3). Furthermore, the
incidence of endometrial cancer appears to be rapidly increasing in
the USA (4). In addition, numerous
previous studies have suggested that endometrial cancer has a
higher incidence in developing countries (5,6). For
most patients newly diagnosed with endometrial cancer, the
prognosis is good and the 5 year survival rate may be as high as
81.1% (7). Stage, grade,
histological subtype, myometrial depth invasion and lymphovascular
space involvement have been identified as major prognostic factors
for endometrial cancer (8,9).
In 1872, Riess et al (10) reported the association between
elevated platelet counts and malignant tumors. To date, various
studies have confirmed that pre-treatment thrombocytosis may be
used as a prognostic indicator for patients with numerous solid
tumor types, including lung, gastric, colorectal, esophageal,
hepatocellular, pancreatic, breast and ovarian cancer,
glioblastoma, renal cell carcinoma, oral squamous cell carcinoma,
breast cancer and ovarian cancer (11–22).
This effect may be due to the role served by platelets in tumor
growth, angiogenesis and metastasis (23–26).
To date, whether pre-treatment thrombocytosis is
able to predict the prognosis of patients with endometrial cancer
remains unclear. To the best of our knowledge, no previous
prospective randomized trials have been performed to evaluate the
pre-treatment platelet count for predicting the prognosis for
endometrial cancer. In addition, in a prior retrospective
controlled trial investigating this association, the number of
patients was limited (27). In the
present study, a pooled analysis of a large number of patients was
performed to investigate the effect of preoperative
thrombocytopenia on the prognosis of endometrial carcinoma. The
present study aimed to investigate the association between
thrombocytosis and the prognosis of patients with endometrial
cancer to provide a reference for clinical therapy.
Materials and methods
Search for relevant studies
On December 17, 2018, studies associated with
endometrial cancer and thrombocytosis were systematically searched
for using the PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), Embase
(https://www.embase.com) and Cochrane Library
databases (http://www.cochranelibrary.com/). In addition,
references to relevant reviews were manually searched in order to
avoid excluding any studies that met the selection criteria. The
terms used for the search were as follows: i) ‘Thrombocytosis’,
‘thrombocythemia’ or ‘platelet count’; and ii) ‘endometrial
cancer’, ‘endometrial neoplasm’ or ‘endometrial carcinoma’.
Furthermore, the language was not limited during the search
process. Two researchers independently searched for relevant
studies and screened the articles according to the inclusion and
exclusion criteria applied in the present study.
Inclusion criteria
The inclusion criteria for the present study were as
follows: i) The study covered the prognosis of endometrial cancer;
ii) the study described the effects of thrombocytosis or platelet
count on the prognosis of endometrial cancer; iii) the study
contained relevant prognostic data, including hazard ratios (HR)
and the corresponding 95% CI for overall survival (OS),
progression-free survival (PFS), disease-free survival (DFS) and
disease-specific survival (DSS); iv) the relevant HR and
corresponding 95% CI were available through conversion.
Exclusion criteria
The following exclusion criteria were applied: i)
Subjects did not have endometrial cancer; ii) the study did not
include the platelet count or any information regarding
thrombocytosis; iii) the study was a case report, review or
meta-analysis; iv) required data were unavailable. In addition,
studies that did not include any data on the prognosis of patients
with endometrial cancer were also excluded.
Data extraction and quality
assessment
Two researchers extracted the data, including
author, publication year, country, sample size, clinical stage,
age, cut-off and follow-up time using a pre-made spreadsheet. In
the present study, the effect of thrombocytosis on OS was used as
the primary outcome, and the impact of thrombocytosis on PFS, DFS
and DSS was considered as a secondary outcome. In the process of
calculating the HR and its 95% CI, multivariate analyses were
initially performed, followed by univariate analyses, and finally
Kaplan-Meier survival curves. Kaplan-Meier survival curve analysis
was performed using Engauge Digitizer 4.1 (http://digitizer.sourceforge.net/). For studies in
which OR or 95% CI could not be obtained directly, OR and 95% CI
were obtained through Kaplan-Meier survival analysis. A
non-randomized study (NRS) rating scale was used to evaluate the
quality of the studies included in the present meta-analysis, as
previously described (28). If
inconsistent results regarding literature searches and data
extraction were identified between two researchers, a third
researcher was consulted to resolve the discrepancy.
Statistical analysis
Stata statistical software (version 13.0; StataCorp,
LLC) was used to perform all statistical analyses and P<0.05 was
considered to indicate statistical significance. Cochran's Q and
I2 tests were performed to quantify the heterogeneity of
the HR. If the heterogeneity was >50%, random-effects models
were selected for pooled analysis; otherwise, a fixed-effects model
was used. In addition, Egger's test was performed to assess the
publication bias and sensitivity analysis was used to examine the
effect of a single study on the final results.
Results
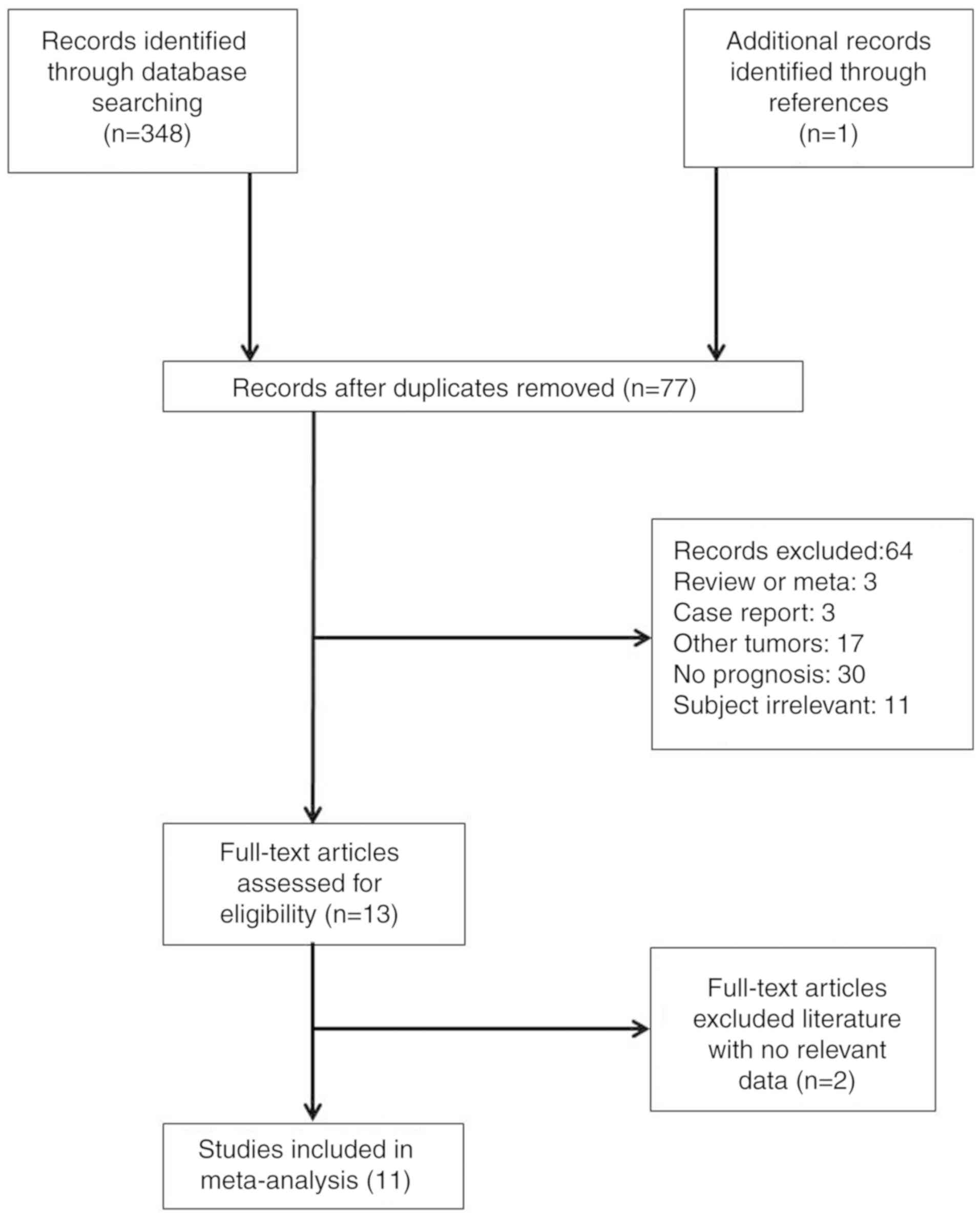
Search and screening of eligible
studies
A total of 349 studies were initially identified by
searching the PubMed, Embase and Cochrane Library databases and
through manual searching. After exclusion of duplicate studies, 77
studies were retained. Subsequently, 64 articles comprising
reviews, meta-analyses, case reports and studies investigating
other tumor types were removed by analyzing the title and
abstracts. The full text of the remaining 13 articles was then
reviewed and two studies lacking sufficient data were excluded.
Finally, 11 studies (29–39) met the inclusion criteria and were
included in the meta-analysis. The process of study selection is
illustrated in Fig. 1.
Basic characteristics of included
studies
In order to understand the basic characteristics of
the studies included, relevant data, including publication year,
country, number of cases, age of the patients and platelet count,
were extracted. Detailed basic information on the studies included
is provided in Table I. In total, 3
of the 11 studies included in the present meta-analysis analyzed
patients exhibiting tumors at stage III–IV and the remaining 8
examined patients with tumors at stage I–IV. These previous studies
were published after 2000 and 5 were published after 2015. The
study by Gorelick et al (37)
analyzed platelet counts as continuous variables and the remaining
studies analyzed platelet counts as categorical variables with
corresponding cut-off values. In total, 9 of the 11 studies
reported the effect of pre-treatment thrombocytosis on the OS of
patients with endometrial cancer.
 | Table I.Basic characteristics of the 11
studies included in the present meta-analysis. |
Table I.
Basic characteristics of the 11
studies included in the present meta-analysis.
| Author (year) | Country | Case number | FIGO stage | Age (years) | Cut-off | Follow-up
timea (months) | Category |
Adjustedb | Survival
analysis | (Refs.) |
|---|
| Takahashi
(2017) | Japan | 508 | I–IV | 58.0±10.5 | >400,000 | Unclear | Endometrial
cancer | Yes | OS | (29) |
| Abu-Zaid
(2017) | Saudi Arabia | 162 | I–IV | 59.0±11.0 | >400,000 | Unclear | Endometrial
cancer | Yes | OS, DFS | (30) |
| Younes (2016) | Israel | 56 | I–IV | 69.4±15.0 | >400,000 | 76.8 | USPC | No | OS | (31) |
| Nakamura
(2016) | Japan | 108 | I–IV | 60.0
(27.0–87.0)a | ≥350,000 | Unclear | Endometrial
cancer | No | OS, PFS | (32) |
| Kizer (2015) | USA | 318 | I–IV | Unclear | >400,000 | 25.6/23.1 | Endometrial
cancer | Yes | DSS, DFS | (33) |
| Heng (2014) | Thailand | 238 | I–IV | 57.9±10.0 | >400,000 | 59.6
(1.0–98.0) | Endometrial
cancer | Yes | OS, DFS | (34) |
| Njølstad
(2013) | Norway | 557 | I–IV | 66.2±11.7 | >390,000 | 55.0
(0.0–97.0) | Endometrial
cancer | No | DSS | (35) |
| Matsuo (2013) | USA | 516 | I–IV | 52.0±10.4 | >400,000 | 43.7 | Endometrial
cancer | No | OS, PFS | (36) |
| Gorelick
(2009) | USA | 29 | III–IV | Unclear | Continuous | Unclear | Endometrial
cancer | Yes: OS No:
PFS | OS,
PFSc | (37) |
| Lerner (2007) | USA | 39 | III–IV | Unclear | >400,000 | Unclear | USPC | Yes: OS | OS | (38) |
| Scholz (2000) | Turkey | 59 | III–IV | 64.8 | >400,000 | Unclear | Endometrial
cancer | No | OSc | (39) |
Quality assessment
To measure the quality of the studies included, an
NRS rating scale was applied. The results of the quality evaluation
are provided in Table II. Among the
11 studies included in the present study, the baseline data of
three studies were comparable and those of the other 8 studies were
only partially comparable. In total, 4 studies did not control for
confrontation factor. Finally, 4 articles had a quality score of 7
and the remaining studies had a score of >7.
 | Table II.Quality evaluation results of 11
studies included in the present meta-analysis. |
Table II.
Quality evaluation results of 11
studies included in the present meta-analysis.
| Study | Grouping
method | Blinding | ITT | Baseline data | Diagnostic
criteria | Confrontation
factor control | Quality
levela | (Refs.) |
|---|
| Takahashi
(2017) | 2 | 0 | 2 | 2 | 2 | 2 | 10 | (29) |
| Abu-Zaid
(2017) | 2 | 0 | 2 | 1 | 2 | 2 | 9 | (30) |
| Younes (2016) | 2 | 0 | 2 | 1 | 2 | 0 | 7 | (31) |
| Nakamura
(2016) | 2 | 0 | 2 | 1 | 2 | 0 | 7 | (32) |
| Kizer (2015) | 2 | 0 | 2 | 2 | 2 | 2 | 10 | (33) |
| Heng (2014) | 2 | 0 | 2 | 1 | 2 | 2 | 9 | (34) |
| Njølstad
(2013) | 2 | 0 | 2 | 1 | 2 | 0 | 7 | (35) |
| Matsuo (2013) | 2 | 0 | 2 | 1 | 2 | 0 | 7 | (36) |
| Gorelick
(2009) | 2 | 0 | 2 | 2 | 2 | 1 | 9 | (37) |
| Lerner (2007) | 2 | 0 | 2 | 1 | 2 | 1 | 8 | (38) |
| Scholz (2000) | 2 | 0 | 2 | 1 | 2 | 2 | 9 | (39) |
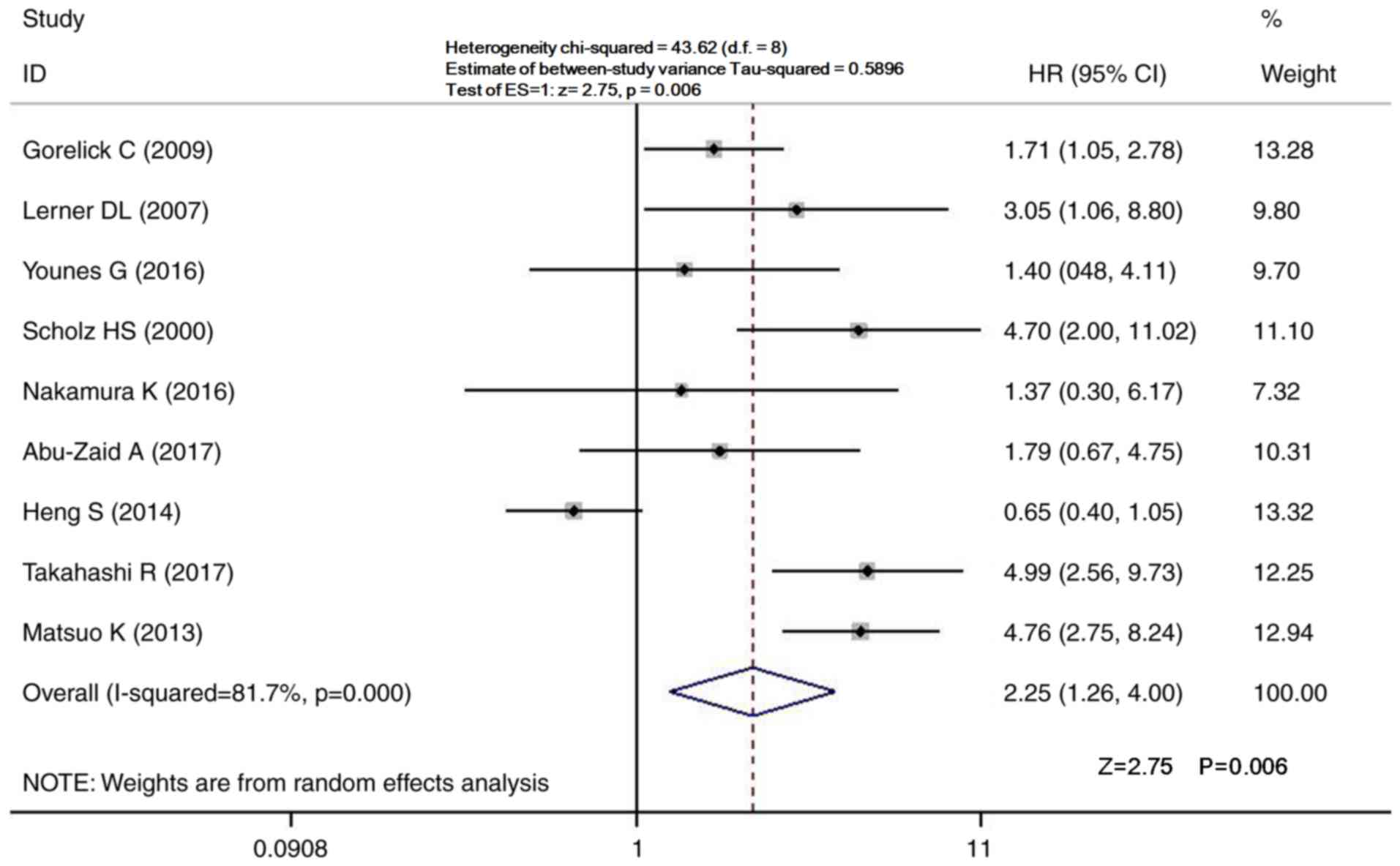
Pooled analysis for OS
A total of 9 studies comprising 1,715 patients
reported the effects of pre-treatment thrombocytosis on the OS of
patients with endometrial cancer (29–32,34,36–39).
Considering the large heterogeneity, a random-effects model was
used to summarize the 9 articles (I2=81.7%; P<0.001;
Fig. 2). The pooled analysis
indicated that pre-treatment thrombocytosis was associated with a
significantly reduced OS of patients with endometrial cancer
(HR=2.25; 95% CI=1.26–4.00; P<0.001; Fig. 2), indicating that thrombocytosis may
affect the prognosis of patients with endometrial cancer.
Pooled analysis for PFS, DFS and
DSS
To determine the effects of pre-treatment
thrombocytosis on other prognostic indicators, PFS, DFS and DSS
were also analyzed. Of the 11 studies included in the present
meta-analysis, 8 studies included relevant data: 3 Studies reported
on PFS, DFS and DSS (32,36,37), 3
on DFS (30,33,34) and
2 on DSS (33,35). The pooled analysis indicated that
pre-treatment thrombocytosis was significantly associated with PFS
and DFS, but not with DSS (PFS, HR=2.60, 95% CI=1.23–5.50, P=0.013;
DFS, HR=2.23, 95% CI=1.45–3.42, P<0.001; DSS, HR=2.17, 95%
CI=0.51–9.27, P=0.296; Supplemental Figs. S1–S3). The present results further confirmed
that pre-treatment thrombocytosis may be used to predict the
prognosis of patients with endometrial cancer.
Subgroup analysis
To further examine the association between
pre-treatment thrombocytosis and prognosis of endometrial cancer in
different patients and studies, a subgroup analysis was performed
based on publication date, the region covered by the study, the
number of cases included in the analysis, the type of cancer
included in analysis, whether it was a multivariate or
single-factor analysis and the platelet count cut-off. Detailed
results of the subgroup analyses are provided in Table III. The publication date and the
number of cases included in the analysis both had a significant
effect on patient prognosis. The results indicated that
pre-treatment thrombocytosis was not associated with OS in patients
from Asia or Europe, however, there appeared to be some association
for patients from America. Patients with uterine serous papillary
carcinoma (USPC) and endometrial cancer were included in the study
cohort. There was no association for USPC patients, but endometrial
cancer patient prognosis was related to pre-operative
thrombocytopenia. No association between pre-operative
thrombocytopenia and prognosis was noted for patients with a
platelet count of ≥350×109/l, however, the total
survival time of patients with a cut-off >400 was related to
pre-operative thrombocytopenia (Table
III).
 | Table III.Subgroup analyses of the association
between pre-treatment thrombocytosis and overall survival. |
Table III.
Subgroup analyses of the association
between pre-treatment thrombocytosis and overall survival.
| Variable | Number of
studies | HR (95%) | Z | P-value | I2
(%) | P-value |
|---|
| Year of
publication |
|
2000–2014 | 5 | 2.916
(1.534–5.543) | 3.26 | 0.001 | 56.9 | 0.055 |
|
2015–2017 | 5 | 2.296
(1.002–5.262) | 1.96 | 0.049 | 88.7 | <0.001 |
| Region |
|
Asia | 5 | 2.207
(0.772–6.311) | 1.48 | 0.140 | 89.0 | <0.001 |
|
Europe | 2 | 2.687
(0.823–8.774) | 1.64 | 0.102 | 66.6 | 0.084 |
|
America | 3 | 2.874
(1.388–5.952) | 2.84 | 0.004 | 73.4 | 0.023 |
| Number of
cases |
|
<150 | 5 | 2.202
(1.388–3.492) | 3.35 | 0.001 | 26.5 | 0.245 |
|
>150 | 5 | 2.779
(1.041–7.420) | 2.04 | 0.041 | 91.2 | <0.001 |
| Type of cancer |
|
USPC | 2 | 2.078
(0.969–4.457) | 1.88 | 0.060 |
2.1 | 0.312 |
|
Endometrial cancer | 8 | 2.598
(1.355–4.982) | 2.88 | 0.004 | 86.0 | <0.001 |
| Analysis |
|
Multivariate | 9 | 1.733
(1.430–2.100) | 5.61 | <0.001 | 50.9 | 0.038 |
| Single
factor | 4 | 1.886
(1.117–3.186) | 2.37 |
0.018 | 63.8 | 0.040 |
| Platelet count
cut-off (×103/mm3) |
|
≥350 | 1 | 1.367
(0.303–6.171) | 0.41 | 0.684 | – | – |
|
>400 | 8 | 2.771
(1.382–5.557) | 2.87 | 0.004 | 85.7 | <0.001 |
|
Continuous | 1 | 1.714
(1.055–2.785) | 2.18 | 0.030 | – | – |
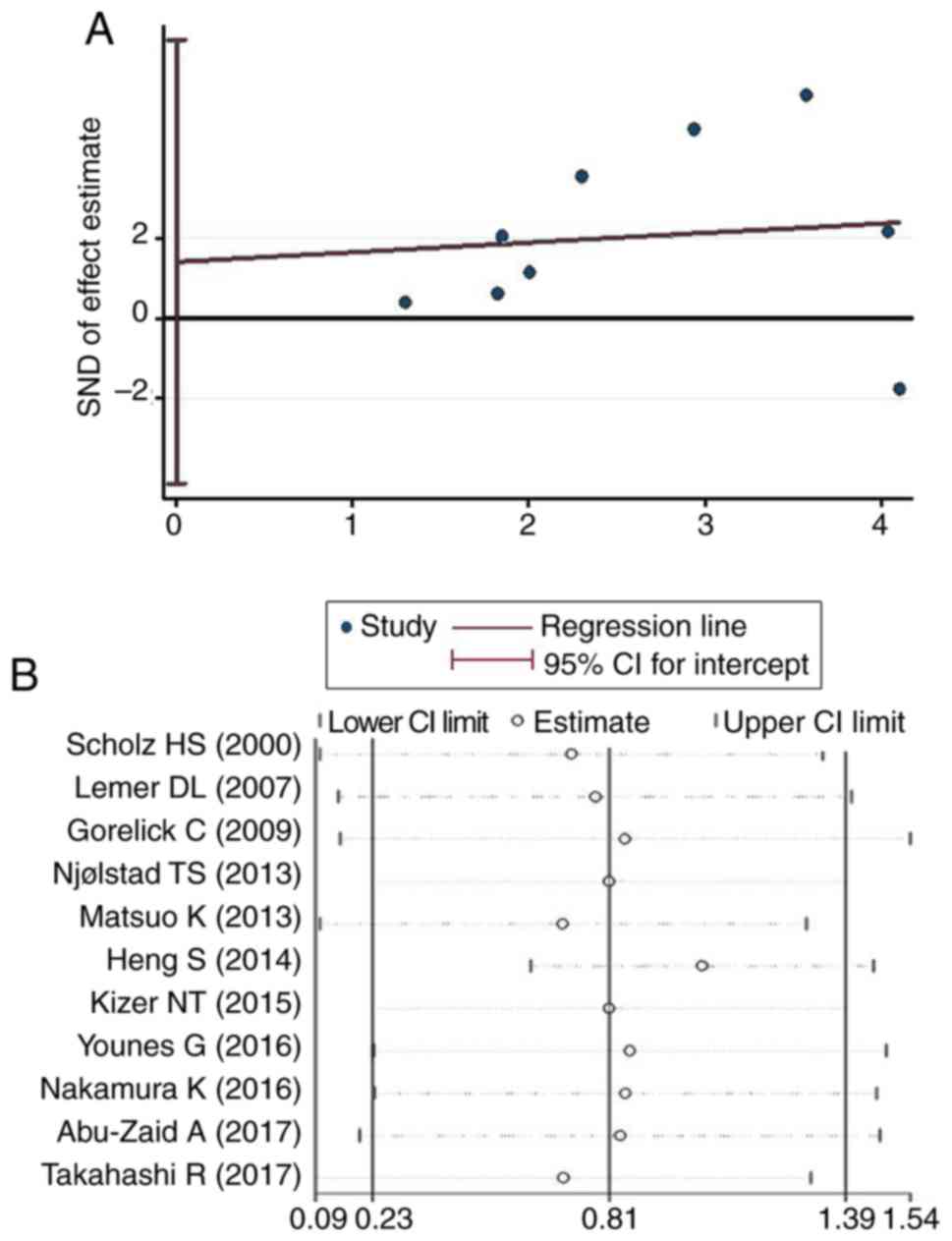
Publication bias and sensitivity
analysis
In order to evaluate the potential publication bias,
the articles included in the present meta-analysis were subjected
to Egger's test. The results indicated no significant publication
bias in the present study (P=0.565). A sensitivity analysis was
also performed to assess the effect of a single study on the final
results. The analysis suggested that the impact of individual
studies on the results of the pooled analysis was negligible,
indicating that the present conclusions are not influenced by
individual articles and exhibit high robustness (Fig. 3).
Discussion
Identification of prognostic markers for patients
with endometrial cancer is important and may facilitate clinical
decisions regarding potential treatments and disease outcome. The
present study performed a pooled analysis of the results of
previously published studies to assess whether pre-treatment
thrombocytosis may be a prognostic indicator for patients with
endometrial cancer. The meta-analysis suggested that pre-treatment
thrombocytosis was significantly associated with OS, PFS and DFS in
patients with endometrial cancer, but was not significantly
associated with DSS. Furthermore, quality assessments, publication
bias analysis and sensitivity analysis of the studies included
confirmed that the present results were reliable. Of note, only two
previous studies reported an association between pre-treatment
thrombocytosis and DSS (33,35). Further studies with more patients are
required in order to investigate the association between
pre-treatment thrombocytosis and DSS.
Previous studies have reported that 7–41% of
patients with endometrial cancer suffer from thrombocytosis with
different cut-offs (29,34,35,37–44). In
addition, one previous study indicated a significant correlation
between thrombocytosis and clinical stages, suggesting that
patients with advanced clinical stage are more likely to suffer
from thrombocytosis (29).
Thrombocytosis may reduce the rate of chemotherapy response in
patients with endometrial cancer, and this effect may explain why
patients with thrombocytosis have a poor prognosis (33). A retrospective study by Borges et
al (45) analyzed ~1,000
patients with lung cancer and indicated that a platelet count of
>440×103/mm3 significantly reduced the
chemotherapy response rate (45).
Furthermore, a previous study suggested that an increase in the
platelet count may reduce the resistance to docetaxel-induced
apoptosis in a mouse model of ovarian cancer (46). In addition, previous studies
suggested that thrombocytosis may increase the risk of
thromboembolism in cancer patients (35,46). The
present study suggested that a platelet count of
≥400×103/mm3 was significantly associated
with a reduced OS of patients with endometrial cancer. Further
studies are required to investigate the molecular mechanisms
underlying the effect of thrombocytosis on the prognosis of
patients with endometrial cancer.
A previous study demonstrated that various cytokines
and growth factors, including thrombopoietin, interleukin-1 (IL-1),
IL-3, IL-6, IL-11 and leukemia inhibitory factors are able to
enhance platelet production (23).
Elevated levels of IL-6 were previously identified to be associated
with poor prognosis of patients with ovarian cancer (47). Various previous studies have
indicated that thrombocytosis may be a paraneoplastic syndrome in
patients with ovarian cancer, and thrombocytosis may stimulate
angiogenesis and lead to tumor progression via increased secretion
of IL-6 from tumor cells (46,48,49). Due
to limited information provided by the studies included, the
present study did not investigate the association between multiple
growth factors and platelet formation in patients with endometrial
cancer. Further studies are required to investigate whether IL-6 is
involved in platelet formation in patients with endometrial cancer
and IL-6 may be a novel potential therapeutic target for treating
endometrial cancer, providing a novel direction for future studies.
To the best of our knowledge, the present study was the first
meta-analysis investigating the association between pre-treatment
thrombocytosis and prognosis in patients with endometrial cancer.
Pooled analysis of prognostic indicators for OS, PFS, DFS and DSS
in patients with endometrial cancer was performed following the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
guidelines (50) and reliable
results were obtained. However, the present study has certain
limitations. First, the analysis included a small number of studies
on uterine serous papillary carcinoma and further studies are
required to examine this type of tumor. In addition, the results of
the present meta-analysis suggested that pre-treatment
thrombocytosis was not associated with the prognosis of patients
with endometrial cancer and a platelet count of
≥350×103/mm3, and this result requires
further investigation using a larger sample size. Furthermore, it
is necessary to analyze the association between thrombocytosis and
the prognosis of stage I–II endometrial cancer. This was not
analyzed in the present study due to the lack of previous studies
reporting on this.
The results of the present systematic review and
meta-analysis indicated that pre-treatment thrombocytosis was
significantly associated with OS in patients with endometrial
cancer at stage III–IV. Furthermore, pre-treatment thrombocytosis
(>400×103 platelets/mm3) was an
independent predictor of OS regardless of the clinical stage. In
addition, patients with pre-treatment thrombocytosis had poor DFS
and PFS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
QY and ZW performed the literature search and
selection, performed the data analysis and wrote the manuscript. TX
and DL helped with the data analysis. YY and HT designed and
supervised the present study and revised the manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Ann Oncol. 26:16–41.
2016. View Article : Google Scholar
|
|
2
|
Gruber S and Thompson W: A
population-based study of endometrial cancer and familial risk in
younger women. Cancer and steroid hormone study group. Cancer
Epidemiol Biomarkers Prev. 5:411–417. 1996.PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lortet-Tieulent J, Ferlay J, Bray F and
Jemal A: International patterns and trends in endometrial cancer
incidence, 1978–2013. J Natl Cancer Inst. 110:354–361. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silverberg E, Boring CC and Squires TS:
Cancer statistics 1990. CA Cancer J Clin. 40:9–26. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gusberg SB: Diagnosis and principles of
treatment of cancer of the endometriumFemale genital cancer.
Gusberg SB, Shingleton HM and Deppe G: New York: Churchill; 337.
1988
|
|
7
|
Colombo N, Preti E, Landoni F, Carinelli
S, Colombo A, Marini C and Sessa C; ESMO Guidelines Working Group,
: Endometrial cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24:33–38. 2013.
View Article : Google Scholar
|
|
8
|
Noone A, et al: SEER cancer statistics
review, 1975–2015, national cancer instituteBethesda, MD:
https://seer.cancer.gov/csr/1975_2015/2018
|
|
9
|
Aristizabal P, Graesslin O, Barranger E,
Clavel-Chapelon F, Haddad B, Luton D, Darai E, Rouzier R and Koskas
M: A suggested modification to FIGO stage I endometrial cancer.
Gynecol Oncol. 133:192–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riess L: Zur pathologischen anatomie des
blutes. Arch Anat Physiol Wiss Med. 39:237–249. 1872.
|
|
11
|
Pedersen LM and Milman N: Prognostic
significance of thrombocytosis in patients with primary lung
cancer. Eur Respir J. 9:1826–1830. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costantini V, Zacharski LR, Moritz TE and
Edwards RL: The platelet count in carcinoma of the lung and colon.
Thromb Haemost. 64:501–505. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikeda M, Furukawa H, Imamura H, Shimizu J,
Ishida H, Masutani S, Tatsuta M and Satomi T: Poor prognosis
associated with thrombocytosis in patients with gastric cancer. Ann
Surg Oncol. 9:287–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Monreal M, Fernandez-Llamazares J, Pinol
M, Julian JF, Broggi M, Escola D and Abad A: Platelet count and
survival in patients with colorectal cancer-a preliminary study.
Thromb Haemost. 79:916–918. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada H, Oohira G, Okazumi S, Matsubara
H, Nabeya Y, Hayashi H, Takeda A, Gunji Y and Ochiai T:
Thrombocytosis associated with poor prognosis in patients with
esophageal carcinoma. J Am Coll Surg. 198:737–741. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen CC, Yang CF, Yang MH, Lee KD, Kwang
WK, You JY, Yu YB, Ho CH, Tzeng CH, Chau WK, et al: Pretreatment
prognostic factors and treatment outcome in elderly patients with
de novo acute myeloid leukemia. Ann Oncol. 16:1366–1373. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suzuki K, Aiura K, Kitagou M, Hoshimoto S,
Takahashi S, Ueda M and Kitajima M: Platelets counts closely
correlate with the disease-free survival interval of pancreatic
cancer patients. Hepatogastroenterology. 51:847–853.
2004.PubMed/NCBI
|
|
18
|
Brockmann MA, Giese A, Mueller K, Kaba FJ,
Lohr F, Weiss C, Gottschalk S, Nolte I, Leppert J, Tuettenberg J
and Groden C: Preoperative thrombocytosis predicts poor survival in
patients with glioblastoma. Neuro Oncol. 9:335–342. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gogus C, Baltaci S, Filiz E, Elhan A and
Beduk Y: Significance of thrombocytosis for determining prognosis
in patients with localized renal cell carcinoma. Urology.
63:447–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu CC, Chang KW, Chou FC, Cheng CY and Liu
CJ: Association of pretreatment thrombocytosis with disease
progression and survival in oral squamous cell carcinoma. Oral
Oncol. 43:283–288. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taucher S, Salat A, Gnant M, Kwasny W,
Mlineritsch B, Menzel RC, Schmid M, Smola MG, Stierer M, Tausch C,
et al: Impact of pretreatment thrombocytosis on survival in primary
breast cancer. Thromb Haemost. 89:1098–1106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye Q, Cheng J, Ye M, Liu D and Zhang Y:
Association of pretreatment thrombocytosis with prognosis in
ovarian cancer: A systematic review and meta-analysis. J Gynecol
Oncol. 30:e52019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buergy D, Wenz F, Groden C and Brockmann
MA: Tumor-platelet interaction in solid tumors. Int J Cancer.
130:2747–2760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gupta GP and Massagué J: Platelets and
metastasis revisited: A novel fatty link. J Clin Invest.
114:1691–1693. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Palumbo JS, Talmage KE, Massari JV, La
Jeunesse CM, Flick MJ, Kombrinck KW, Jirousková M and Degen JL:
Platelets and fibrin (ogen) increase metastatic potential by
impeding natural killer cell-mediated elimination of tumor cells.
Blood. 105:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sierko E and Wojtukiewicz MZ: Platelets
and angiogenesis in malignancy. Semin Thromb Hemost. 30:95–108.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ayhan A, Bozdag G, Taskiran C, Gultekin M,
Yuce K and Kucukali T: The value of preoperative platelet count in
the prediction of cervical involvement and poor prognostic
variables in patients with endometrial carcinoma. Gynecol Oncol.
103:902–905. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
TS Z: Applied methodology for
evidence-based medicineCentral South University; 2014
|
|
29
|
Takahashi R, Mabuchi S, Kuroda H, Kozasa
K, Yokoi E, Matsumoto Y and Kimura T: The Significance of
pretreatment thrombocytosis and its association with neutrophilia
in patients with surgically treated endometrial cancer. Int J
Gynecol Cancer. 27:1399–1407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Abu-Zaid A, Alsabban M, Abuzaid M, AlOmar
O, Salem H and Al-Badawi IA: Preoperative thrombocytosis as a
prognostic factor in endometrioid-type endometrial carcinoma. Ann
Saudi Med. 37:393–400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Younes G, Segev Y, Begal J, Auslender R,
Goldberg Y, Amit A and Lavie O: The prognostic significance of
hematological parameters in women with uterine serous papillary
carcinoma (USPC). Eur J Obstet Gynecol Reprod Biol. 199:16–20.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakamura K, Nakayama K, Ishikawa M,
Katagiri H, Minamoto T, Ishibashi T, Ishikawa N, Sato E, Sanuki K,
Yamashita H, et al: High pretreatment plasma D-dimer levels are
related to shorter overall survival in endometrial carcinoma. Eur J
Obstet Gynecol Reprod Biol. 201:89–93. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kizer NT, Hatem H, Nugent EK, Zhou G,
Moore K, Heller P, Mutch DG and Thaker PH: Chemotherapy response
rates among patients with endometrial cancer who have elevated
serum platelets. Int J Gynecol Cancer. 25:1015–1022. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heng S and Benjapibal M: Preoperative
thrombocytosis and poor prognostic factors in endometrial cancer.
Asian Pac J Cancer Prev. 15:10231–10236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Njølstad TS, Engerud H, Werner HM,
Salvesen HB and Trovik J: Preoperative anemia, leukocytosis and
thrombocytosis identify aggressive endometrial carcinomas. Gynecol
Oncol. 131:410–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsuo K, Yessaian AA, Lin YG, Pham HQ,
Muderspach LI, Liebman HA, Morrow CP and Roman LD: Predictive model
of venous thromboembolism in endometrial cancer. Gynecol Oncol.
128:544–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gorelick C, Andikyan V, Mack M, Lee YC and
Abulafia O: Prognostic significance of preoperative thrombocytosis
in patients with endometrial carcinoma in an inner-city population.
Int J Gynecol Cancer. 19:1384–1389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lerner DL, Walsh CS, Cass I, Karlan BY and
Li AJ: The prognostic significance of thrombocytosis in uterine
papillary serous carcinomas. Gynecol Oncol. 104:91–94. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Scholz HS, Petru E, Gücer F, Haas J,
Tamussino K and Winter R: Preoperative thrombocytosis is an
independent prognostic factor in stage III and IV endometrial
cancer. Anticancer Res. 20:3983–5398. 2000.PubMed/NCBI
|
|
40
|
Gucer F, Moser F, Tamussino K, Reich O,
Haas J, Arikan G, Petru E and Winter R: Thrombocytosis as a
prognostic factor in endometrial carcinoma. Gynecol Oncol.
70:210–214. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tamussino KF, Gucer F, Reich O, Moser F,
Petru E and Scholz HS: Pretreatment hemoglobin, platelet count, and
prognosis in endometrial carcinoma. Int J Gynecol Cancer.
11:236–240. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luomaranta A, Leminen A and Loukovaara M:
Prediction of lymph node and distant metastasis in patients with
endometrial carcinoma: A new model based on demographics,
biochemical factors, and tumor histology. Gynecol Oncol. 129:28–32.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kaloglu S, Guraslan H, Tekirdag AI,
Dagdeviren H and Kaya C: Relation of preoperative thrombocytosis
between tumor stage and grade in patients with endometrial cancer.
Eurasian J Med. 46:164–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tuomi T, Pasanen A, Luomaranta A, Leminen
A, Bützow R and Loukovaara M: Risk-stratification of endometrial
carcinomas revisited: A combined preoperative and intraoperative
scoring system for a reliable prediction of an advanced disease.
Gynecol Oncol. 137:23–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Borges M, Sculier JP, Paesmans M, Richez
M, Bureau G, Dabouis G, Lecomte J, Michel J, Van Cutsem O,
Schmerber J, et al: Prognostic factors for response to chemotherapy
containing platinum derivatives in patients with unresectable
non-small cell lung cancer (NSCLC). Lung Cancer. 16:21–33. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stone RL, Nick AM, McNeisch IA, Balkwill
F, Han HD, Bottsford-Miller J, Rupairmoole R, Armaiz-Pena GN, Pecot
CV, Coward J, et al: Paraneoplastic thrombocytosis in ovarian
cancer. N Engl J Med. 366:610–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Scambia G, Testa U, Benedetti PP, Foti E,
Martucci R, Gadducci A, Perillo A, Facchini V, Peschle C and
Mancuso S: Prognostic significance of interleukin 6 serum levels in
patients with ovarian cancer. Br J Cancer. 71:354–356. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gastl G, Plante M, Finstad CL, Wong GY,
Federici MG, Bander NH and Rubin SC: High IL-6 levels in ascitic
fluid correlate with reactive thrombocytosis in patients with
epithelial ovarian cancer. Br J Haematol. 83:433–441. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zeimet AG, Marth C, Müller-Holzner E,
Daxenbichler G and Dapunt O: Significance of thrombocytosis in
patients with epithelial ovarian cancer. Am J Obstet Gynecol.
170:549–554. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Knobloch K, Yoon U and Vogt PM: Preferred
0reporting items for systematic reviews and m20eta-analyses
(PRISMA) statement and publication bias. J Craniomaxillofac Surg.
39:91–92. 2011. View Article : Google Scholar : PubMed/NCBI
|