Introduction
Threatened premature labor (TPTL) is a sign of
giving birth in the middle and late stages of pregnancy before
normal delivery (<37 weeks). It is reported that 65% of pregnant
women with TPTL will give birth prematurely (before 37 weeks)
(1), while ~30% of hospitalized
patients related to pregnancy are due to TPTL (2), and premature labor is still the leading
cause of neonatal death and attack (3,4). Some
studies have shown that ~30% of premature labor is the result of
spontaneous premature labor, and only ~10–15% of women with
symptoms of premature labor will give birth within the next 2–7
days (5). Finding more effective
treatment methods for premature labor is a serious problem that
needs to be faced clinically.
Allylestrenol (AT) is an artificially synthesized
progesterone, which can effectively treat abortion, TPTL,
intrauterine growth restriction (6).
Some studies have pointed out that AT has selective β-adrenergic
effect mediated by nerves on myometrium β2 receptor, reducing
myometrium activity, thus rapidly and effectively relaxing
hypertonic myometrium within 24 h, and avoiding life-threatening
emergencies of mothers, such as pulmonary edema and myocardial
ischemia. Therefore, AT is a drug with minimal side effects and
good patient compliance in progesterone use (7,8).
Ritodrine (RD) can effectively inhibit the contraction of uterine
smooth muscle, reduce uterine activity and prolong pregnancy, and
is a commonly used anti-uterine contraction drug for TPTL treatment
(9). Some studies have comfirmed
that AT combined with RD in the treatment of habitual abortion has
achieved better efficacy than that of AT alone (10). In addition, studies have also
verified that the initiation of labor is closely related to the
regulation of inflammatory factors. Progesterone can maintain
pregnancy by mediating the expression of relevant inflammatory
factors, which can not only control the symptoms of premature
labor, but also eliminate the real causes of labor (11).
Therefore, through prospective analysis, this study
explores the efficacy of AT combined with RD on TPTL and its
influence on inflammatory factors in peripheral blood, so as to
provide reference for clinical treatment of TPTL.
Patients and methods
Patient data
Data of 206 patients with TPTL admitted to Zhongshan
Hospital Affiliated to Fudan University from January 2017 to July
2018 were prospectively analyzed. They were aged 20–40 years, and
were divided into two groups according to different drug
application methods. Among them, 106 patients received AT combined
with RD (research group) and 100 patients received AT combined with
magnesium sulfate (MgSO4) (control group). Inclusion
criteria were as follows: cases conformed to TPTL diagnostic
criteria, patients with complete clinical data, those who did not
use other combined drugs, those with no contraindication for
continuing pregnancy or using fetal protection drugs. Exclusion
criteria were as follows: patients with abnormal heart, liver, lung
and kidney functions, patients with hypertension, diabetes,
hyperthyroidism and other diseases, multiple births,
polyhydramnios, congenital malformation of children, and patients
with mental diseases or abnormal brain judgment. This study
informed all patients and their families by letter or telephone and
they signed an informed consent. The study was approved by the
Ethics Committee of Qingpu Branch of Zhongshan Hospital Affiliated
to Fudan University (Shanghai, China).
Intervention methods
Pregnant women were injected dexamethasone 6 mg
intramuscularly after admission, 12 h each time, 4 times. The
control group was injected with AT+MgSO4. Treatment:
MgSO4 injection (Yangzhou Zhongbao Pharmaceutical Co.,
Ltd., SFDA approval no. H32024805) was used for treatment. The
first loading dose was 8 g/h intravenous drip, followed by 1–2 g/h
maintenance treatment until 12–24 h after uterine contraction
inhibition, and the blood magnesium concentration was maintained at
2–3.5 mmol/l; at the same time, they were given AT tablets orally
(Changzhou Siyao Pharmaceutical Co., Ltd., SFDA approval no.
H20113293), one tablet (5 mg) each time, three times a day, until
37 weeks of pregnancy or childbirth. The research group was treated
with AT+RD: 100 mg of RD injection (Guangdong Xianqiang
Pharmaceutical Co., Ltd., SFDA approval no. H20090302) mixed with
500 ml of 5% glucose injection intravenously with an initial dose
of 0.05 mg/min and an addition of 0.05–0.35 mg/min per minute until
0.10–0.40 mg/min. The treatment was maintained until 12–24 h after
uterine contraction was inhibited. At the same time, oral
administration of AT tablets was the same as that of the control
group for the same number of times and dose until 37 weeks of
pregnancy or childbirth.
Observation indicators
General information of patients were collected,
including age, height, weight, and gestational weeks. Enzyme-linked
immunosorbent assay (Shanghai Xinfan Biotechnology Co., Ltd.,
XF130618) was used to detect the changes in the expression levels
of IL-17, IL-10 and IL-6 in peripheral blood of patients before and
after treatment. Prolonged pregnancy time, success rate of fetal
protection and average delivery time of patients were recorded. The
adverse pregnancy conditions of patients in the two groups were
compared, including the Apgar score (12), birth weight and adverse conditions of
the newborns, and postpartum hemorrhage and postpartum hospital
stays of the research group were recorded.
Statistical methods
SPSS 19.0 (Asia Analytics Formerly SPSS China) was
used for analysis. Measurement data were expressed as [n (%)], and
comparison of the rates between the two groups adopted
χ2 test. Enumeration data were expressed as mean ± SD.
Comparison between the two groups was conducted by
independent-samples t-test, comparison at different time points in
the group was conducted by repeated measures analysis of variance,
and back testing was conducted by LSD test. A P<0.05 was
considered to indicate a statistically significant difference.
Results
General information
The gestational weeks of patients in the two groups
were between 27 and 33 weeks, and there was no significant
difference in age, height, weight, gestational weeks and mean
arterial pressure between them (P>0.05) (Table I).
 | Table I.General information. |
Table I.
General information.
|
| Control group
(n=100) | Research group
(n=106) | χ2/t | P-value |
|---|
| Age | 27.42±5.65 | 27.68±5.49 | 0.335 | 0.738 |
| Height (cm) | 158.57±5.65 | 159.14±5.84 | 0.735 | 0.463 |
| Weight (kg) | 55.36±5.48 | 54.69±5.98 | 0.837 | 0.404 |
| Gestational weeks
(week) | 29.68±2.89 | 29.44±3.10 | 0.574 | 0.567 |
| Mean arterial
pressure (mmHg) | 80.56±15.44 | 82.67±14.96 | 0.996 | 0.320 |
| Cervical length
(mm) | 13.42±4.26 | 13.56±4.01 | 0.243 | 0.808 |
| Abdominal
circumference (cm) | 24.84±2.26 | 24.66±2.37 | 0.557 | 0.578 |
| Fetal heart rate
(times/min) | 120.65±21.77 | 123.85±20.64 | 1.803 | 0.280 |
| History of premature
labor [n (%)] |
|
| 0.011 | 0.918 |
| Yes | 54 (54) | 58 (54.72) |
|
|
| No | 46 (46) | 48 (45.28) |
|
|
Analysis of pregnancy
The fetus protection rate in the research group was
significantly higher than that in the control group, and the
difference was statistically significant (P<0.05). The prolonged
pregnancy time in the research group was significantly better than
that in the control group (P<0.05). The average delivery time in
the research group was also higher than that in the control group
(P<0.05) (Table II).
 | Table II.Analysis of pregnancy. |
Table II.
Analysis of pregnancy.
|
| Control group
(n=100) | Research group
(n=106) | χ2/t | P-value |
|---|
| Fetal protection [n
(%)] |
|
| 4.903 |
0.027 |
|
Success | 85 (85) | 98 (92.45) |
|
|
|
Failure | 15 (15) | 8 (7.55) |
|
|
| Prolonged pregnancy
time (days) | 21.54±18.68 | 34.68±23.58 | 4.416 | <0.001 |
| Average delivery time
(weeks) | 35.21±3.68 | 37.54±3.86 | 4.429 | <0.001 |
Analysis of inflammatory factors in
peripheral blood
After treatment, the levels of IL-17, IL-10 and IL-6
in serum of the two groups were significantly lower than those
before treatment (P<0.05). The expression levels of IL-17, IL-10
and IL-6 in serum of the research group were significantly lower
than those of the control group (P<0.05) (Table III).
 | Table III.Analysis of inflammatory factors in
peripheral blood (pg/ml). |
Table III.
Analysis of inflammatory factors in
peripheral blood (pg/ml).
|
| Control group
(n=100) | Research group
(n=106) | t | P-value |
|---|
| IL-17 |
| Before
treatment | 37.25±8.67 | 38.47±8.47 | 1.021 |
0.308 |
| After
treatment |
29.68±7.38a |
22.51±6.68a | 7.318 | <0.001 |
| IL-10 |
| Before
treatment | 56.67±12.45 | 57.58±13.22 | 0.620 |
0.536 |
| After
treatment |
46.62±12.84a |
32.74±12.65a | 7.814 | <0.001 |
| IL-6 |
| Before
treatment | 58.24±12.65 | 57.09±13.49 | 0.630 |
0.529 |
| After
treatment |
45.25±11.68a |
34.68±12.55a | 6.248 | <0.001 |
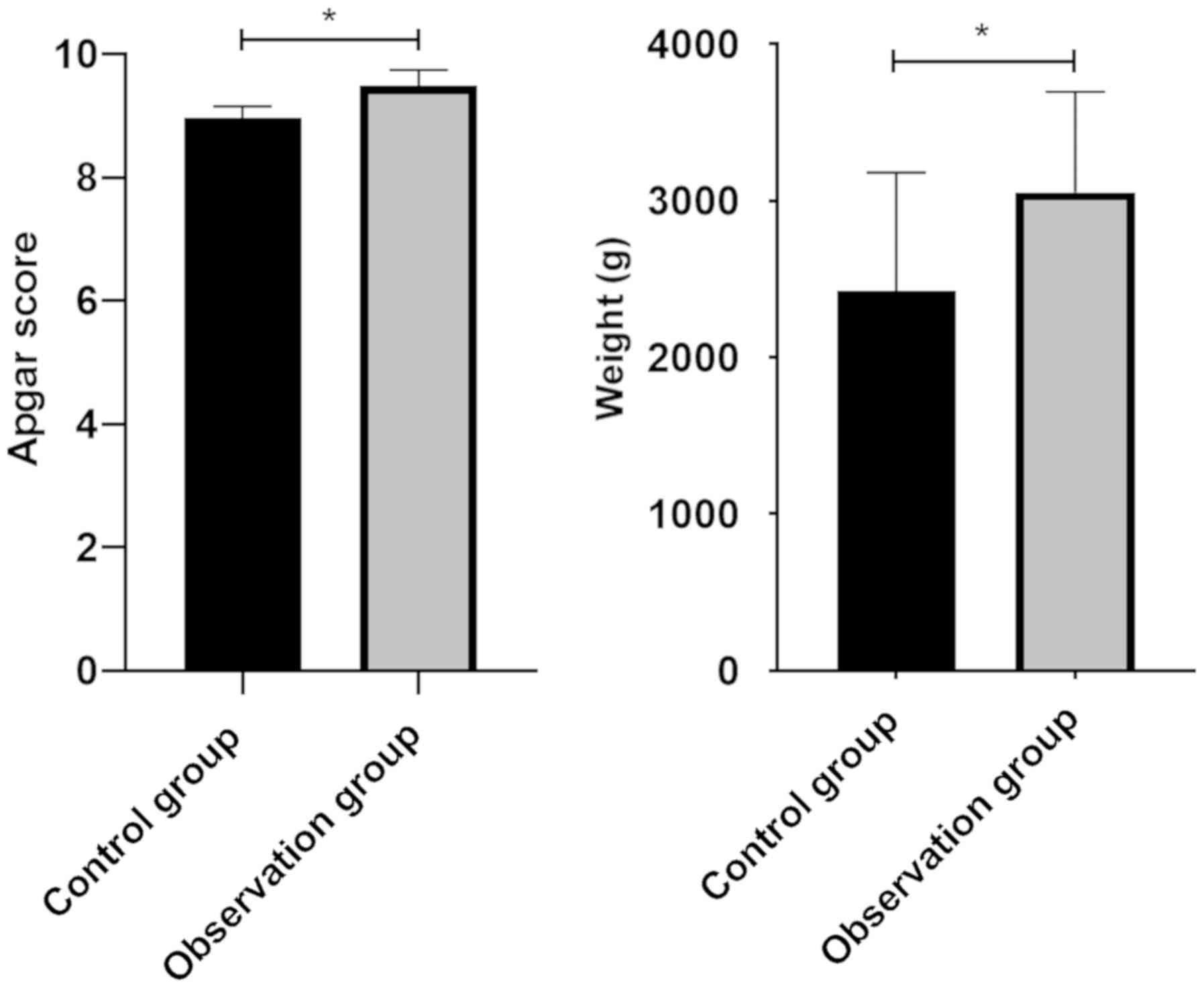
Analysis of Apgar score and birth
weight of newborns
Apgar scores of newborns in research group and
control group were 9.48±0.26 and 8.97±0.18, respectively, and birth
weight was 3054.51±641.98 g and 2423.26±756.10 g, respectively.
Apgar scores and neonatal weight of patients in the research group
were significantly higher than those in the control group, and the
difference was statistically significant (P<0.05) (Fig. 1).
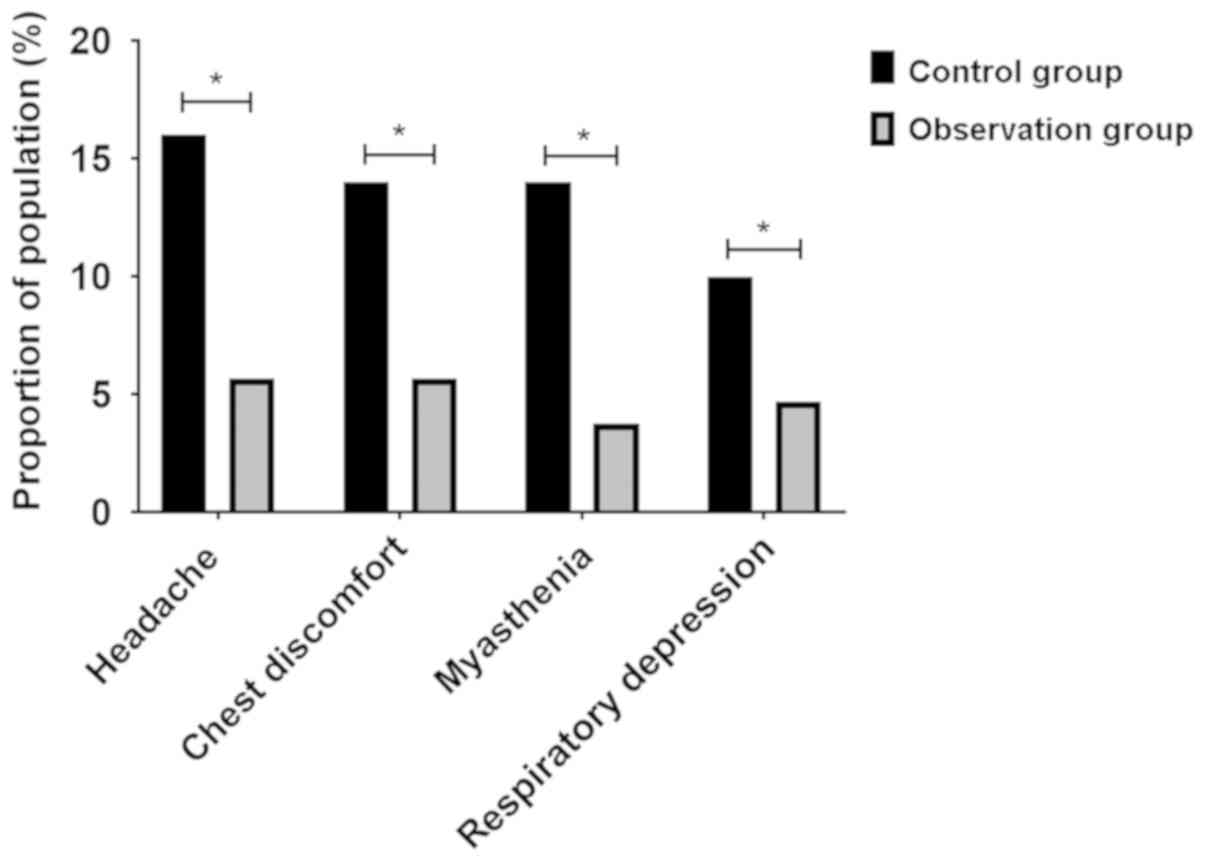
Analysis of adverse reactions of
patients during treatment
During treatment, the number of patients with
adverse reactions such as headache, chest discomfort, myasthenia
and respiratory depression in the research group were 6 (5.66%), 6
(5.66%), 4 (3.77%) and 5 (4.72%), while the number of patients with
adverse reactions in the control group were 16 (16%), 14 (14%), 14
(14%) and 15 (15%), respectively. The proportion of patients in the
research group with adverse reactions was significantly lower than
that in the control group, and the difference was statistically
significant (P<0.05) (Fig.
2).
Analysis of adverse factors
There were 2 cases of neonatal death in the control
group, but none in the research group, and no significant
difference. However, the newborns in the research group with
malformation and asphyxia were significantly fewer than those in
the control group, and the difference was statistically significant
(P<0.05) (Table IV).
 | Table IV.Analysis of neonatal adverse events
[n (%)]. |
Table IV.
Analysis of neonatal adverse events
[n (%)].
|
| Control group
(n=100) | Research group
(n=106) | χ2 | P-value |
|---|
| Death | 2 (2.00) | 0 | 2.020 | 0.155 |
| Malformation | 5 (5.00) | 0 | 5.229 | 0.022 |
| Asphyxia | 8 (8.00) | 2 (1.89) | 3.914 | 0.048 |
Comparison of postpartum hemorrhage
and postpartum hospital stays
Postpartum hemorrhage and postpartum hospital stays
in the research group were significantly fewer than those in the
control group, and the difference was statistically significant
(P<0.05) (Table V).
 | Table V.Comparison of postpartum hemorrhage
and postpartum hospital stays. |
Table V.
Comparison of postpartum hemorrhage
and postpartum hospital stays.
|
| Control group
(n=100) | Research group
(n=106) | χ2 | P-value |
|---|
| Postpartum
hemorrhage (ml) | 270.52±34.66 | 164.85±25.67 | 24.748 | <0.001 |
| Postpartum hospital
stays (days) | 12.63±4.62 |
8.52±3.54 |
7.113 | <0.0011 |
Discussion
TPTL is the main cause of perinatal morbidity and
mortality in many countries, including the USA. Neonatal mortality,
respiratory distress syndrome, intraventricular hemorrhage and
other diseases are negatively correlated with gestational age at
birth (13), and a study indicates
that TPTL is a risk factor for impaired cognitive development at
the age of 2 years. Cognitive ability of premature children is
significantly lower than that of full-term children (14). TPTL seriously threatens the life and
health of newborns and affects cognitive growth. Therefore,
clinical treatment for TPTL requires better efficacy. Some studies
have pointed out that AT can effectively prolong the pregnancy time
of TPTL patients and can effectively reduce the messenger
nucleotide level of oxytocin receptor (15). In addition, progesterone has a
limited effect on acute uterine contraction, but it may play a role
in preventing premature delivery or be regarded as a sensitizer of
other uterine contraction agents (16). RD, as the only drug approved by the
US Food and Drug Administration for anti-uterine contraction, has
significant anti-uterine contraction efficacy on TPTL (17). There are many clinical reports on the
treatment of TPTL with combination drugs. For example, RD combined
with nifedipine can prolong pregnancy >7 days more effectively
than RD alone (18), and there is no
obvious difference in the efficacy and adverse reactions between RD
and MgSO4 (19). However,
there are few reports on AT combined with RD in the treatment of
TPTL. Therefore, through prospective analysis, this study explored
the efficacy of AT combined with RD in the treatment of TPTL and
its influence on inflammatory factors in peripheral blood, so as to
provide reference for clinical treatment of TPTL.
This study showed that after treatment, the
pregnancy rate, prolonged pregnancy time and average delivery time
in the research group were significantly higher than those in the
control group. The Apgar score and neonatal weight of patients in
the research group were significantly better than those in the
control group. During treatment, the adverse reactions of patients
in the research group and overall adverse conditions of newborns
were significantly better than those in the control group.
Under normal circumstances, embryos are not rejected
by mothers, which depends on the immune tolerance of mother-fetus
interface and the balance between cytokines. Once this immune
tolerance pattern is destroyed, habitual abortion will occur
(20). The cervical vagina or
intrauterine infection process during pregnancy is an extremely
fragile state for the mothers and fetuses. The immune defense
process induces the pro-inflammatory environment and damages the
immune privilege in the uterine cavity. Nearly 30% of premature
women have microbial invasion or inflammation in the amniotic
cavity, which results in increase of interleukin and tumor necrosis
factor, leading to disruption of fetal tolerance (21). Progesterone has a strong
immunoregulation effect and is of great significance to
physiological immune tolerance when lying in bed (22). Progesterone has an effect on
interleukin production in vaginal and cervical epithelial cells,
inhibits vaginal epithelial cell base and bacterial stimulation of
interleukin production, and induces changes in the concentration of
inflammatory factors in cervical secretions (23,24). We
studied and measured the changes of IL-17, IL-10 and IL-6 levels in
serum of the two groups. Our study indicated that the levels of
IL-17, IL-10 and IL-6 in serum of the two groups after treatment
were significantly lower than those before treatment. The
expression levels of IL-17, IL-10 and IL-6 in serum of the research
group after treatment were significantly lower than those in the
control group. AT combined with RD can effectively reduce the level
of inflammatory factors, prevent the immune destruction of the body
and effectively maintain the body balance. This is one of the
reasons why the efficacy of patients in the research group is
obviously better than that of the control group. In addition, this
study indicated that IL-6, IL-8 and IL-10 might be developed as
factors for predicting TPTL in the future, which also verified the
role of inflammatory factors in TPTL (25).
In the present study, we obtained results showing
that the effects of AT combined with RD on TPTL were better than
that of AT combined with MgSO4. However, further studies
are necessary with larger sample size for confirmation.
In conclusion, AT combined with RD in the treatment
of TPTL can significantly reduce the expression levels of IL-17,
IL-10 and IL-6, reduce adverse pregnancy conditions, prolong
gestational weeks, and has higher safety and better application
value.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL wrote the manuscript. QL and CL conceived and
designed the study. QL and HJ were responsible for the collection
and analysis of the experimental data. CL and HJ interpreted the
data and drafted the manuscript. QL and CL revised the manuscript
critically for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qingpu Branch of Zhongshan Hospital Affiliated to Fudan University
(Shanghai, China). Patients who participated in this research had
complete clinical data. Signed informed consents were obtained from
the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Roos C, Schuit E, Scheepers HC,
Bloemenkamp KW, Bolte AC, Duvekot HJ, van Eyck J, Kok JH, Kwee A,
Merién AE, et al for APOSTEL-II Study Group, : Predictive factors
for delivery within 7 days after successful 48-hour treatment of
threatened preterm labor. AJP Rep. 5:e141–e149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heng YJ, Taylor L, Larsen BG, Chua HN,
Pung SM, Lee MW, Tucholska M, Tate S, Kupchak P, Pennell CE, et al:
Albumin decrease is associated with spontaneous preterm delivery
within 48 h in women with threatened preterm labor. J Proteome Res.
14:457–466. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Melamed N, Hiersch L, Gabbay-Benziv R,
Bardin R, Meizner I, Wiznitzer A and Yogev Y: Predictive value of
cervical length in women with twin pregnancy presenting with
threatened preterm labor. Ultrasound Obstet Gynecol. 46:73–81.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter J, Tribe RM, Shennan AH and Sandall
J: Threatened preterm labour: Women's experiences of risk and care
management: A qualitative study. Midwifery. 64:85–92. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wagner P, Sonek J, Heidemeyer M, Schmid M,
Abele H, Hoopmann M and Kagan KO: Repeat measurement of cervical
length in women with threatened preterm labor. Geburtshilfe
Frauenheilkd. 76:779–784. 2016.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malhotra N, Garg R, Malhotra N and
Malhotra J: Oral allylestrenol: A pregnancy-supporting progestogen.
J South Asian Fed Obstet Gynecol. 9:297–303. 2017.
|
|
7
|
Joshi P and Nayak A: Role of progesterone
in threatened preterm labour. Indian Obstetr Gynaecol. 4:16–23.
2014.
|
|
8
|
Dewailly D, Lujan ME, Carmina E, Cedars
MI, Laven J, Norman RJ and Escobar-Morreale HF: Definition and
significance of polycystic ovarian morphology: A task force report
from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum
Reprod Update. 20:334–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takekawa D, Jinushi K, Kitayama M and
Hirota K: Rebound hyperkalemia after cessation of ritodrine in a
parturient undergoing cesarean section. JA Clin Rep. 3:32017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan JL and Yang L: Efficacy observation
of allylestrenol combined with ritodrine in treatment of habitual
abortion. Zhongguo Yiyuan Yongyao Pingjia Yu Fenxi. 15:1580–1582.
2015.(In Chinese).
|
|
11
|
Zhu RY, Wong YC and Yong EL: Sonographic
evaluation of polycystic ovaries. Best Pract Res Clin Obstet
Gynaecol. 37:25–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iliodromiti S, Mackay DF, Smith GC, Pell
JP and Nelson SM: Apgar score and the risk of cause-specific infant
mortality: A population-based cohort study. Lancet. 384:1749–1755.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Berghella V, Palacio M, Ness A, Alfirevic
Z, Nicolaides KH and Saccone G: Cervical length screening for
prevention of preterm birth in singleton pregnancy with threatened
preterm labor: Systematic review and meta-analysis of randomized
controlled trials using individual patient-level data. Ultrasound
Obstet Gynecol. 49:322–329. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paules C, Pueyo V, Martí E, de Vilchez S,
Burd I, Calvo P and Oros D: Threatened preterm labor is a risk
factor for impaired cognitive development in early childhood. Am J
Obstet Gynecol. 216:157. e1–157. e7. 2017. View Article : Google Scholar
|
|
15
|
Xu YJ, Ren LD, Zhai SS, Ran LM, Hu LL, Luo
XH, Hong T, Liu R, Yu YR and Ban YJ: OXTR and ZEB1 expression
before and after progesterone dosing in pregnant women with
threatened premature labor. Eur Rev Med Pharmacol Sci.
21:3164–3168. 2017.PubMed/NCBI
|
|
16
|
van Vliet EO, Boormans EM, de Lange TS,
Mol BW and Oudijk MA: Preterm labor: Current pharmacotherapy
options for tocolysis. Expert Opin Pharmacother. 15:787–797. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kareli D, Pouliliou S, Liberis A, Nikas I,
Psillaki A, Kontomanolis E, Nikolettos N, Galazios G and Lialiaris
T: Genotoxic effect of tocolytic drug ritodrine in combination with
smoking during pregnancy. J Matern Fetal Neonatal Med.
29:3496–3505. 2016.PubMed/NCBI
|
|
18
|
Kim MJ, Hwang I, Bae JY and Seong WJ: The
influence of ritodrine alone or in combination with nifedipine on
maternal cardiovascular side effects and pregnancy outcomes. Clin
Exp Obstet Gynecol. 41:537–540. 2014.PubMed/NCBI
|
|
19
|
Kim MK, Lee SM, Oh JW, Kim SY, Jeong HG,
Kim SM, Park CW, Jun JK, Hahn SK and Park JS: Efficacy and side
effect of ritodrine and magnesium sulfate in threatened preterm
labor. Obstet Gynecol Sci. 61:63–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nigro G, Mazzocco M, Mattia E, Di Renzo
GC, Carta G and Anceschi MM: Role of the infections in recurrent
spontaneous abortion. J Matern Fetal Neonatal Med. 24:983–989.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garcia-Ruíz G, Flores-Espinosa P,
Preciado-Martínez E, Bermejo-Martínez L, Espejel-Nuñez A,
Estrada-Gutierrez G, Maida-Claros R, Flores-Pliego A and
Zaga-Clavellina V: In vitro progesterone modulation on bacterial
endotoxin-induced production of IL-1β, TNFα, IL-6, IL-8, IL-10,
MIP-1α, and MMP-9 in pre-labor human term placenta. Reprod Biol
Endocrinol. 13:1152015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carp HJA: Progestogens and pregnancy loss.
Climacteric. 21:380–384. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koucký M, Malíčková K, Cindrová-Davies T,
Smíšek J, Vráblíková H, Černý A, Šimják P, Slováčková M, Pařízek A
and Zima T: Prolonged progesterone administration is associated
with less frequent cervicovaginal colonization by Ureaplasma
urealyticum during pregnancy - Results of a pilot study. J
Reprod Immunol. 116:35–41. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alimohamadi S, Javadian P, Gharedaghi MH,
Javadian N, Alinia H, Khazardoust S, Borna S and Hantoushzadeh S:
Progesterone and threatened abortion: A randomized clinical trial
on endocervical cytokine concentrations. J Reprod Immunol.
98:52–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shah J and Baxi B: Identification of
biomarkers for prediction of preterm delivery. J Med Soc. 30:3–14.
2016. View Article : Google Scholar
|