Introduction
Cutaneous squamous cell carcinoma (CSCC), which
represents 20% of all cutaneous malignancies, is the second most
common skin cancer (1–3). In addition, the incidence of CSCC is
rising in the UK, with the mean annual increase of 5% between 2013
and 2015 (4). Although CSCC commonly
occurs on the skin of the head and neck (5), there is also a risk of occurrence in
the lymph nodes and metastasis to other organs (3,6).
Patients with metastatic CSCC have a poor prognosis (6). The standard treatment for CSCC is
systemic chemotherapy (6,7); the platinum compound cisplatin used
either alone or in combination with other agents is regarded as a
standard chemotherapeutic agent for CSCC therapy (8). However, cisplatin treatment may induce
a variety of side effects, including eventual resistance to
cisplatin and the relapse of most cancer types after therapy
(9). Therefore, novel and effective
anti-CSCC therapeutic strategies are urgently required.
Natural herbal medicines have been used as
anticancer agents (10). Avicularin
(AL), a bioactive flavonol, can be isolated from many medicinal
herbs, including Lespedeza cuneata, Lindera erythrocarpa and
Psidium guajava (11). AL
exhibits diverse pharmacological properties including antioxidant,
antiallergic, anti-inflammatory, hepatoprotective and antitumor
effects (12–14). AL has been found to have a
significant role in reducing the progression of type 2 diabetes
(15). The biological activities of
other flavonols, such as quercetin in the aglycone form have been
investigated as a possible treatment strategy for cancer, including
breast cancer and leukemia (16).
However, the biological properties of AL are not fully understood,
therefore the present study examined the role of AL in regulating
CSCC development.
The present study investigated the effect of AL on
CSCC cells. The present results suggested that AL significantly
inhibited proliferation and increased apoptosis in CSCC cells. In
addition, AL significantly suppressed the MEK/NF-κB signaling
pathway in a concentration-dependent manner.
Materials and methods
Cell culture and treatment
DMEM (Gibco; Thermo Fisher Scientific, Inc) with 10%
FBS (Gemini Bio-Products, Inc.), 10 U/ml penicillin-G and 10 mg/ml
streptomycin (Gemini Bio-Products, Inc.) was used to culture the
CSCC cell line SCC13 (kind gift from Dr James Rheinwald, Brigham
and Women's Hospital, Harvard Medical School, Boston, MA, USA) at
37°C with 5% CO2. SCC13 cells were treated with
different concentrations of AL (0, 10, 30, 100 or 300 µM; purity
>99%; Chengdu Best-Reagent Chemical, Co., Ltd.) (17) at 37°C for 24, 48 and 72 h (18) prior to subsequent
experimentation.
Cell Counting Kit-8 (CCK-8) cell
viability assay
A CCK-8 assay (Dojindo Molecular Technologies, Inc.)
was used to measure cell viability according to the manufacturer's
protocol. SCC13 cells were plated into 96-well plates at
5×103 cells/well and incubated at 37°C for 24 h.
Subsequently, the cells were treated with various concentrations
(0, 10, 30, 100 or 300 µM) of AL for 24, 48 and 72 h at 37°C. Then,
10 µl CCK-8 assay solution was added to each well (Dojindo
Molecular Technologies, Inc.) and the wells were incubated at 37°C
for a 1 h. A microplate reader (Synergy2; BioTeK Instruments, Inc.)
was used to measure the optical density at 450 nm. The
IC50 value of AL to SCC13 cells at 48 h was calculated.
Each experiment was performed in triplicate.
Apoptosis analysis
To detect SCC13 cell apoptosis, the cells were
treated with or without different concentrations of AL (10, 30, 100
or 300 µM) at 37°C for 48 h. Then, 5 µl Annexin V-FITC and 10 µl
propidium iodide (cat. no. 70-AP101-100; Hangzhou Multi Sciences
Biotech Co., Ltd.) were used to stain the cells at room temperature
for 30 min following the manufacturer's protocol. Cell apoptosis
was analyzed using a BD FACSCalibur™ flow cytometer (Becton,
Dickinson and Company) and WinMDI software (version 2.5; Purdue
University Cytometry Laboratories) was used to analyze the
data.
Western blot analysis
SCC13 cells were treated with or without AL (10, 30,
100 or 300 µM) at 37°C for 48 h. Total cellular proteins from SCC13
cells were extracted using RIPA buffer (Beyotime Institute of
Biotechnology) supplemented with protease inhibitor (Beyotime
Institute of Biotechnology). A bicinchoninic acid protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.) was used to evaluate the
protein concentrations and 100°C water was used to heat the samples
for 10 min to denature the proteins. Then, 12% SDS-PAGE gels were
used to separate the proteins (40 µg per lane), which were then
electrophoretically transferred to PVDF membranes (EMD Millipore).
Membranes were blocked at room temperature for 1 h with 5% non-fat
milk and blotted overnight at 4°C with the following primary
antibodies (all from Cell Signaling Technology, Inc.): E-cadherin
(1:1,000; cat no. 3195), N-cadherin (1:1,000; cat no. 13116),
matrix metalloproteinase (MMP-9; 1:1,000; cat no. 13667), vimentin
(1:1,000; cat no. 12826), Bcl-2 (1:1,000; cat no. 4223), Bax
(1:1,000; cat no. 5023), phosphorylated (p)-mitogen-activated
protein kinase kinase (p-MEK; 1:1,000; cat no. 3958), MEK (1:1,000;
cat no. 8727), p-p65 (1:1,000; cat no. 3033), p65 (1:1,000; cat no.
8242) and β-actin (1:1,000; cat no. 4970). Membranes were then
incubated with the anti-rabbit IgG horseradish peroxidase-linked
secondary antibody (cat no. 7074; 1:2,000; Cell Signaling
Technology, Inc.) for 2.5 h at room temperature. Enhanced
chemiluminescence reagent (EMD Millipore) was used to visualize the
protein bands. The band density was quantified with Gel-Pro
Analyzer densitometry software (version 6.3; Media Cybernetics,
Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) was used to prepare the total RNA from
SCC13 cells according to the manufacturer's protocol. A
PrimeScript™ RT reagent kit (Takara Bio, Inc.) was used to perform
the RT. The temperature protocol for the reverse transcription
reaction was as follows: Primer annealing at 25°C for 5 min, cDNA
synthesis at 42°C for 60 min and termination at 80°C for 2 min.
qPCR analysis was carried out using SYBR Premix Ex Taq II (Takara
Bio, Inc.) following the manufacturer's instructions. The reaction
conditions for PCR were: Initial denaturation at 95°C for 10 min,
followed by 40 cycles of 15 sec at 95°C, 72°C for 30 sec and 78°C
for 1.5 min. GAPDH was used as an internal control. All PCR primer
sequences were obtained as required and listed as follows:
E-cadherin forward, 5′-CGAGAGCTACACGTTCACGG-3′ and reverse,
5′-GGGTGTCGAGGGAAAAATAGG-3′; N-cadherin forward,
5′-TTTGATGGAGGTCTCCTAACACC-3′ and reverse,
5′-ACGTTTAACACGTTGGAAATGTG-3′; MMP-9 forward,
5′-AGACCTGGGCAGATTCCAAAC3′ and reverse, 5′-CGGCAAGTCTTCCGAGTAGT-3′;
vimentin forward, 5′-GACGCCATCAACACCGAGTT-3′ and reverse,
5′-CTTTGTCGTTGGTTAGCTGGT-3′; Bcl-2, forward
5′-TTGGATCAGGGAGTTGGAAG-3′ and reverse, 5′-TGTCCCTACCAACCAGAAGG-3′
and Bax forward, 5′-CGTCCACCAAGAAGCTGAGCG-3′ and reverse,
5′-CGTCCACCAAGAAGCTGAGCG-3′. The 2−ΔΔCq quantification
method (19) was used to analyze the
relative gene expression.
Statistical analysis
Data are presented as the mean ± SD. SPSS 16.0
software (SPSS, Inc.) was used to perform the statistical analysis.
One-way ANOVA test followed by Tukey's post hoc test was used to
assess the differences between multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
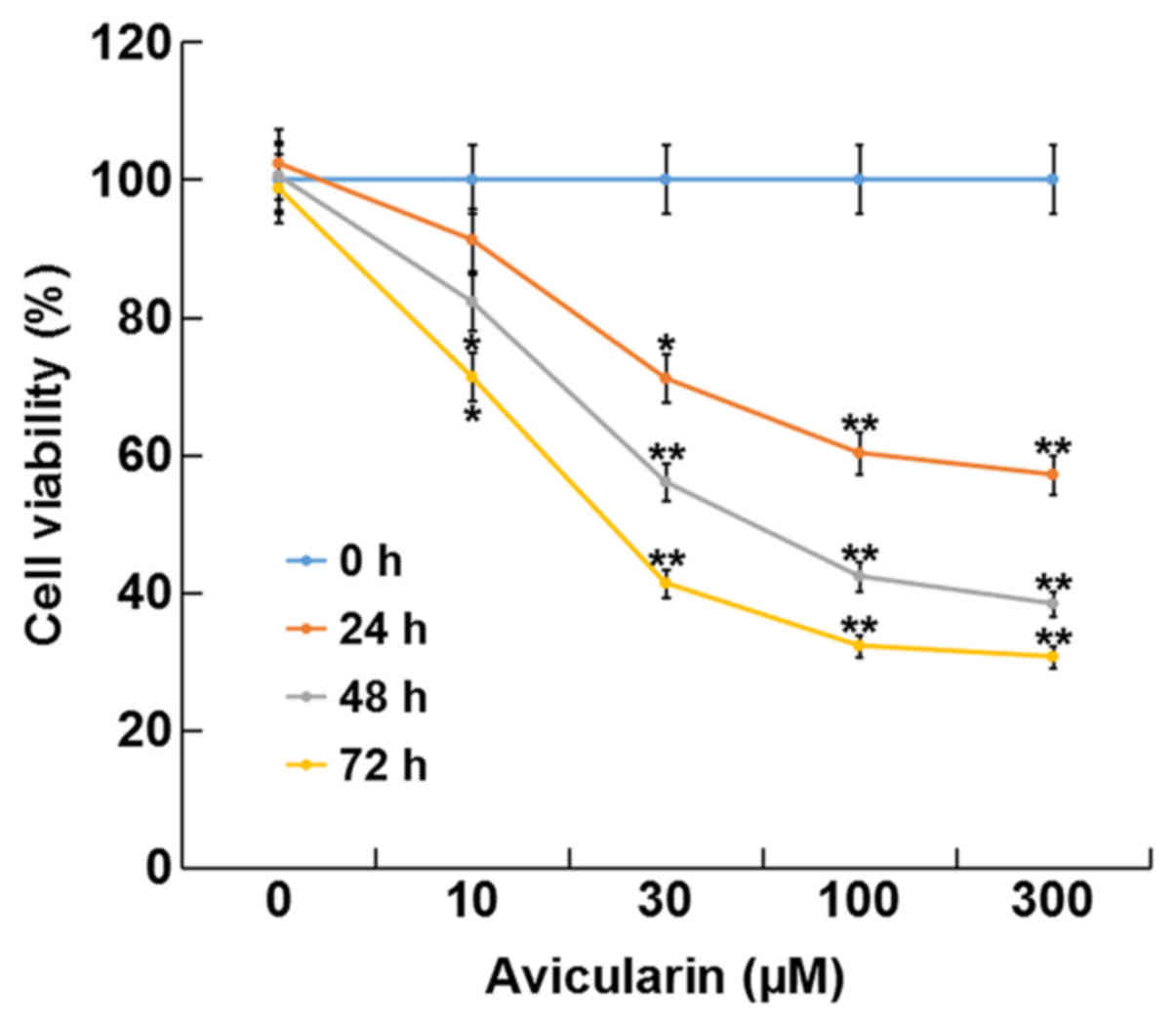
AL inhibits SCC13 cell viability
The present study investigated the effect of AL on
CSCC cell viability using a CCK-8 assay. The present results
suggested that treatment with AL at different concentrations (10,
30, 100 or 300 µM) for 24, 48 and 72 h significantly inhibited cell
viability in a dose- and time-dependent manner compared with
control cells without AL treatment (Fig.
1). The present results suggested that AL had cytotoxic effects
on SCC13 cells in a dose- and time-dependent manner. In addition,
the present results indicated that the IC50 for 48 h of
AL in the SCC13 cells was 80.27 µM.
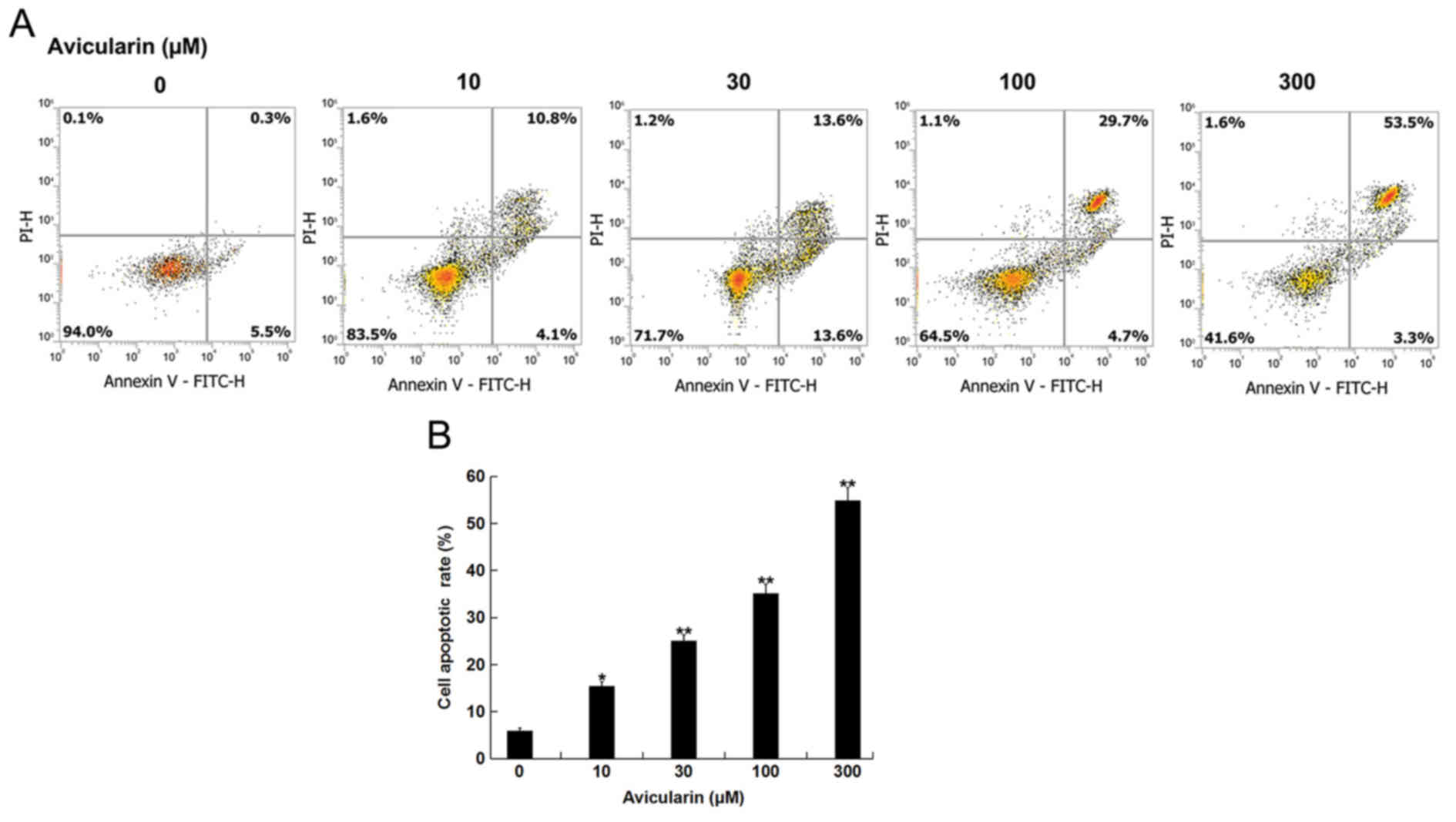
AL increases the apoptotic rate of
SCC13 cells
The present study investigated whether AL affected
CSCC cell apoptosis. SCC13 cells were treated with different
concentrations of AL (10, 30, 100 or 300 µM) for 48 h, and then
cell apoptosis was analyzed. AL dose-dependently increased the
apoptotic ratio of SCC13 cells (Fig.
2). Therefore, the present results suggested that AL increased
CSCC cell apoptosis.
Avicularin regulates the
epithelial-mesenchymal transition (EMT) of SCC13 cells
To investigate whether AL has a role in EMT
regulation, the present study evaluated the expression levels of
the EMT markers E-cadherin, N-cadherin, MMP-9 and vimentin. The
present results suggested that AL increased E-cadherin protein
expression level and decreased N-cadherin, MMP-9 and vimentin
protein expression levels in SCC13 cells in a dose-dependent manner
(Fig. 3A-E). Similar results for the
mRNA expression levels were indicated by RT-qPCR analysis (Fig. 3F-I).
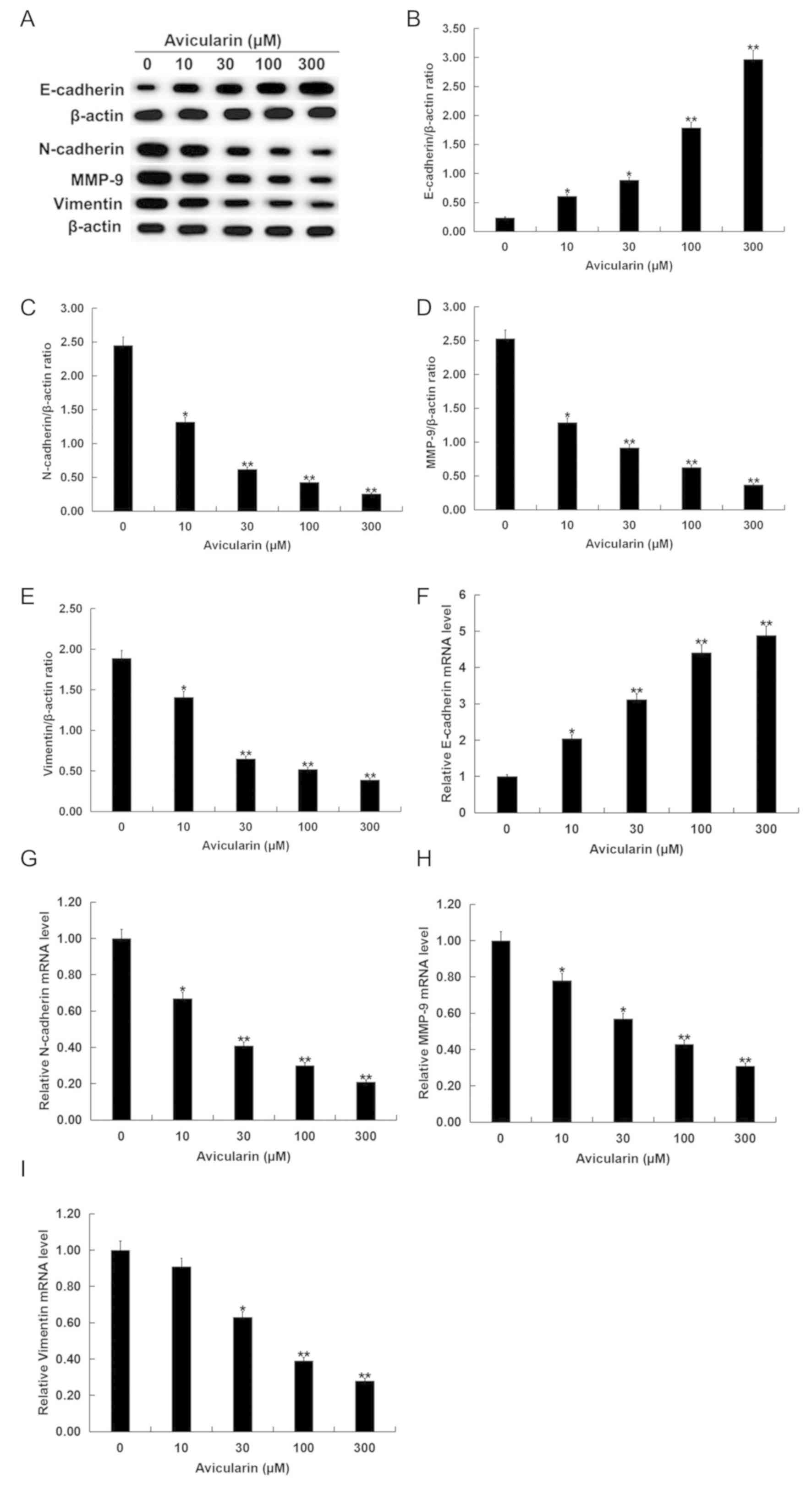 | Figure 3.Avicularin regulates the expression
levels of EMT-related genes in SCC13 cells. SCC13 cells were
treated with different concentrations of avicularin (0, 10, 30, 100
or 300 µM) for 48 h. (A) Western blot analysis results of the
protein expression levels of EMT-related genes. Quantification of
the protein expression levels of (B) E-cadherin, (C) N-cadherin,
(D) MMP-9 and (E) vimentin. Reverse-transcription-quantitative PCR
results of the mRNA expression levels of (F) E-cadherin, (G)
N-cadherin, (H) MMP-9 and (I) vimentin. Each experiment was
performed at least three times. Data are presented as the mean ±
SD. *P<0.05, **P<0.01 vs. 0 µM avicularin. MMP-9, matrix
metalloproteinase-9; EMT, epithelial-mesenchymal transition. |
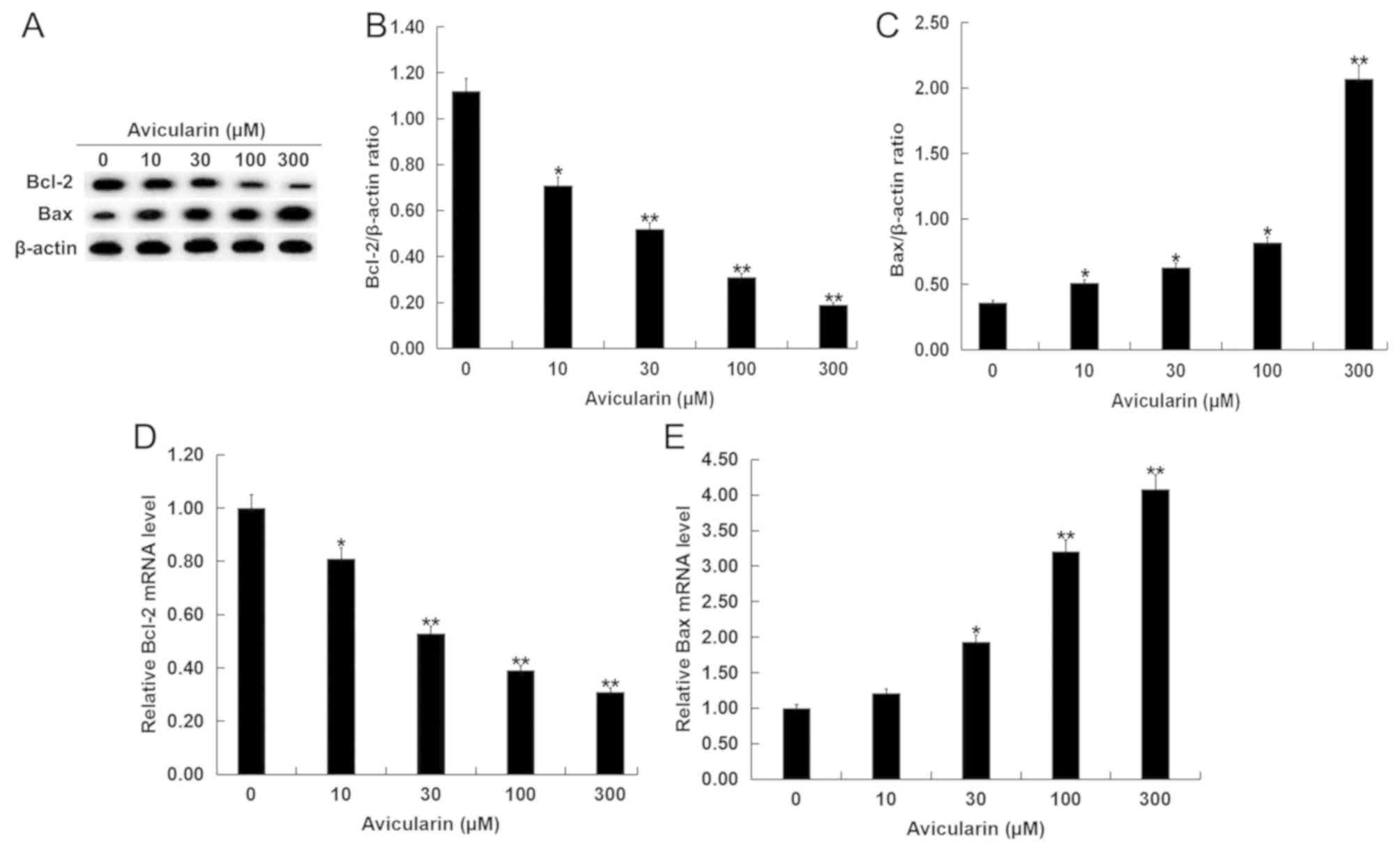
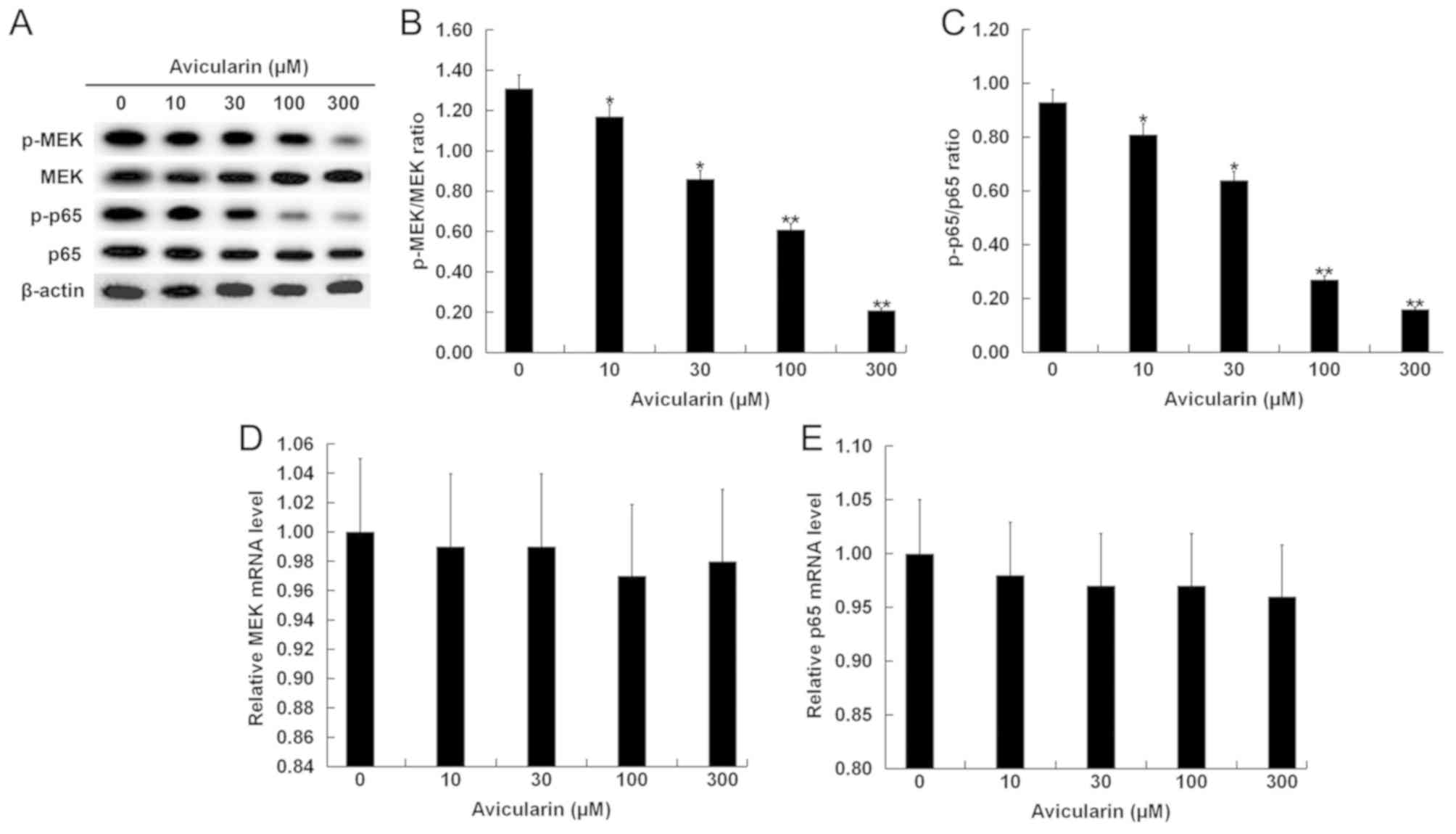
Avicularin regulates the expression
levels of apoptosis-related genes and suppresses the MEK/NF-κB
signaling pathway
The present study investigated the molecular
mechanisms by which AL may affect SCC13 cells. SCC13 cells were
treated with different concentrations of AL for 48 h, and then the
expression levels of Bcl-2 and Bax and the relative proteins in
MEK/NF-κB signaling pathway were measured. The present results
suggested that Bcl-2, which plays an important role in apoptosis
(20), was decreased in SCC13 cells
after AL treatment (Fig. 4A and B).
In addition, Bax protein expression level was significantly
increased by AL treatment in a dose-dependent manner (Fig. 4A and C). A similar result was
identified at the mRNA expression level of Bcl-2 and Bax (Fig. 4D and E). Moreover, the expression
levels of the MEK/NF-κB signaling-related proteins p-MEK and p-p65
were significantly decreased (Fig.
5A-C), while AL treatment had no significant effect on the mRNA
expression levels of MEK and p65 in SCC13 cells (Fig. 5D and E).
Discussion
A large number of flavonoids, which are natural
plant polyphenols, are found in fruits and vegetables (11–14).
Naturally occurring flavonoids are a promising source for drug
development, as they have been shown to have anticancer,
antioxidant, anti-inflammatory and neuroprotective effects
(18,21). Aglycone forms of flavonoid exhibit a
more potent free radical scavenging activity compared with their
glycoside forms (22). However, the
biological activities of AL remain largely unknown. Quercetin has
been shown to have a variety of beneficial effects such as
suppression of neuronal apoptosis (23) and adipogenesis (24). Currently, the effect of AL on CSCC is
not fully understood. Therefore, the present study investigated the
effect of AL on CSCC to understand its biological activity and
potentially facilitate the development of a theoretical basis for
the treatment of CSCC.
The present study investigated the impact of AL on
SCC13 cell viability and apoptosis. The present results suggested
that AL inhibited cell viability and induced apoptosis in
dose-dependent manner, indicating that AL induced cytotoxicity and
apoptosis in CSCC cells. Subsequently, RT-qPCR and western blot
analysis were used to detect the expression levels of genes related
to EMT, including N-cadherin, E-cadherin, MMP-9 and vimentin to
evaluate whether AL had an effect on the EMT of SCC13 cells. The
present results suggested that AL increased E-cadherin expression
level and decreased N-cadherin, MMP-9 and vimentin expression
levels in a dose-dependent manner; therefore, AL may regulate some
biological functions in CSCC cells. Apoptosis is the main molecular
mechanism of chemotherapy-induced cell death (25). Thus, defects in apoptosis can enhance
resistance to chemotherapy and increase the survival rate of cancer
cells (26). Bax is a major
pro-apoptotic member of the Bcl-2 family, which regulates apoptosis
in both cancer and healthy cells (27). Moreover, Bcl-2 is an essential
antiapoptotic signal that can contribute to tumor growth (28,29). To
investigate whether AL was involved in the regulation of CSCC cell
apoptosis, the present study analyzed the expression levels of Bax
and Bcl-2 in SCC13 cells. The present results suggested that AL
reduced Bcl-2 expression level, but increased Bax expression level
in cells; therefore AL may induce CSCC cell apoptosis by regulating
Bcl-2 and Bax. The present study investigated the signaling
pathways involved in the effect of AL on CSCC by examining the
expression levels of p-MEK and p-p65, which are associated with the
MEK/NF-κB signal pathway (30,31). The
present results suggested that AL inhibited the activation of the
MEK/NF-κB signaling pathway, thus AL may have an anticancer role in
CSCC.
In summary, the present results suggested that AL
inhibited cell viability, induced apoptosis and prevented SCC13
cell EMT in a dose-dependent manner. The present results indicated
that AL may repress the MEK/NF-κB signaling pathway to regulate
cell apoptosis-related and EMT-related genes in SCC13 cells. The
present results may provide a theoretical basis for AL treatment of
patients with CSCC. However, the present study is only a
preliminary study of the effect of AL on CSCC, and further research
is needed in other CSCC cell lines and in in vivo
experiments. In addition, the IC50 of AL in CSCC cells
should be determined and the molecular mechanism of AL inhibition
of the MEK/NF-κB requires further investigation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data sets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
YW contributed to study design, data collection,
statistical analysis, data interpretation and manuscript
preparation. MZL and SGC contributed to data collection and
statistical analysis. QW contributed to data collection,
statistical analysis and manuscript preparation. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McGuire JF, Ge NN and Dyson S: Nonmelanoma
skin cancer of the head and neck I: Histopathology and clinical
behavior. Am J Otolaryngol-Head Neck Med Surg. 30:121–133.
2009.
|
|
2
|
Martinez JC and Cook JL: High-risk
cutaneous squamous cell carcinoma without palpable lymphadenopathy:
Is there a therapeutic role for elective neck dissection? Dermatol
Surg. 33:410–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinberg AS, Ogle CA and Shim EK:
Metastatic cutaneous squamous cell carcinoma: An update. Dermatol
Surg. 33:885–899. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Venables ZC, Nijsten T, Wong KF, Autier P,
Broggio J, Deas A, Harwood CA, Hollestein LM, Langan SM, Morgan E,
et al: Epidemiology of basal and cutaneous squamous cell carcinoma
in the U.K. 2013–15: A cohort study. Br J Dermatol. 181:474–482.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trakatelli M, Ulrich C, Del Marmol V,
Euvrard S, Stockfleth E and Abeni D: Epidemiology of nonmelanoma
skin cancer (NMSC) in Europe: Accurate and comparable data are
needed for effective public health monitoring and interventions. Br
J Dermatol. 156:1–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alam M and Ratner D: Cutaneous
squamous-cell carcinoma. N Engl J Med. 344:975–983. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Langer CJ: Targeted therapy in head and
neck cancer: State of the art 2007 and review of clinical
applications. Cancer. 112:2635–2645. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trodello C, Pepper JP, Wong M and Wysong
A: Cisplatin and cetuximab treatment for metastatic cutaneous
squamous cell carcinoma: A systematic review. Dermatol Surg.
43:40–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang L, Wu S, Zhang Q, Liu F and Wu P:
23,24-Dihydrocucurbitacin B induces G2/M cell-cycle arrest and
mitochondria dependent apoptosis in human breast cancer cells
(Bcap37). Cancer Lett. 256:267–278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buqui GA, Gouvea DR, Sy SK, Voelkner A,
Singh RS, da Silva DB, Kimura E, Derendorf H, Lopes NP and Diniz A:
Pharmacokinetic evaluation of avicularin using a model-based
development approach. Planta Med. 81:373–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujimori K and Shibano M: Avicularin: A
plant flavonoid, suppresses lipid accumulation through repression
of C/EBPα-activated GLUT4-mediated glucose uptake in 3T3-L1 cells.
J Agric Food Chem. 6:5139–5147. 2013. View Article : Google Scholar
|
|
13
|
Vo VA, Lee JW, Chang JE, Kim JY, Kim NH,
Lee HJ, Kim SS, Chun W and Kwon YS: Avicularin inhibits
lipopolysaccharide-induced inflammatory response by suppressing ERK
phosphorylation in RAW 264.7 macrophages. Biomol Ther. 20:5322012.
View Article : Google Scholar
|
|
14
|
Zhang WM, Li RF, Sun M, Hu DM, Qiu JF and
Yan YH: UPLC-MS/MS method for determination of avicularin in rat
plasma and its application to a pharmacokinetic study. J Chromatogr
B Analyt Technol Biomed Life Sci. 965:107–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu X, Ouyang W, Miao J, Xiong P, Feng K,
Li M, Cao Y and Xiao H: Dietary avicularin alleviated type 2
diabetes in mice. FASEB J. 31:46–47. 2017.
|
|
16
|
Srivastava S, Somasagara RR, Hegde M,
Nishana M, Tadi SK, Srivastava M, Choudhary B and Raghavan SC:
Quercetin, a natural flavonoid interacts with DNA, arrests cell
cycle and causes tumor regression by activating mitochondrial
pathway of apoptosis. Sci Rep. 6:240492016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Zheng H, Zheng M, Liu X and Yu J:
Protective effect of avicularin on rheumatoid arthritis and its
associated mechanisms. Exp Ther Med. 16:5343–5349. 2018.PubMed/NCBI
|
|
18
|
Ghasemzadeh A and Ghasemzadeh N:
Flavonoids and phenolic acids: Role and biochemical activity in
plants and human. J Med Plants Res. 5:6697–6703. 2011.
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schenk RL, Strasser A and Dewson G: BCL-2:
Long and winding path from discovery to therapeutic target. Biochem
Biophys Res Commun. 482:459–469. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharififar F, Dehghn-Nudeh G and
Mirtajaldini M: Major flavonoids with antioxidant activity from
Teucrium polium L. Food Chem. 112:885–888. 2009. View Article : Google Scholar
|
|
22
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suematsu N, Hosoda M and Fujimori K:
Protective effects of quercetin against hydrogen peroxide-induced
apoptosis in human neuronal SH-SY5Y cells. Neurosci Lett.
504:223–227. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahn J, Lee H, Kim S, Park J and Ha T: The
anti-obesity effect of quercetin is mediated by the AMPK and MAPK
signaling pathways. Biochem Biophys Res Commun. 373:545–549. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cotter TG: Apoptosis and cancer: The
genesis of a research field. Cancer. 9:501–507. 2009.PubMed/NCBI
|
|
26
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim H, Tu HC, Ren D, Takeuchi O, Jeffers
JR, Zambetti GP, Hsieh JJ and Cheng EH: Stepwise activation of BAX
and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis.
Mol Cell. 36:487–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leverson JD, Phillips DC, Mitten MJ,
Boghaert ER, Diaz D, Tahir SK, Belmont LD, Nimmer P, Xiao Y, Ma XM,
et al: Exploiting selective BCL-2 family inhibitors to dissect cell
survival dependencies and define improved strategies for cancer
therapy. Sci Transl Med. 7:279ra402015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han L, Zhang EB, Yin DD, Kong R, Xu TP,
Chen WM, Xia R, Shu YQ and De W: Low expression of long noncoding
RNA PANDAR predicts a poor prognosis of non-small cell lung cancer
and affects cell apoptosis by regulating Bcl-2. Cell Death Dis.
6:e16652015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van der Noord VE, McLaughlin RP, Smid M,
Foekens JA, Martens JWM, Zhang Y and van de Water B: An increased
cell cycle gene network determines MEK and Akt inhibitor double
resistance in triple-negative breast cancer. Sci Rep. 9:133082019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|