Introduction
Acute myocardial infarction (AMI) has become the
leading cause of death worldwide, and it is also the most serious
type of coronary artery disease (1).
Regarding the treatment of this disease, early evaluation of
treatment effects and outcomes is required in order to select the
patient's further individualized treatment (2).
Cardiac troponin I (cTnI), the standard marker for
myocardial necrosis (3), is one of
the important diagnostic indicators for patients with AMI (3). However, due to a delayed release of
cTnI, its sensitivity within the first hour of AMI is not
sufficient for diagnosis, which is therefore being referred to as
the ‘troponin-blind period’ (3). The
acute phase of the inflammatory response is an important step in
the development of AMI (4).
Hypersensitive C-reactive protein (hs-CRP) is a highly sensitive
acute inflammatory protein produced by the liver (5), which may be used as an independent risk
factor for predicting adverse cardiovascular events. A number of
studies have indicated an increase in serum hs-CRP in patients with
AMI (6,7).
To date, only a few studies have assessed the
changes of cTnI and hs-CRP in patients after treatment for AMI.
Koskinas et al (8) reported
that in patients with ST segment elevation MI, hs-CRP was
significantly decreased after intensive statin treatment compared
with that prior to treatment, suggesting that the curative effect
may be assessed by using the indexes of absolute values or the
extent of change of hs-CRP. In the present study, the utility of
the absolute values of cTnI and hs-CRP, as well as changes of cTnI
and hs-CRP, prior to and after treatment [extent of change (C) and
change rate (Cr)] in evaluating the early treatment efficacy for
AMI was investigated.
Materials and methods
Patients
A total of 145 patients with AMI treated at the
Ninth People's Hospital of Chongqing between December 2011 and
January 2015 were enrolled in the present study. The diagnostic
criteria for AMI applied were the World Health Organization's
‘Clinical Diagnostic Criteria for Coronary Heart Disease’, 2003
edition (9). The inclusion criteria
were as follows: i) Patients with ‘non-ST segment-elevation MI’ at
first onset or hospitalized within 12 h of onset; and ii) patients
with a similar first-course treatment strategy. Patients with
comorbidities, including diabetes, malignant tumors, chronic
respiratory diseases and coagulopathy, and those who received
special or long-term treatments, were excluded. The present study
was approved by the Ethics Committee of the Chongqing Health
Economics Association (Chongqing, China). Informed consent was
obtained from each patient.
AMI treatments, including dilation of blood vessels,
thrombolytic therapy, regulation of heart rhythm and anti-shock
therapy (Table I), were performed in
strict accordance with the Guidelines for the Treatment of AMI
(2001 edition by the Chinese Medical Association Cardiovascular
Society) (10). The first phase of
AMI treatment lasted for 7–10 days. Two indicators, including cTnI
and hs-CRP, were respectively measured within 2 h of
hospitalization (the start value) and in the early morning of the
last day of the first treatment (the end value). Effective
treatment in the first phase of treatment was defined as follows:
i) Electrocardiogram indicated that the ST segment or the T wave
returned to normal and ii) the symptoms of chest tightness or pain
disappeared. Patients were divided into the effective group (n=69)
and the ineffective group (n=76) based on the early therapeutic
efficacy (10). All participants
were followed up every three months for one year post-discharge.
They were followed up by phone or outpatient visit. The mortality
and re-infarction rates were recorded.
 | Table I.Treatment of patients and the
proportion of patients in the effective and ineffective groups. |
Table I.
Treatment of patients and the
proportion of patients in the effective and ineffective groups.
| Group | Dilation of blood
vessels | Thrombolytic
therapy | Regulation of heart
rhythm | Anti-shock
therapy |
|---|
| Effective group
(n=69) | 69 (100) | 49 (71.01) | 69 (100) | 12 (17.39) |
| Ineffective group
(n=76) | 76 (100) | 58 (76.32) | 76 (100) | 10 (13.16) |
| Total (n=145) | 145 (100) | 107 (73.79) | 145 (100) | 22 (15.17) |
Detection of cTnI and hs-CRP
The serum cTnI was detected using the Architect
i4000SR plus automated immunoassay system (Abbott Diagnostics). The
serum hs-CRP was measured with a Hitachi automatic biochemical
analyzer 7600 (Hitachi, Ltd.). The measurements of cTnI and hs-CRP
were strictly in accordance with the instructions of their
corresponding kits, which were the Cardiac Troponin I kit (Abbott
Ireland Diagnostics Division) and the Hypersensitive C-reactive
Protein kit (Maccura Biotechnology Co.). According to the
instructions, when cTnI was <0.3 ng/ml and hs-CRP was <8
mg/l, the test result was considered negative; otherwise, it was
considered positive.
Definition
C represents the extent of change prior to and after
treatment, while Cr represents the change rate after treatment. The
formula for calculating C or Cr of cTnI was as follows:
C(cTnI)=cTnI(post)-cTnI(pre).
If C(cTnI)≥0,
Cr(cTnI)=C(cTnI)/cTnI(post); if
C(cTnI)<0,
Cr(cTnI)=C(cTnI)/cTnI(pre).
cTnI(post) is the value of cTnI after
treatment, while cTnI(pre) is the value of cTnI prior to
treatment. C(cTnI) is the extent of change of cTnI after
treatment and Cr(cTnI) is the change rate of cTnI.
Similarly, C(hs-CRP) and
Cr(hs-CRP) were calculated as follows.
C(hs-CRP)=hs-CRP(post)-hs-CRP(pre).
If C(hs-CRP)≥0,
Cr(hs-CRP)=C(hs-CRP)/hs-CRP(post);
if C(hs-CRP)<0,
Cr(hs-CRP)=C(hs-CRP)/hs-CRP(pre).
If the value of cTnI or hs-CRP was greater than the
upper limit of its reference value, it was considered as positive;
otherwise, it was defined as negative. If cTnI(post) and
hs-CRP(post) were positive, the combined detection of
cTnI(post) and hs-CRP(post) was positive;
otherwise, it was defined as negative. If the value of
C(cTnI) or C(hs-CRP) was no less than zero,
it was defined as positive; otherwise, it was defined as negative.
If C(cTnI) and C(hs-CRP) were positive, the
combined detection of C(cTnI) and C(hs-CRP)
was defined as positive; otherwise, it was defined as negative
(2).
Statistical analysis
The data were analyzed by using SPSS 17.00 (SPSS
Inc.). The non-parametric independent-samples t-test was used to
analyze the differences in cTnI, hsCRP, C and Cr between the
effective group and the ineffective group. The chi-squared test and
the odds ratio analysis were used to determine the difference in
the positive rate (PR) of cTnI(post),
hs-CRP(post), (cTnI+hs-CRP)(post),
C(cTnI), C(hs-CRP) and
C(cTnI+hs-CRP) between the effective and ineffective
groups. Multivariate logistic regression analysis was performed by
using four models. The treatment groups (the effective or the
ineffective group) were used as dependent variables and five
indicators (sex, age, treatment days and the value of cTnI and
hs-CRP) were used as independent variables. Model I was based on
the values of cTnI(post) and hs-CRP(post);
Model II was based on the PR of cTnI(post) and
hs-CRP(post); Model III was based on the
C(cTnI) and C(hs-CRP); Model IV was based on
the PR of C(cTnI) and C(hs-CRP). Receiver
operating characteristic (ROC) curves for cTnI(post) and
hs-CRP(post), C(cTnI), C(hs-CRP),
Cr(cTnI) and Cr(hs-CRP) to distinguish
between the effective and ineffective groups were respectively
analyzed. Based on the corresponding thresholds, the area under the
ROC curve (AUC) for cTnI(post) and
hs-CRP(post), C(cTnI), C(hs-CRP),
Cr(cTnI) and Cr(hs-CRP), as well as the
sensitivities and specificities, were respectively calculated.
P<0.05 was considered to indicate statistical significance.
Results
Basic clinical characteristics
The characteristics of patients of the effective and
ineffective groups were compared. No significant differences in age
(72.30±10.998 years vs. 72.96±11.384 years), duration of treatment
(7.97±4.759 days vs. 6.93±3.689 days) and sex ratio (42/27 male vs.
49/27 female) were identified between the effective group and the
ineffective group (P>0.05). Following 12 months after discharge,
valid follow-up data were obtained for 33 patients in the effective
group, where 3 incidences of AMI and 2 mortalities occurred, and
for 41 patients in the ineffective group, including 5 incidences of
AMI and 3 mortalities. The mortality and re-infarction rates were
not significantly different between the two groups. Therefore,
there were no significant differences between the two groups
regarding their baseline characteristics and follow-up data
(Table II).
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Characteristic | Effective group
(n=69) | Ineffective group
(n=76) | P-value |
|---|
| Age (years) | 72.30±10.998 | 72.96±11.384 | 0.882 |
| Sex
(male/female) | 42/27 | 49/27 | 0.732 |
| Duration of
treatment (days) | 7.97±4.759 | 6.93±3.689 | 0.166 |
Significant differences in
cTnI(post), hs-CRP(post), C(cTnI),
C(hs-CRP), Cr(cTnI) and Cr(hs-CRP)
between the effective and ineffective groups
To identify any differences of
cTnI(pre/post), hs-CRP(pre/post), C(cTnI
or hs-CRP) and Cr(cTnI or hs-CRP)
between the effective group and the ineffective group,
non-parametric independent-samples t-tests were performed. Prior to
treatment for AMI, there were no significant differences in the
levels of cTnI(pre) and hs-CRP(pre) between
the two groups (P>0.05; Table
III). However, after treatment for AMI, significant differences
in the levels of cTnI(post) and hs-CRP(post)
were determined between the effective group and the ineffective
group. Similarly, the levels of C(cTnI),
C(hs-CRP), Cr(cTnI) and Cr(hs-CRP)
were also significantly different between the two groups
(P<0.01). After one course of treatment, six indicators,
including cTnI(post), hs-CRP(post),
C(cTnI), C(hs-CRP), Cr(cTnI) and
Cr(hs-CRP), were significantly improved in the
patients.
 | Table III.cTnI and hs-CRP prior to and after
treatment in the effective and ineffective groups, as well as the
extent of change and the rate of change. |
Table III.
cTnI and hs-CRP prior to and after
treatment in the effective and ineffective groups, as well as the
extent of change and the rate of change.
|
Parameter/group | Number of
patients | Mean±SD | Median | P-value |
|---|
|
cTnI(pre) |
|
|
| 0.094 |
|
Effective group | 68 | 10.75±15.17 | 2.78 |
|
|
Ineffective group | 72 | 7.93±13.18 | 0.93 |
|
|
cTnI(post) |
|
|
| <0.001 |
|
Effective group | 68 | 2.92±7.28 | 0.49 |
|
|
Ineffective group | 72 | 15.81±16.93 | 9.42 |
|
|
hs-CRP(pre) |
|
|
| 0.972 |
|
Effective group | 23 | 40.65±63.46 | 9.7 |
|
|
Ineffective group | 31 | 37.54±44.84 | 14.3 |
|
|
hs-CRP(post) |
|
|
| <0.001 |
|
Effective group | 23 | 15.60±22.57 | 4.3 |
|
|
Ineffective group | 31 | 55.00±47.49 | 51.5 |
|
|
C(cTnI) |
|
|
| <0.001 |
|
Effective group | 68 | −7.83±11.80 | −1.96 |
|
|
Ineffective group | 72 | 7.88±18.29 | 2.03 |
|
|
C(hs-CRP) |
|
|
| <0.001 |
|
Effective group | 23 | −25.05±54.75 | −7.7 |
|
|
Ineffective group | 31 | 17.45±32.50 | 7.6 |
|
|
Cr(cTnI) |
|
|
| <0.001 |
|
Effective group | 68 | −54.29±50.28 | −70.69 |
|
|
Ineffective group | 72 | 34.53±63.38 | 55.41 |
|
|
Cr(hs-CRP) |
|
|
| <0.001 |
|
Effective group | 23 | −31.82±57.85 | −52.54 |
|
|
Ineffective group | 31 | 39.71±44.09 | 46.08 |
|
Significant differences in
PR-cTnI(post), PR-hs-CRP(post),
PR-(cTnI+hs-CRP)(post) as well as PR-C(cTnI),
PR-C(hs-CRP) and PR-C(cTnI+hs-CRP) between
the effective and the ineffective group
To test whether the PR of the various indicators
differs between the effective and the ineffective group,
chi-squared tests were performed. The PR-cTnI(post),
PR-hs-CRP(post), PR-(cTnI+hs-CRP)(post), as
well as PR-C(cTnI), PR-C(hs-CRP) and
PR-C(cTnI+hs-CRP), were significantly different between
the effective group and the ineffective group (P<0.01; Table IV). The odds ratios to indicate
effective treatment for the PR-C(cTnI),
PR-C(hs-CRP) and particularly the
PR-C(cTnI+hs-CRP) were much higher than those for the
PR-cTnI(post), PR-hs-CRP(post) and
PR-(cTnI+hs-CRP)(post). Thus, the use of
PR-C(cTnI) and PR-C(hs-CRP) may be better
than that of PR-cTnI(post) and
PR-hs-CRP(post) for evaluating the early efficacy of AMI
treatment. Furthermore, the combination of PR-C(cTnI)
and PR-C(hs-CRP) had the best value in evaluating the
early efficacy of AMI treatment.
 | Table IV.Odds of the positive rate to indicate
effective treatment compared between the effective group and the
ineffective group. |
Table IV.
Odds of the positive rate to indicate
effective treatment compared between the effective group and the
ineffective group.
|
Parameter/status | Effective group, n
(%) | Ineffective group,
n (%) | P-value | Odds ratio | 95% CI |
|---|
|
PR-cTnI(post) |
|
| 0.008 | 2.728 | 1.277–5.83 |
| N | 27 (65.85) | 14 (34.15) |
|
|
|
| P | 41(41.41) | 58 (58.59) |
|
|
|
|
PR-hs-CRP(post) |
|
| 0.002 | 6.481 | 1.908–22.014 |
| N | 14 (70.00) | 6 (30.00) |
|
|
|
| P | 9 (26.47) | 25 (73.53) |
|
|
|
|
PR-(cTnI+hs-CRP)(post) |
|
| 0.001 | 6.063 | 1.935–18.994 |
| N | 32 (62.75) | 19 (37.25) |
|
|
|
| P | 5 (21.74) | 18 (78.26) |
|
|
|
|
PR-C(cTnI) |
|
| <0.001 | 20.921 | 8.464–51.711 |
| N | 60 (75.95) | 19 (24.05) |
|
|
|
| P | 8 (13.11) | 53 (86.89) |
|
|
|
|
PR-C(hs-CRP) |
|
| <0.001 | 11.806 | 3.255–42.821 |
| N | 17 (73.91) | 6 (26.09) |
|
|
|
| P | 6 (19.35) | 25 (80.65) |
|
|
|
|
PR-C(cTnI+hs-CRP) |
|
| <0.001 | 58.095 | 7.340–459.787 |
| N | 61 (74.39) | 21 (25.61) |
|
|
|
| P | 1 (4.76) | 20 (95.24) |
|
|
|
Efficacy evaluation by four
models
In order to explore the impact of various factors on
the early efficacy of AMI treatment, multivariate logistic
regression analysis was performed. The groups (effective and
ineffective group) were used as the dependent variable and the five
indicators [sex, age, treatment days, cTnI(post) and
hs-CRP(post)] were used as independent variables. As
presented in Table V, the total
effective rate in Model I was 75.5%, and it was indicated that the
hs-CRP(post) may be used as an independent factor for
efficacy evaluation in Model I (P<0.05). The total effective
rate in Model II was 73.5% and according to this model, the
positive rate of cTnI(post) may be used as an
independent factor for evaluation of the treatment efficacy
(P<0.05). The total effective rate in Model III was 75.5% and it
was indicated that C(hs-CRP) may be used as an
independent factor for efficacy evaluation (P<0.05). The total
effective rate in Model IV was 83.7% and according to this model,
the PR-C(cTnI) may be used as an independent factor for
evaluation of efficacy (P<0.01). The analysis suggested that,
among the various factors that may be indicative of the early
efficacy of AMI treatment, hsCRP(post),
C(hs-CRP), PR-cTnI(post) and
PR-C(cTnI) were the best indicators.
 | Table V.Effects of sex, age and treatment
days, as well as parameters associated with cTnI and hs-CRP, on the
clinical outcomes of patients. |
Table V.
Effects of sex, age and treatment
days, as well as parameters associated with cTnI and hs-CRP, on the
clinical outcomes of patients.
|
| Model I | Model II | Model III | Model IV |
|---|
|
|
|
|
|
|
|---|
| Variable | B | Wals F | P-value | B | Wals F | P-value | B | Wals F | P-value | B | Wals F | P-value |
|---|
| Sex (male,
female) | 0.742 | 0.946 | 0.331 | 0.832 | 1.199 | 0.274 | 0.797 | 1.075 | 0.300 | 1.993 | 2.833 | 0.092 |
| Age (years) | −0.018 | 0.281 | 0.596 | 0.001 | 0.001 | 0.981 | −0.019 | 0.300 | 0.584 | 0.017 | 0.128 | 0.721 |
| Treatment days | −0.061 | 0.547 | 0.459 | −0.096 | 1.406 | 0.236 | −0.088 | 1.179 | 0.278 | −0.062 | 0.257 | 0.612 |
|
cTnI(post) | 0.063 | 3.567 | 0.059 | – | – | – | – | – | – | – | – | – |
|
hsCRP(post) | 0.031 | 6.066 | 0.014 | – | – | – | – | – | – | – | – | – |
|
PR-cTnI(post) (P,N) | – | – | – | 1.802 | 5.469 | 0.019 | – | – | – | – | – | – |
|
PR-hs-CRP(post) (P,N) | – | – | – | 1.436 | 3.269 | 0.070 | – | – | – | – | – | – |
|
C(cTnI) | – | – | – | – | – | – | 0.036 | 1.668 | 0.197 | – | – | – |
|
C(hs-CRP) | – | – | – | – | – | – | 0.037 | 5.222 | 0.022 | – | – | – |
|
PR-C(cTnI) (P,N) | – | – | – | – | – | – | – | – | – | 4.239 | 8.997 | 0.003 |
|
PR-C(hs-CRP) (P,N) | – | – | – | – | – | – | – | – | – | 0.629 | 0.321 | 0.571 |
ROC analysis of cTnI(post),
C(cTnI) and Cr(cTnI) to determine the early
efficacy of AMI therapy
The ROC curves for cTnI(post),
C(cTnI) and Cr(cTnI) to distinguish between
the effective and ineffective groups were separately analyzed. For
cTnI(post), the AUC was 0.775 (95% CI: 0.695–0.854,
P<0.001) at the best cut-off value of 2.459 ng/ml. The
corresponding sensitivity was 70.4% and the specificity was 80.9%.
For C(cTnI), the AUC was 0.826 (95% CI: 0.755–0.898,
P<0.001) at the best cut-off value of 0.001 ng/ml. The
corresponding sensitivity was 73.2% and the specificity was 89.7%.
For Cr(cTnI), the AUC was 0.851 (95% CI: 0.786–0.916,
P<0.001) and the cut-off value was −17.725%. The corresponding
sensitivity was 77.5% and the specificity was 83.8% (Fig. 1; Table
VI).
 | Table VI.ROC analysis of
cTnI(post), C(cTnI), Cr(cTnI),
hs-CRP(post), C(hs-CRP) and
Cr(hs-CRP) to determine the early efficacy of AMI
therapy. |
Table VI.
ROC analysis of
cTnI(post), C(cTnI), Cr(cTnI),
hs-CRP(post), C(hs-CRP) and
Cr(hs-CRP) to determine the early efficacy of AMI
therapy.
| Groups | AUC | 95% CI | P-value | Best cut off
value | Corresponding
sensitivity (%) | Corresponding
specificity (%) |
|---|
|
cTnI(post) | 0.775 | 0.695–0.854 | <0.001 | 2.459 ng/ml | 70.4 | 80.9 |
|
C(cTnI) | 0.826 | 0.755–0.898 | <0.001 | 0.001 ng/ml | 73.2 | 89.7 |
|
Cr(cTnI) | 0.851 | 0.786–0.916 | <0.001 | −17.725% | 77.5 | 83.8 |
|
hs-CRP(post) | 0.785 | 0.660–0.911 | <0.001 | 14.85 mg/l | 74.2 | 78.3 |
|
C(hs-CRP) | 0.837 | 0.727–0.946 | <0.001 | 1.75 mg/l | 74.2 | 87.0 |
|
Cr(hs-CRP) | 0.826 | 0.708–0.945 | <0.001 | −13.565% | 87.1 | 73.9 |
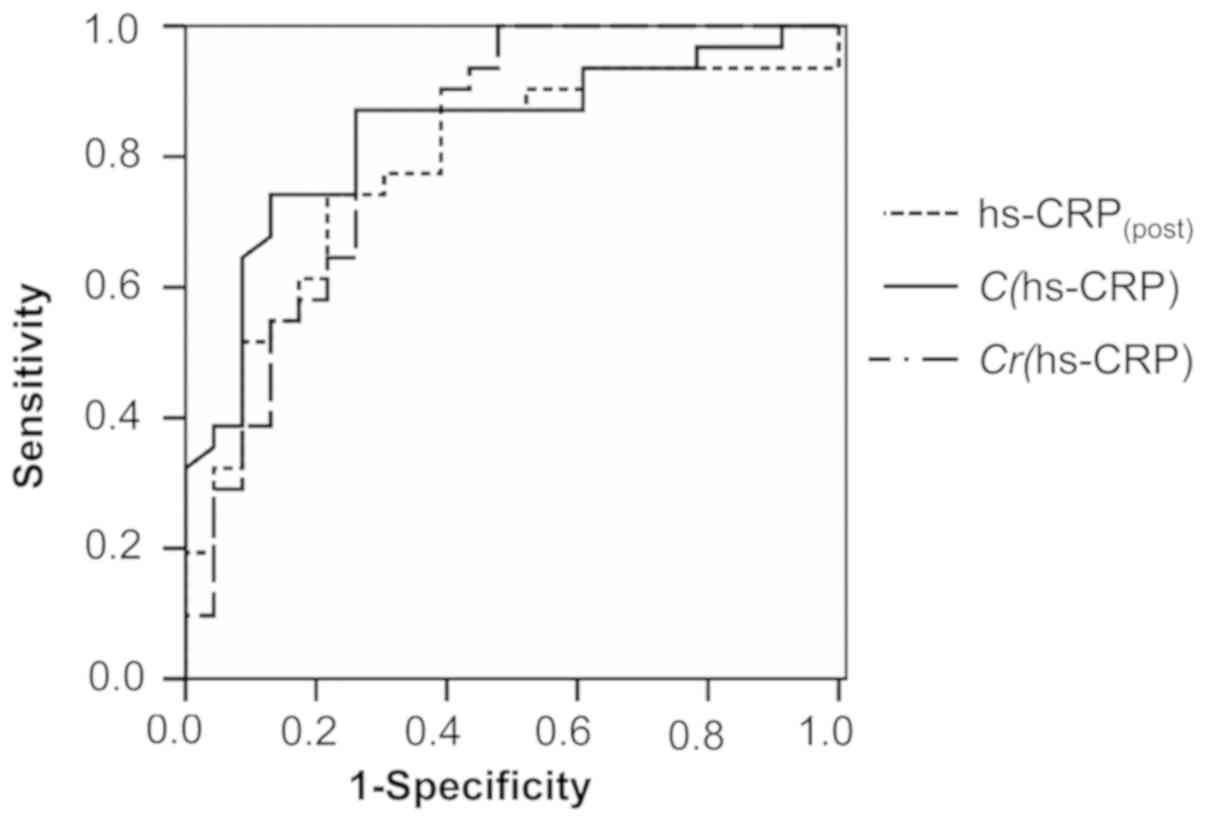
Similarly, the ROC curves for
hs-CRP(post), C(hs-CRP) and
Cr(hs-CRP) were also drawn (Fig. 2). For hs-CRP(post), the
AUC was 0.785 (95% CI: 0.660–0.911, P<0.001) at the best cut-off
value of 14.850 mg/l; the sensitivity was 74.2% and the specificity
was 78.3%. For C(hs-CRP), the AUC was 0.837 (95% CI:
0.727–0.946, P<0.001) at the cut-off value of 1.750 mg/l; the
sensitivity and specificity were 74.2 and 87.0%, respectively. For
Cr(hs-CRP), the AUC was 0.826 (95% CI: 0.708–0.945,
P<0.001) at the cut-off value of −13.565%, with a sensitivity of
87.1% and a specificity of 73.9%. The AUCs of those above-mentioned
indicators ranged from 0.775 to 0.851, indicating that they all had
a certain predictive effect. Of note, the AUCs of those six
indicators exhibited a trend: Cr(cTnI) >
C(hs-CRP) > Cr (hs-CRP)=C(cTnI)
> hs-CRP(post) > cTnI(post), indicating
that the C and Cr values of the two indicators were more suitable
for assessment of the therapeutic effect than their absolute
values.
Discussion
In the present study, the utility of cTnI and hs-CRP
in evaluating the early efficacy of AMI treatment was evaluated.
The levels of cTnI and hs-CRP were measured in patients with AMI
treated between 2011 and 2015. The results suggested that after
treatment, the levels of cTnI and hs-CRP, as well as their C and
Cr, were markedly decreased in the effective group (P<0.01). The
hs-CRP(post) and C(hs-CRP), as well as the
PR-cTnI(post) and PR-C(cTnI), may be used as
independent factors for evaluating the early therapeutic efficacy
(P<0.05). The present study also confirmed that the C and Cr of
cTnI and hs-CRP had better sensitivity and specificity for
assessing the early treatment efficacy of AMI than the absolute
values.
AMI is the most severe manifestation of coronary
artery disease (1), mostly due to
atherosclerotic plaque rupture. Atherosclerosis is thought to be
caused by lipids invading the arterial wall, which deposit between
smooth muscle cells, collagen or elastin fibers, and stimulate
fibrous tissue proliferation (11).
Once plaques rupture, platelets bind to collagen through cell
surface receptors to accelerate thrombus formation, promote
platelet recruitment and adhesion, and aggravate coronary stenosis
(12). In the clinic, patients with
MI commonly present with chest pain and acute circulatory
dysfunction, and the condition seriously endangers the life of aged
individuals affected (2).
Current methods for assessing the early treatment
effects of AMI are not sensitive and comprise serum enzymes
including aspartate aminotransferase (AST), lactate dehydrogenase
(LDH) and their isoenzymes (13,14).
Since AST and LDH are distributed in numerous organs throughout the
body, their diagnostic specificity is poor (15). Creatine kinase isoenzymes (CK-MB) may
reflect the subtle changes of myocardial injury; thus, the abnormal
increase of serum CK-MB may be used for early prediction or
therapeutic evaluation of myocardial injury; however, its
specificity is low due to its expression in multiple tissues
(16). In addition, the degree of ST
segment changes in the aVF lead has certain significance in the
prognostication of patients with AMI, but its sensitivity is not
high either (17).
hs-CRP, an acute-phase reactant, is significantly
elevated in the plasma of patients with AMI or MI (18). Numerous prospective studies have
demonstrated that CRP may independently predict cardiovascular
events (19,20), and it therefore has important
clinical value in the diagnosis of MI, as well as the diagnosis of
ischemic stroke and sudden cardiac death (21). In addition, cTnI has been reported to
hold great value in assessing thrombolytic effects, predicting
infarct size and identifying unstable angina (22). For instance, Twerenbold et al
(23) reported that detection of
hypersensitive cTnI is able to accurately quantify the extent of
myocardial damage. Xu et al (24) indicated that the serum levels of
hs-CRP and cTnI in patients with AMI were significantly higher than
those in healthy controls, and they were significantly lower in the
treatment-effective group than in the treatment-ineffective group.
Similarly, in the present study, no significant difference in the
levels of hs-CRP and cTnI prior to AMI treatment was identified
between the two groups; however, after AMI treatment, their levels
were significantly lower in the effective group than those in the
ineffective group, and it was suggested that
hs-CRP(post), C(hs-CRP), the
PR-cTnI(post) and the PR-C(cTnI) may be used
as independent factors to predict early treatment outcomes in
patients with AMI. Muhlestein et al (25) demonstrated that the change of
Erythrocyte Distribution Width was of higher evaluation value for
the early efficacy of AMI treatment, which is similar to the
methodology used in the current study. Consistently, the present
study also indicated the significant differences in the C and Cr of
hs-CRP and cTnI between the effective group and the ineffective
group after AMI treatment, while there were no differences prior to
AMI treatment, indicating that these indicators are able to reflect
the early therapeutic effect. Simultaneously, there were
significant differences in the level of hs-CRP and cTnI and their
positive rates between the effective and ineffective groups.
Further analysis suggested that the combined detection of hs-CRP
and cTnI and their combined PRs also exhibited significant
differences between the effective and ineffective groups. In
addition, the multivariate logistic regression analysis revealed
that hs-CRP(post), C(hs-CRP),
PR-cTnI(post) and PR-C(cTnI) may serve as
independent factors for evaluating the early response to AMI
treatment. Furthermore, the ROC curve analysis of the present study
revealed that the AUC, sensitivity and specificity for the C and Cr
of cTnI and hs-CRP for evaluating the early treatment efficacy of
AMI than those for hs-CRP(post) and
cTnI(post). In addition, the specificity and sensitivity
of the C and Cr of cTnI and hsCRP were better than their absolute
values. However, further studies are required to confirm this. The
present results suggest that introduction of the C or Cr of hs-CRP
and cTnI in the evaluation of early therapeutic effects in patients
with AMI may dynamically reflect early treatment responses, which
may further facilitate the development of more individualized and
accurate treatments.
At present, complete data on traditional indicators
(including AST, LDH and CK-MB) are not available. Thus, it was not
possible to compare the utility of modified cTnI and hs-CRP
parameters in predicting the efficacy of AMI treatment to that of
existing markers (e.g. AST, LDH and CK-MB). However, it has been
reported that the diagnostic and prognostic value of cTnI and
hs-CRP in patients with AMI is better than that of AST, LDH and
CK-MB. For instance, Fan et al (26) demonstrated that the specificity and
sensitivity of cTnI in the diagnosis of AMI was much better than
that of CK-MB. Jia et al (27) also indicated that cTnI was a better
indicator for diagnosis and differential diagnosis of patients with
AMI due to its higher sensitivity and specificity compared with
that of AST, CK-MB and LDH. Therefore, cTnI may be an ideal
biomarker for AMI. In addition, the level of serum cTnI was
reported to not be affected by age, sex, site of myocardial damage
or thrombolytic drugs (28). Aseri
et al (29) suggested that
hs-CRP is a more significant predictor for myocardial damage than
AST, CK-MB and LDH, and it may be a useful prognostic marker in
acute coronary syndrome. In the clinic, the indicators used to
evaluate early treatment effects in patients after AMI include, but
are not limited to, cTnI, hs-CRP, AST, LDH and CK-MB (13,14,24).
However, to the best of our knowledge, no previous study has
directly compared the role of cTnI and hs-CRP in evaluating
treatment efficacy to that of other traditional indicators
(including AST, LDH and CK-MB), and therefore, further studies are
warranted.
Of note, the present study has certain limitations.
First, the acquisition time of cTnI and hs-CRP data in certain
patients did not meet the requirements set by the experimental
design, resulting in data loss. Second-treatment duration times
were different among each patient, lowering the reliability of
conclusion. Furthermore, the overall sample size was relatively
small. In addition, the follow-up was relatively short, and the
amount of patients lost to follow-up was relatively high, thus the
role of hs-CRP and cTnI in evaluating the long-term efficacy of AMI
treatment was not determined. Finally, comorbidities in the
patients with AMI were not analyzed in the present study. In some
AMI patients with comorbidities, the hs-CRP and cTnI values may be
altered, which could impact the evaluation of these parameters.
Further studies are therefore warranted.
At present, the levels of hsCRP and cTnI are
frequently used in the clinic to evaluate the early treatment
efficacy of AMI; however, less attention is paid to their changes
prior to and after treatment. The present study indicated that the
value of the C and Cr of hsCRP and cTnI in the evaluation of the
early therapeutic efficacy of AMI treatment is improved compared
with that of the absolute values, providing a reference for their
clinical application.
Acknowledgements
Not applicable.
Funding
The present study was supported by the 2017 Key
Project of the Chongqing Health Economics Association (grant. no.
YWJK2017-1).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
LW, BL, MZ, XC and KH designed the study. BL, JY
and LC collected and analyzed the data. LW and MZ prepared the
manuscript. LL, JY and MZ performed the statistical analysis. LW,
JY and KH searched the literature. MZ collected the funds. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Chongqing Health Economics Association (Chongqing,
China). Informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reed GW, Rossi JE and Cannon CP: Acute
myocardial infarction. Lancet. 389:197–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu J, Wang L, Peng Y, Xiong M, Cai X, Luo
J and Zhang M: Dynamic monitoring of erythrocyte distribution width
(RDW) and Platelet Distribution Width (PDW) in treatment of acute
myocardial infarction. Med Sci Monit. 23:5899–5906. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raskovalova T, Twerenbold R, Collinson PO,
Keller T, Bouvaist H, Folli C, Giavarina D, Lotze U, Eggers KM,
Dupuy AM, et al: Diagnostic accuracy of combined cardiac troponin
and copeptin assessment for early rule-out of myocardial
infarction: A systematic review and meta-analysis. Eur Heart J
Acute Cardiovasc Care. 3:18–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramesh G, Sai NVB, Gururaj P, Bhupal R and
Patel N: Association of metabolic syndrome and level of hs-CRP,
Lp(a), and serum ferritin in young Asian patients (≤45 years) with
acute myocardial infarction. Interv Med Appl Sci. 10:65–69.
2018.PubMed/NCBI
|
|
5
|
Yoshinaga R, Doi Y, Ayukawa K and Ishikawa
S: High-sensitivity C reactive protein as a predictor of inhospital
mortality in patients with cardiovascular disease at an emergency
department: A retrospective cohort study. BMJ Open. 7:e0151122017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Wang L, Wang Q, Xin Z, Liu Y and
Zhao Q: Diagnostic value of carotid artery ultrasound and
hypersensitive C-reactive protein in Type 2 diabetes mellitus
patients with acute myocardial infarction in Chinese population.
Medicine (Baltimore). 97:e123342018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yip HK, Wu CJ, Chang HW, Yang CH, Yeh KH,
Chua S and Fu M: Levels and values of serum high-sensitivity
C-reactive protein within 6 hours after the onset of acute
myocardial infarction. Chest. 126:1417–1422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koskinas KC, Zaugg S, Yamaji K,
García-García HM, Taniwaki M, Klingenberg R, Moschovitis A, Lüscher
TF, van Tits LJ, Matter CM, et al: Changes of coronary plaque
composition correlate with C-reactive protein levels in patients
with ST-elevation myocardial infarction following high-intensity
statin therapy. Atherosclerosis. 247:154–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blomström-Lundqvist C, Scheinman MM, Aliot
EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH,
Lerman BB, et al: ACC/AHA/ESC guidelines for the management of
patients with supraventricular arrhythmias-executive summary. A
report of the American college of cardiology/American heart
association task force on practice guidelines and the European
society of cardiology committee for practice guidelines (writing
committee to develop guidelines for the management of patients with
supraventricular arrhythmias) developed in collaboration with
NASPE-Heart Rhythm Society. J Am Coll Cardiol. 42:1493–1531. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao RL: Guidelines for the diagnosis and
treatment of acute myocardial infarction. Chin J Cardiol.
29:710–725. 2001.
|
|
11
|
Swirski FK and Nahrendorf M: Leukocyte
behavior in atherosclerosis, myocardial infarction, and heart
failure. Science. 339:161–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mackman N: Triggers, targets and
treatments for thrombosis. Nature. 451:914–918. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baars T, Neumann U, Jinawy M, Hendricks S,
Sowa JP, Kälsch J, Riemenschneider M, Gerken G, Erbel R, Heider D
and Canbay A: In acute myocardial infarction liver parameters are
associated with stenosis diameter. Medicine (Baltimore).
95:e28072016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun T, Zhang L, Li X, Chen F, Li Y, Ma X
and Yu F: MicroRNA-1 and circulating microvesicles mediate the
protective effects of dantonic in acute myocardial infarction rat
models. Front Physiol. 9:6642018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elsman P, Zijlstra F, Miedema K, Hoorntje
JC, Dikkeschei LD, Slingerland RJ, Reiffers S, de Boer MJ and
Suryapranata H: The predictive value of cumulative lactate
dehydrogenase release within the first 72 h of acute myocardial
infarction in patients treated with primary angioplasty. Ann Clin
Biochem. 41:142–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Young I and Rifai N: High-sensitivity
C-reactive protein and cardiovascular disease. Clin Chem.
55:201–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cichota LC, Moresco RN, Duarte MM and da
Silva JE: Evaluation of ischemia-modified albumin in anemia
associated to chronic kidney disease. J Clin Lab Anal. 22:1–5.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ridker PM, Cushman M, Stampfer MJ, Tracy
RP and Hennekens CH: Inflammation, aspirin, and the risk of
cardiovascular disease in apparently healthy men. N Engl J Med.
336:973–979. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dibra A, Mehilli J, Schwaiger M, Schühlen
H, Bollwein H, Braun S, Neverve J, Schömig A and Kastrati A:
Predictive value of basal C-reactive protein levels for myocardial
salvage in patients with acute myocardial infarction is dependent
on the type of reperfusion treatment. Eur Heart J. 24:1128–1133.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Suleiman M, Khatib R, Agmon Y, Mahamid R,
Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H
and Aronson D: Early inflammation and risk of long-term development
of heart failure and mortality in survivors of acute myocardial
infarction predictive role of C-reactive protein. J Am Coll
Cardiol. 47:962–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stumpf C, Sheriff A, Zimmermann S,
Schaefauer L, Schlundt C, Raaz D, Garlichs CD and Achenbach S:
C-reactive protein levels predict systolic heart failure and
outcome in patients with first ST-elevation myocardial infarction
treated with coronary angioplasty. Arch Med Sci. 13:1086–1093.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XG, Wang NS and Gao XP: Risk factors
for cardiovascular disease in patients with chronic kidney disease.
J Clin Kidney Dis. 10:220–222. 2010.
|
|
23
|
Twerenbold R, Boeddinghaus J, Nestelberger
T, Wildi K, Rubini Gimenez M, Badertscher P and Mueller C: Clinical
use of high-sensitivity cardiac troponin in patients with suspected
myocardial infarction. J Am Coll Cardiol. 70:996–1012. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu GX, Wang F and Yang YQ: Clinical
significance of detection of plasma LP (a) Hcy hs-CRP and cTnI
levels in patients with acute myocardial infarction. Int J
Laboratory Med. 2466–2468. 2014.
|
|
25
|
Muhlestein JB, Lappe DL, Anderson JL,
Muhlestein JB, Budge D, May HT, Bennett ST, Bair TL and Horne BD:
Both initial red cell distribution width (RDW) and change in RDW
during heart failure hospitalization are associated with length of
hospital stay and 30-day outcomes. Int J Lab Hematol. 38:328–337.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan J, Ma J, Xia N, Sun L, Li B and Liu H:
Clinical value of combined detection of CK-MB, MYO, cTnI and plasma
NT-proBNP in diagnosis of acute myocardial infarction. Clin Lab.
63:427–433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia CY, Wang L, Mao ZG, Zhang JL and Zhang
L: Combined myocardial injury markers for diagnosis of acute
myocardial infarction. Sichuan Da Xue Xue Bao Yi Xue Ban.
40:1082–1085. 2009.(In Chinese). PubMed/NCBI
|
|
28
|
Vogiatzis I, Dapcevic I, Datsios A,
Koutsambasopoulos K, Gontopoulos A and Grigoriadis S: A comparison
of prognostic value of the levels of ProBNP and troponin T in
patients with Acute Coronary Syndrome (ACS). Med Arch. 70:269–273.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aseri ZA, Habib SS, Alhomida AS and Khan
HA: Relationship of high sensitivity C-reactive protein with
cardiac biomarkers in patients presenting with acute coronary
syndrome. J Coll Physicians Surg Pak. 24:387–391. 2014.PubMed/NCBI
|