Introduction
Ischemic stroke is a cerebrovascular disease that
seriously threatens human health. High rates of incidence,
mortality, disability and recurrence, as well as many serious
complications are the characteristics of ischemic stroke. With the
improved life quality and fast-paced lifestyle, the incidence of
ischemic stroke has markedly increased and the disease onset is
becoming younger. So far, thrombolysis is the most effective
treatment for ischemic stroke (1,2).
However, thrombolysis has a strict time window limitation. Only 5%
of ischemic stroke patients are eligible for thrombolysis, and
unfortunately, they suffer from thrombolysis-related bleeding risk
(3). Therapeutic efficacy of
thrombolysis is not satisfactory. Only 50% ischemic stroke patients
are able to achieve recanalization after thrombolysis, and they may
also experience revascularization in the future (4). Therefore, it is urgent to search for
novel targets for clinical treatment of ischemic stroke.
In recent years, many studies have reported that
microRNAs (miRNAs) are able to regulate pathological processes of
cerebral ischemia in different stages (5). miRNAs are a class of non-coding small
RNAs with 19–23 nt in length that are highly conserved in sequence
(6). miRNAs degrade or inhibit
translation of mRNAs at post-transcriptional level and are
completely or incompletely complementary to them. They participate
in various biological progressions, including development,
differentiation, innate immune response and adaptive immune
response (7). Currently, there are
hundreds of miRNAs discovered in human brain, which are involved in
the development and pathophysiology of the nervous system (8).
miRNA-324-5p is located on chromosome 17p13.1.
Deficiency of miRNA-324-5p in medulloblastoma is proved to be
associated with del (17p) (9). In
addition, studies have confirmed that miRNA-324-5p regulates
metastasis, invasion, stemness and drug-resistance of
hepatocellular carcinoma (10,11). The
role of miRNA-324-5p in ischemic stroke, however, remains
unclear.
Patients and methods
Sample collection
A total of 80 cases of acute ischemic stroke
patients admitted to the Third People's Hospital of Wuxi (Wuxi,
China) from July 2015 to March 2017 were enrolled. Moreover, 80
cases of healthy controls undergoing physical examination at the
same period were enrolled as controls. Written informed consent was
obtained before the study, which was approved by the Ethic
Committee of the Third People's Hospital of Wuxi.
Analysis of differentially expressed
miRNAs
Analysis of differentially expressed miRNAs was
performed using the GEO2R online analysis tool (http://www.ncbi.nlm.nih.gov/geo/geo2r/)
of the GEO database. GEO2R identifies the differentially expressed
genes by variance analysis and t-test using the R project for
statistical computing. Genes with a fold difference ≥1.5 and
P<0.05 were considered to be differentially expressed.
Primary neuron extraction
After anesthetizing and disinfecting the pregnant
rats, gestational sac was harvested and placed in sterile Hank's
Balanced Salt Solution (HBSS). The epidermis and skull were cut
with ophthalmology, and the cortical brain tissue was harvested
using an elbow microscopic sputum. After peeling off meninges and
blood vessels, cortical tissues were cut, digested in 0.125%
trypsin at 37°C for 15 min and cultured. Primary neurons were
seeded in a 6-well plate and NB27 medium was applied 2 h later. At
7 days of culture, primary neurons were collected for subsequent
experiments.
Establishment of oxygen-glucose
deprivation (OGD) model in primary neurons
Primary neurons were cultured in glucose-free
Dulbecco's modified Eagles medium (DMEM) (Gibco; Thermo Fisher
Scientific, Inc.) and maintained in a three-gas incubator for 2 h.
Neurons were then incubated in glucose-containing DMEM and
maintained at 37°C, 5% CO2 incubator for 12, 24 and 48
h, respectively.
Glucose uptake determination
Primary neurons were seeded in the 24-well plate and
glucose uptake percentage was determined using the glucose
oxidase-peroxidase method. Neurons were stimulated with synthetic
insulin (100 nmol/l) for 6 h. The supernatant of each well was
collected, centrifuged at 1,500 × g at 4°C for 5 min and subjected
to absorbance determination. Percentage of glucose uptake was
finally calculated.
Caspase-3 activation
determination
Primary neurons were lysed in 100 µl of lysate
solution and the supernatant transferred into the pre-cold
Eppendorf (EP) tube, then incubated with 10 µl of Ac-DEVD-pNA (2
mmol/l) for 60–120 min. After color change, absorbance at 405 nm
was determined.
Cell transfection
Transfection was performed when the confluence was
up to 80–90% following the instructions of Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.). The final dose of
miRNA-324-5p mimics and inhibitor was adjusted to 50 nmol/l.
Cell apoptosis determination
Primary neurons were incubated with 10 µl of Annexin
V fluorescein isothiocyanate (FITC) and 5 µl of propidium iodide
(PI) in the dark. Finally, cells were suspended in 1X binding
buffer for 20 min in dark, followed by flow cytometry detection
(Partec AG).
Western blot analysis
Total protein was extracted from cell lysis,
quantified and electrophoresed. After transferring on
polyvinylidene fluoride (PVDF) membranes (EMD Millipore), they were
incubated with primary antibodies at 4°C. The following day,
membranes were incubated with the corresponding secondary antibody
for 2 h. Bands were exposed with enhanced chemiluminescence, and
integral optical density was analyzed by gel imaging analysis
system (NIH).
RNA extraction and quantitative
real-time polymerase chain reaction (qRT-PCR)
We used TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) to extract total RNA. Cell lysis was mixed with
chloroform, centrifuged at 12,000 × g at 4°C for 10 min and the
precipitate was incubated with isopropanol. After centrifugation,
the precipitate was washed with 75% ethanol, air dried and diluted
in diethyl pyrocarbonate (DEPC) water (Beyotime). The extracted RNA
was subjected to reverse transcription using the Revert Aid First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) and
amplified by SYBR®-Green Master Mix (Takara).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the
internal reference. Primer sequences were as follows: miRNA-324-5p,
forward, GCTATCACAGAGCATTTTCTCAT and reverse,
TGCACCAAACACGACTTTTAACC; RAN, forward, GGT GGTACTGGAAAAACGACC and
reverse, CCCAAG GTGGCTACATACTTCT.
Dual-luciferase reporter gene
assay
RAN 3′-untranslated region (3′UTR) containing the
wild-type or mutant-type sequences of the miRNA-324-5p target
binding sites was cloned into the luciferase reporter vector,
respectively. It was co-transfected with miRNA-324-5p mimics/NC in
neurons for 48 h, followed by luciferase activity
determination.
Cell counting kit-8 (CCK-8) assay
Primary neurons were seeded in a 96-well plate with
5×103 cells per well. At the appointed time-points, 10
µl of CCK-8 (Dojindo) solution was added to each well and incubated
at 37°C for 2 h. The wavelength at 450 nm was detected by a
microplate reader.
Statistical analysis
Data were analyzed by Statistical Product and
Service Solutions (SPSS) 20.0 statistical software (IBM, Corp.).
Quantitative data were represented as mean ± standard deviation
(mean ± SD) and analyzed by the t-test. P<0.05 was considered as
statistically significant.
Results
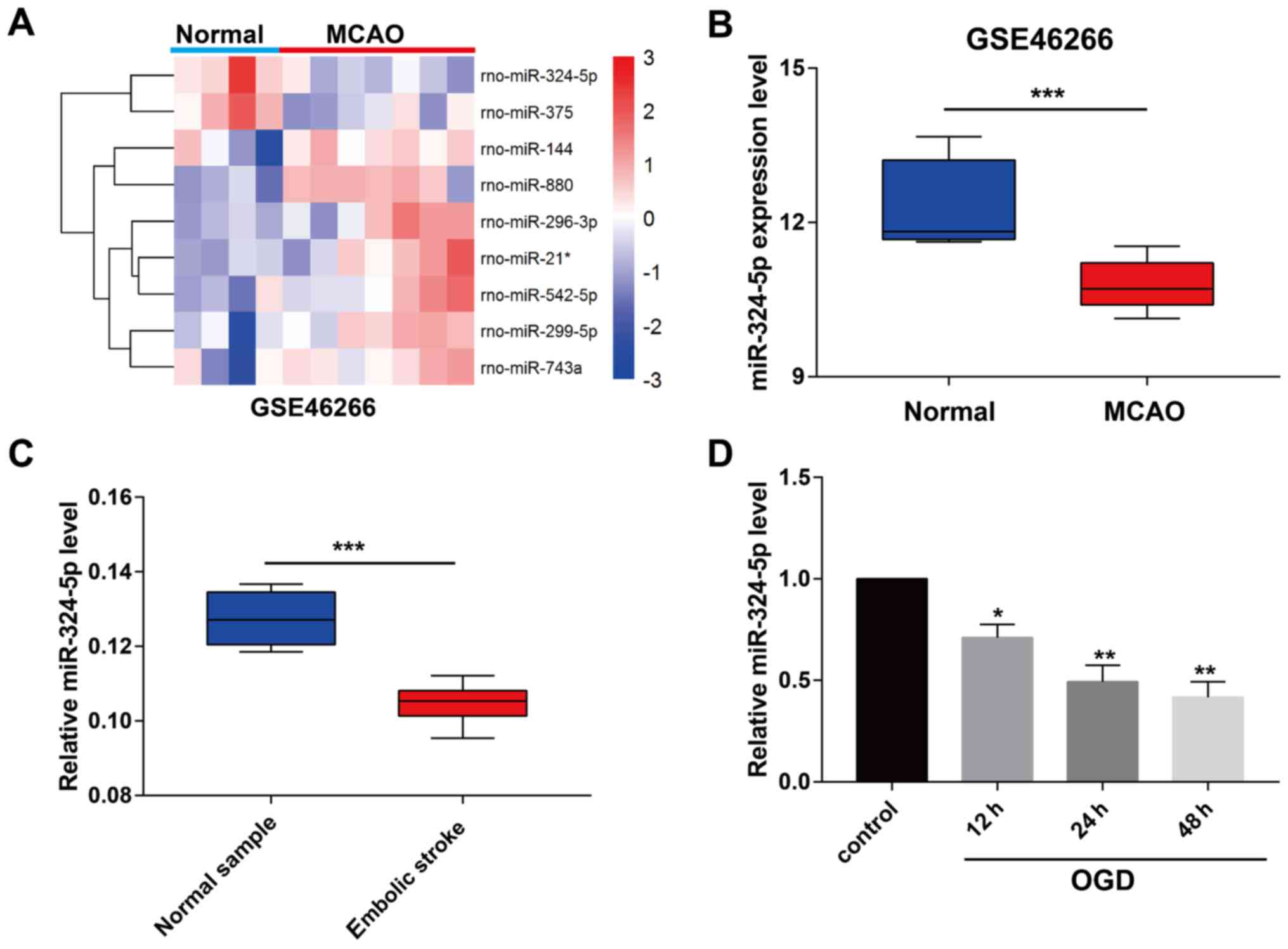
Downregulated miRNA-324-5p in ischemic
stroke
We downloaded the miRNA profile GSE46266 from the
GEO database and analyzed the differentially expressed miRNAs in
ischemic stroke rats (Fig. 1A). It
is indicated that miRNA-324-5p was markedly downregulated in MCAO
rats relative to controls (Fig. 1B).
Peripheral blood samples of ischemic stroke patients and normal
samples were collected in the Third People's Hospital of Wuxi.
Identically, miRNA-324-5p level remained lower in blood samples of
ischemic stroke patients (Fig. 1C).
Subsequently, we constructed the in vitro model of ischemic
stroke by OGD induction in primary rat neurons. As qRT-PCR data
revealed, miRNA-324-5p level was downregulated by OGD induction,
and gradually decreased with the prolongation of reperfusion
(Fig. 1D).
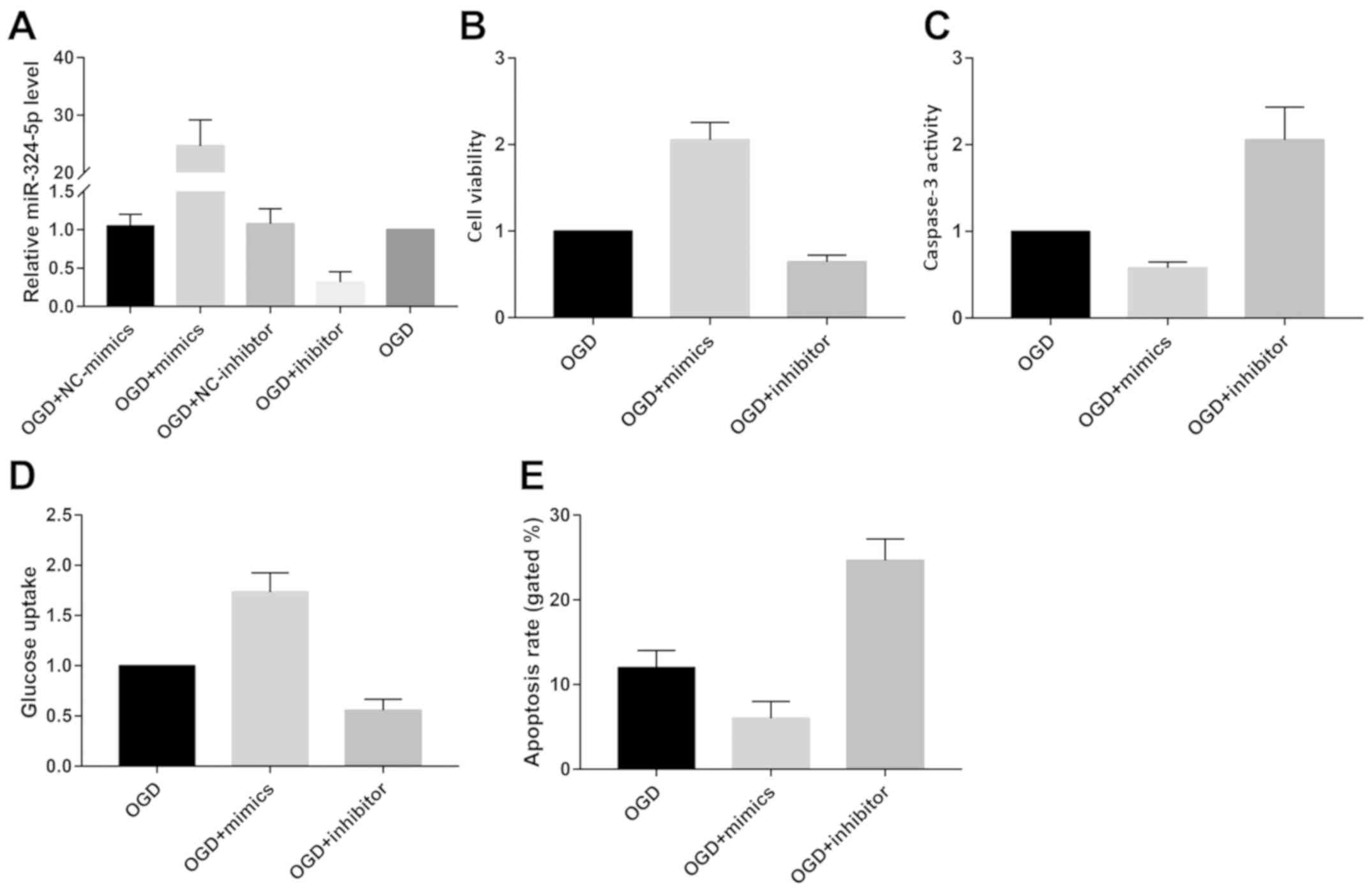
miRNA-324-5p participates in
OGD-induced cerebral ischemic injury
To elucidate the biological function of
miRNA-324-5p, we first transfected miRNA-324-5p mimics or inhibitor
in OGD-induced primary neurons to test their transfection efficacy
(Fig. 2A). Viability was remarkably
elevated in OGD-induced primary neurons overexpressing
miRNA-324-5p. Conversely, knockdown of miRNA-324-5p achieved the
opposite trend (Fig. 2B). Glucose
uptake was accelerated by miRNA-324-5p overexpression (Fig. 2D). However, we observed inhibited
neuronal apoptosis after miRNA-324-5p overexpression as the
decreased caspase-3 activity and apoptotic rate revealed (Fig. 2C and E).
miRNA-324-5p inhibits RAN
expression
miRNA is capable of inhibiting the transcription and
translation of target mRNAs by binding to them. Here, we predicted
the binding between miRNA-324-5p and RAN by bioinformatics method
(Fig. 3A). Luciferase activity was
remarkably reduced in cells co-transfected with RAN-WT and
miRNA-324-5p mimics, whereas it did not change in those transfected
with RAN-WT, indicating the binding of RAN to miRNA-324-5p
(Fig. 3B). Both mRNA and protein
levels of RAN were negatively regulated by miRNA-324-5p (Fig. 3C and D).
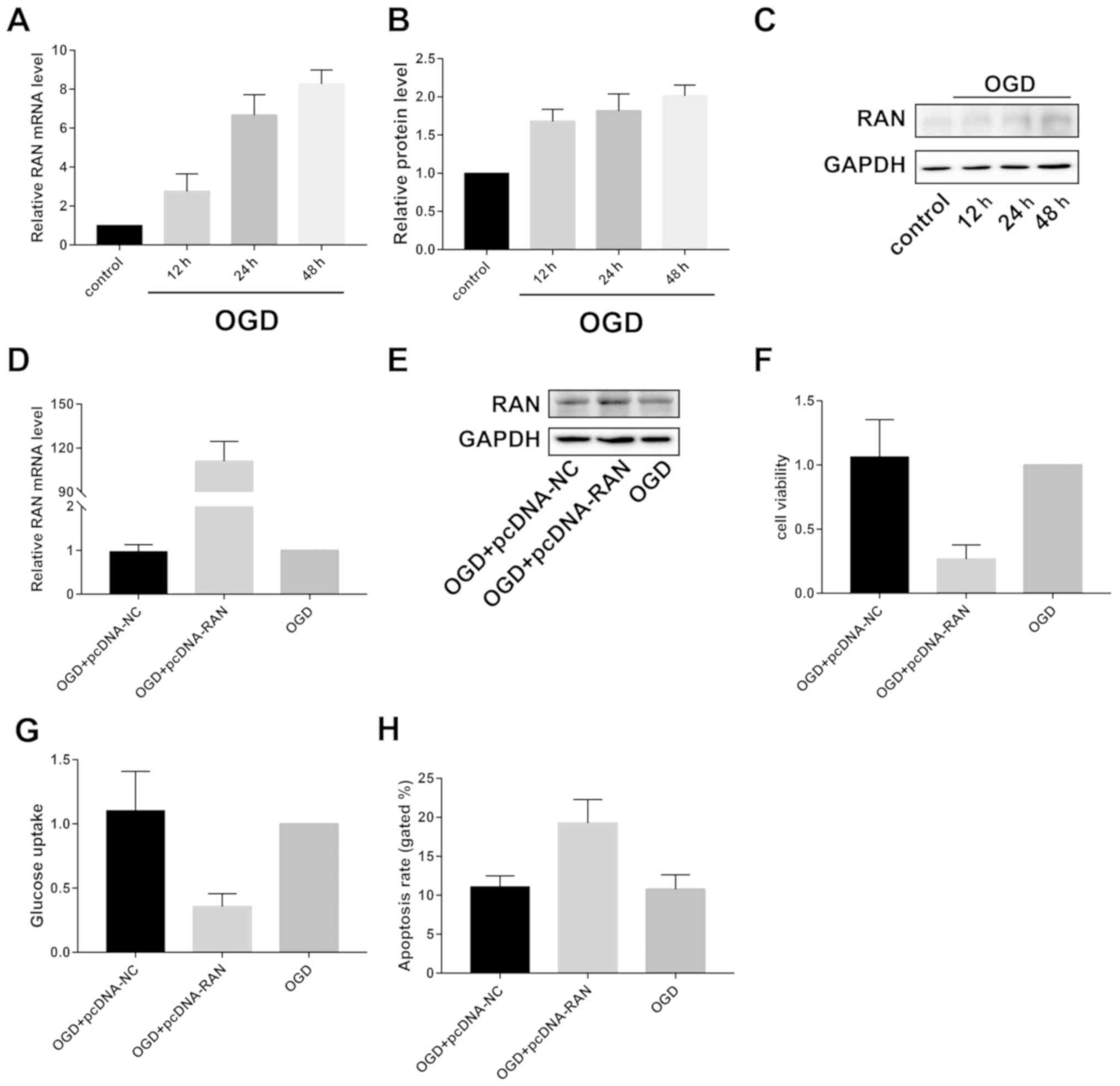
RAN overexpression accelerates
OGD-induced cerebral ischemic injury
Contrary to the expression pattern of miRNA-324-5p,
RAN was gradually upregulated by OGD induction at both mRNA and
protein levels (Fig. 4A-C).
Transfection of pcDNA-RAN sufficiently upregulated RAN level in
OGD-induced primary neurons (Fig. 4D and
E). It was found that RAN overexpression decreased viability
and glucose uptake, but enhanced apoptotic rate of primary neurons
(Fig. 4F-H).
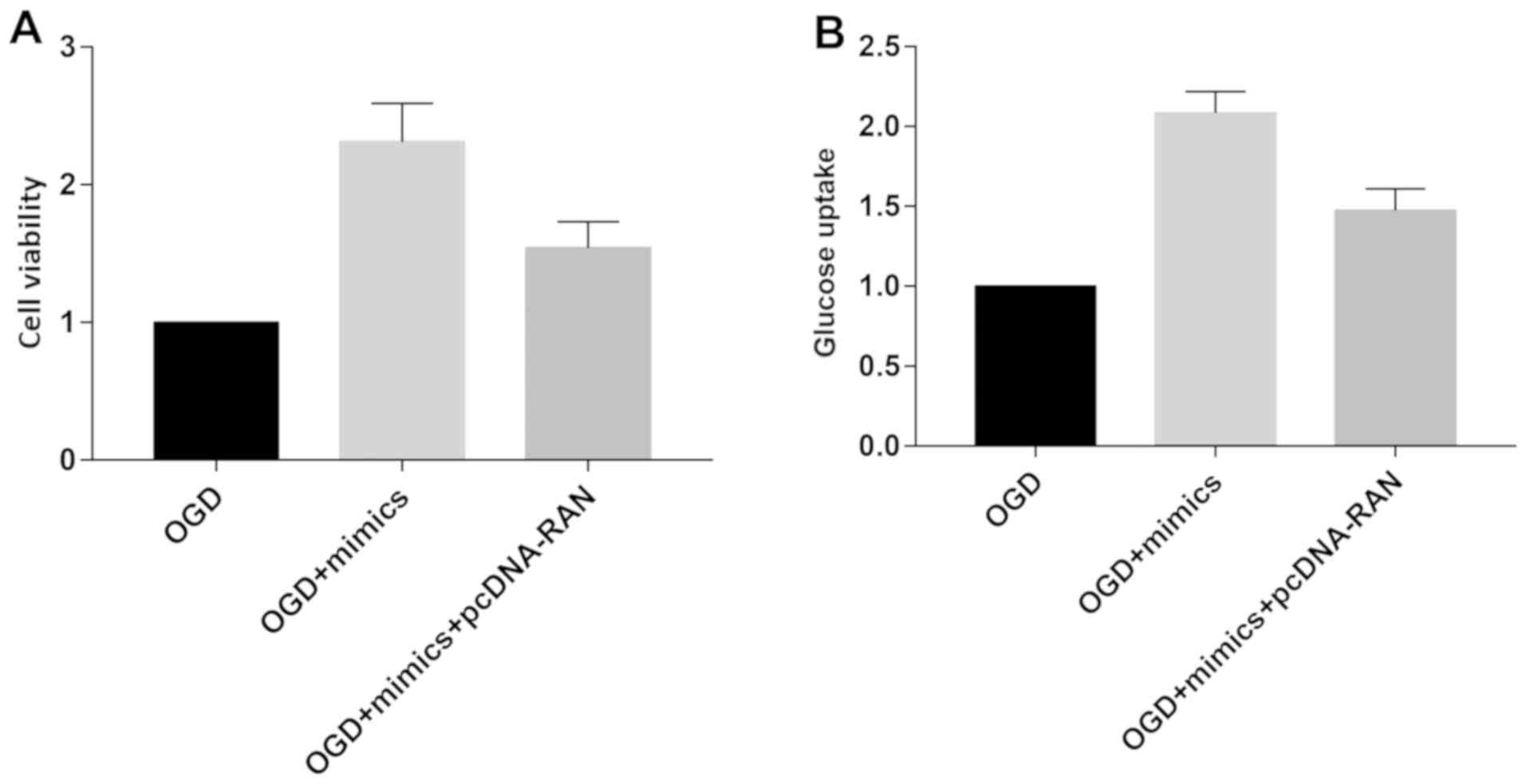
We speculate the involvement of RAN in miRNA-324-5p-
mediated ischemic stroke. OGD-induced primary neurons were
transfected with miRNA-324-5p mimics or miRNA-324-5p mimics +
pcDNA-RAN. Increased viability and glucose uptake due to
miRNA-324-5p overexpression were partially reversed by RAN
overexpression (Fig. 5A and B). The
data demonstrated that miRNA-324-5p alleviated ischemic stroke by
downregulating RAN.
Discussion
Ischemic brain injury involves complex pathological
processes. miRNAs, as novel biological hallmarks, have been
identified to be crucial in different stages of cerebral ischemic
injury (12–15). In vitro and in vivo
experiments have demonstrated that overexpression of miRNA-134
exacerbates cell death and apoptosis. miR-134 deficiency in
OGD-induced N2A cells and ischemic brain tissues upregulate the
protein level of HSPA128. Moreover, miR-134 knockdown could
markedly reduce brain infarct size, alleviate nerve cell damage and
elevate neurological function score in mice (16). Sun et al (17) reported that miR-124 overexpression
remarkably enlarges cerebral infarction area and downregulated
miR-124 exerts a protective role in ischemic stroke by inhibiting
neuronal apoptosis. Overexpression of miR-424 alleviates ischemic
brain damage by inhibiting G1/S phase transition and microglial
activation (18). A relevant study
screened out the target gene of miRNA-181, GRP78, a classic marker
of endoplasmic reticulum stress. miRNA-181 is downregulated in the
cerebral ischemic penumbra, which accelerates the progression of
cerebral injury by inducing neuronal apoptosis through upregulating
GRP78 (19). Differentially
expressed miRNAs in circulating cerebral ischemia may serve as
diagnostic and prognostic hallmarks (20–22).
In this study, we found that miRNA-324-5p was
closely related to cerebral ischemic injury. miRNA-324-5p was
identified to be downregulated in the selected GEO profile,
peripheral blood of stroke patients and OGD-induced primary
neurons. Overexpression of miRNA-324-5p accelerated viability,
induced apoptosis and strengthened glucose uptake ability of
OGD-induced neurons. Subsequently, RAN was predicted to be the
target gene of miRNA-324-5p, which was negatively regulated by
it.
RAN is a 25 kDa GTPase, distributed in nucleus by
binding to GTP or cytoplasm by binding to GDP. RAN exerts
biological functions in eukaryotic cells, including nuclear
transport, mitosis and formation of nuclear membrane and nuclear
pore complexes (23). Recent studies
have shown that RAN is associated with cell fates, such as cell
death, proliferation, differentiation, immortalization and
tumorigenesis. RAN dysfunction will lead to unlimited cell
proliferation. By analyzing serous epithelial ovarian cancer, RAN
expression was shown negatively correlated to disease prognosis
(24). Moreover, transfection of
siRNA RAN reduces viability of human-derived tumor cell lines
(H1299, DLD-1), suggesting that RAN is an effective antitumor
target (25). An in vitro
experiment demonstrated that RAN knockdown inhibits HepG2 cells to
proliferate, exerting remarkable antitumor function (26). The specific role of RAN in ischemic
stroke remains unclear. Our results show that RAN overexpression
inhibited viability, glucose uptake and induced apoptosis of
OGD-induced primary neurons. Importantly, RAN overexpression
partially reversed the regulatory effect of miRNA-324-5p on
ischemic stroke.
Due to the limited experimental conditions, we only
examined the biological effects of miRNA-324-5p in vitro.
Its protective effect on cerebral ischemia-reperfusion injury and
post-ischemic adaptation require validation in an in vivo
model.
In conclusion, miRNA-324-5p is downregulated at
post-stroke, which aggravates the progression of stroke by
inhibiting neuronal proliferation and glucose uptake via
upregulating RAN.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SG, JG and YW designed the study and performed the
experiments, SG, LH and LK collected the data, JG and MD analyzed
the data, SG, JG and YW prepared the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Third People's Hospital of Wuxi (Wuxi, China). Signed informed
consents were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Joliat GR, Halkic N, Pantet O and
Ben-Hamouda N: Ischemic stroke and ST-elevation myocardial
infarction revealing infective endocarditis. Eur Rev Med Pharmacol
Sci. 21:4640–4641. 2017.PubMed/NCBI
|
|
2
|
Zhang B, Sun XJ and Ju CH: Thrombolysis
with alteplase 4.5–6 hours after acute ischemic stroke. Eur Neurol.
65:170–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koroshetz WJ: Tissue plasminogen activator
for acute ischemic stroke. N Engl J Med. 334:1405–1406. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andonova S, Kirov F and Bachvarov C: The
impact of recanalization on ischemic stroke outcome: A clinical
case presentation. Perspect Med. 1:455–458. 2012. View Article : Google Scholar
|
|
5
|
Ouyang YB and Giffard RG: MicroRNAs
regulate the chaperone network in cerebral ischemia. Transl Stroke
Res. 4:693–703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardoso AL, Guedes JR, Pereira de Almeida
L and Pedroso de Lima MC: miR-155 modulates microglia-mediated
immune response by down-regulating SOCS-1 and promoting cytokine
and nitric oxide production. Immunology. 135:73–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berezikov E, Thuemmler F, van Laake LW,
Kondova I, Bontrop R, Cuppen E and Plasterk RH: Diversity of
microRNAs in human and chimpanzee brain. Nat Genet. 38:1375–1377.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mukhopadhyay D and Riezman H:
Proteasome-independent functions of ubiquitin in endocytosis and
signaling. Science. 315:201–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao L, Xie B, Yang X, Liang H, Jiang X,
Zhang D, Xue P, Chen D and Shao Z: miR-324-5p suppresses
hepatocellular carcinoma cell invasion by counteracting ECM
degradation through post-transcriptionally downregulating ETS1 and
SP1. PLoS One. 10:e01330742015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu HS, Zong HL, Shang M, Ming X, Zhao JP,
Ma C and Cao L: MiR-324-5p inhibits proliferation of glioma by
target regulation of GLI1. Eur Rev Med Pharmacol Sci. 18:828–832.
2014.PubMed/NCBI
|
|
12
|
Zhao H, Tao Z, Wang R, Liu P, Yan F, Li J,
Zhang C, Ji X and Luo Y: MicroRNA-23a-3p attenuates oxidative
stress injury in a mouse model of focal cerebral
ischemia-reperfusion. Brain Res. 1592:65–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li LJ, Huang Q, Zhang N, Wang GB and Liu
YH: miR-376b-5p regulates angiogenesis in cerebral ischemia. Mol
Med Rep. 10:527–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XS, Chopp M, Zhang RL, Tao T, Wang XL,
Kassis H, Hozeska-Solgot A, Zhang L, Chen C and Zhang ZG: MicroRNA
profiling in subventricular zone after stroke: MiR-124a regulates
proliferation of neural progenitor cells through Notch signaling
pathway. PLoS One. 6:e234612011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buller B, Liu X, Wang X, Zhang RL, Zhang
L, Hozeska-Solgot A, Chopp M and Zhang ZG: MicroRNA-21 protects
neurons from ischemic death. FEBS J. 277:4299–4307. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chi W, Meng F, Li Y, Wang Q, Wang G, Han
S, Wang P and Li J: Downregulation of miRNA-134 protects neural
cells against ischemic injury in N2A cells and mouse brain with
ischemic stroke by targeting HSPA12B. Neuroscience. 277:111–122.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan
JL and Liu X: MicroRNA-124 protects neurons against apoptosis in
cerebral ischemic stroke. CNS Neurosci Ther. 19:813–819.
2013.PubMed/NCBI
|
|
18
|
Zhao H, Wang J, Gao L, Wang R, Liu X, Gao
Z, Tao Z, Xu C, Song J, Ji X, et al: MiRNA-424 protects against
permanent focal cerebral ischemia injury in mice involving
suppressing microglia activation. Stroke. 44:1706–1713. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX,
White RE, Sun X and Giffard RG: miR-181 regulates GRP78 and
influences outcome from cerebral ischemia in vitro and in vivo.
Neurobiol Dis. 45:555–563. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li P, Teng F, Gao F, Zhang M, Wu J and
Zhang C: Identification of circulating microRNAs as potential
biomarkers for detecting acute ischemic stroke. Cell Mol Neurobiol.
35:433–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long G, Wang F, Li H, Yin Z, Sandip C, Lou
Y, Wang Y, Chen C and Wang DW: Circulating miR-30a, miR-126 and
let-7b as biomarker for ischemic stroke in humans. BMC Neurol.
13:1782013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sepramaniam S, Tan JR, Tan KS, DeSilva DA,
Tavintharan S, Woon FP, Wang CW, Yong FL, Karolina DS, Kaur P, et
al: Circulating microRNAs as biomarkers of acute stroke. Int J Mol
Sci. 15:1418–1432. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagai M and Yoneda Y: Small GTPase Ran and
Ran-binding proteins. Biomol Concepts. 3:307–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ouellet V, Le Page C, Guyot MC, Lussier C,
Tonin PN, Provencher DM and Mes-Masson AM: SET complex in serous
epithelial ovarian cancer. Int J Cancer. 119:2119–2126. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morgan-Lappe SE, Tucker LA, Huang X, Zhang
Q, Sarthy AV, Zakula D, Vernetti L, Schurdak M, Wang J and Fesik
SW: Identification of Ras-related nuclear protein, targeting
protein for xenopus kinesin-like protein 2, and stearoyl-CoA
desaturase 1 as promising cancer targets from an RNAi-based screen.
Cancer Res. 67:4390–4398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Wang R, Wilcox D, Zhao X, Song J,
Lin X, Kohlbrenner WM, Fesik SW and Shen Y: Tumor vasculature is a
key determinant for the efficiency of nanoparticle-mediated siRNA
delivery. Gene Ther. 19:775–780. 2012. View Article : Google Scholar : PubMed/NCBI
|