Introduction
Acetaminophen (APAP) is an effective analgesic and
antipyretic (1,2). APAP is safe at therapeutic doses, and
higher doses can be provided to patients for short durations
(3). However, the incidence of APAP
poisoning is increasing (4). In the
United States and in the United Kingdom, APAP overdose has been
reported as a major cause of drug-induced liver failure (5,6). Since
APAP overdose causes severe damage to liver and kidney cells in
humans and experimental animals (7,8), a
number of studies have focused on the prevention or treatment of
APAP-induced liver failure (9,10).
The mechanism of APAP-induced hepatotoxicity has
been established and extensively reviewed (11,12). The
liver failure that follows APAP ingestion is not due to the drug
itself, but to a toxic metabolite, N-acetyl-p-benzoquinone imine,
produced by the cytochrome P450 group of enzymes in the liver. This
metabolite is normally rendered harmless through its interaction
with glutathione (GSH), an endogenous antioxidant (11,12)
However, when this APAP metabolite is overproduced, GSH stores in
the liver become depleted, the metabolite accumulates, and tissue
injury occurs (13). As a result,
APAP overdose stimulates the apoptotic and/or necrotic death
signaling pathways in cellular models (14,15).
Additionally, APAP overdose increases oxidative stress and reactive
oxygen species (ROS) levels, decreases GSH levels, induces
mitogen-activated protein kinase (MAPK) signaling pathways, and
activates caspase cascades (15–17).
Furthermore, APAP overdose leads to liver failure, promoting lipid
peroxidation and transcriptional activation of inflammatory factors
(11,18). Based on this mechanism, several
inhibitors of APAP overdose-induced liver and kidney failure, such
as N-acetylcysteine (NAC) have been developed (19).
Seaweeds have received increased research attention,
since the majority contain polysaccharides, proteins, vitamins and
minerals with diverse biological activities (20). Pyropia yezoensis, a marine red
alga, is cultured and consumed mainly in China, Japan and Korea
(21). P. yezoensis produces
free radicals and other potent oxidizing agents without causing
serious photodynamic damage if exposed to adverse environmental
conditions, such as a high light intensity or oxygen concentration
(22,23). Therefore, P. yezoensis
produces compounds that protect against external factors, including
environmental pollutants, stresses and UV radiation (22,23).
P. yezoensis has antioxidant (24,25),
antitumor (26,27) and anti-inflammatory activities
(28,29), and protects against neuronal
senescence (30,31), photoaging (22,23) and
cytotoxicity (32,33).
A 14-kDa glycoprotein extracted from P.
yezoensis reportedly protects against hepatotoxicity in rats
with APAP-induced liver injury (33). After the protein is purified from the
glycoprotein by protein sequencing and mass spectrometry, 10- and
7-kDa proteins are obtained (34).
Treatment of the 10-kDa protein (protein ID PYP1; Rhod_EST
AV429545) with digestive enzymes, including chymotrypsin, pepsin
and trypsin, yields several peptides, which have been screened to
identify those with protective effects (34).
Studies on the protective effects of P.
yezoensis peptides in APAP-induced hepatotoxicity have produced
inconclusive results (33,34). Therefore, the present study
investigated the protective effects of P. yezoensis peptides
on APAP-induced liver injury in HepG2 human liver cancer cells, as
well as the underlying molecular mechanisms.
Materials and methods
Peptide synthesis
The peptide PYP1-4 (A-T-R-D-P-E-P-T-A-V-D-P-N) from
P. yezoensis was commercially synthesized by Peptron
Corporation and purified to >95% purity. PYP1-4 was purified
using a Shimadzu Prominence high-performance liquid chromatography
system with a C18 column (Capcell Pak; Shiseido Co., Ltd.), using
the Class-VP software (version 6.14; Shimadzu Corporation). PYP1-4
was first dissolved in 0.1% trifluoroacetic acid/water at 1 mg/ml
and 40 µl of the solution was then injected into the HPLC system.
The HPLC system condition was as follows: Acetonitrile gradient,
10–40%; flow rate, 1 ml/min, temperature, 50°C; and UV detection,
220 nm. The molecular weight of PYP1-4 was 1,382 Da as determined
by mass spectrometry (HP 1100 Series LC/MSD; Agilent Technologies,
Inc.) using ionization mode (positive + H, 1.0079 Da; negative - H,
−1.0079 Da) and multiple reaction monitoring (300–2,300 m/z). The
synthesized peptides was reconstituted in water (10 mg/ml) and
stored at −50°C.
Cell culture
HepG2 liver cancer cells (cat. no. HB-8065) were
purchased from the American Type Culture Collection. The cells were
cultured at 37°C with 5% CO2 in minimum essential medium
(MEM; Sigma-Aldrich; Merck KGaA) supplemented with 10% FBS
(GenDEPOT) containing 50 µg/ml penicillin, 25 µg/ml amphotericin B
and 50 µg/ml streptomycin. The medium was replaced every 2
days.
Cell viability assay
Cell viability was estimated using a Cyto X Cell
Viability Assay kit (cat. no. CYT3000; LPS solution). Cells were
seeded in 96-well plates at 2×104 cells/well in 100 µl
medium and allowed to attach for 24 h at 37°C. Attached cells were
then treated with PYP1-4 (125, 250 or 500 ng/ml) and 15 mM APAP
(A7085; Sigma-Aldrich; Merck KGaA) in serum-free MEM (SFM) for 18 h
at 37°C. Cyto X solution was added to the cells, followed by
incubation for 1 h at 37°C and the absorbance at a wavelength of
450 nm was measured using a FilterMAX F5 microplate reader
(Molecular Devices LLC). Morphological changes to the cells were
subsequently observed using a light microscope (magnification,
×200; Eclipse TS100-F; Nikon Corporation).
Nitric oxide (NO) assay
The nitrite concentration in culture medium was
determined spectrophotometrically as described previously by Lee
et al (29). Briefly, cells
were seeded in 48-well plates at 2×106 cells/well and
incubated for 24 h at 37°C. The cells were treated with PYP1-4
(125, 250 or 500 ng/ml) and 15 mM APAP in SFM for 18 h at 37°C.
Subsequently, 100 µl culture medium were transferred to a 96-well
plate, and 100 µl Griess reagent (G4410; Sigma-Aldrich; Merck KGaA)
was added. The plate was incubated for 10 min at 37°C, following
which absorbance at a wavelength of 540 nm was measured using a
FilterMAX F5 microplate reader.
Intracellular ROS assay
The intracellular ROS concentration was assayed
using the ROS-sensitive fluorescent dye 2′,7′-dichlorofluorescein
diacetate (DCF-DA; cat. no. 35845; Sigma-Aldrich; Merck KGaA).
Cells were seeded in 96-well plates at 2×105 cells/well
and incubated for 24 h at 37°C. The cells were treated with PYP1-4
(125, 250 or 500 ng/ml) and 15 mM APAP in SFM for 18 h at 37°C.
Subsequently, the cells were incubated with 10 µM DCF-DA at 37°C
for 30 min. The fluorescence intensities of stained cells were
measured using a FilterMAX F5 microplate reader at excitation and
emission wavelengths of 485 and 535 nm, respectively.
Apoptosis assay
Apoptosis was assayed using the Muse®
Annexin V and Dead Cell Assay Kit (cat. no. MCH100105; BD
Biosciences). The cells were harvested and washed twice with PBS,
and stained with FITC Annexin V and propidium iodide for 15 min at
room temperature. The percentage of apoptotic cells was determined
using Annexin V and dead cell software program of Muse™ Cell
Analyzer system (2013; EMD Milipore).
Western blot analysis
HepG2 cells were cultured for 18 h at 37°C in SFM
containing 0, 125, 250 or 500 ng/ml PYP1-4 and 15 mM APAP. The
cells were washed with PBS and lysed in RIPA lysis buffer (50 mM
Tris-HCl, 1 mM EDTA, 150 mM sodium chloride, 1% NP-40 and 0.25%
sodium deoxycholate; pH 7.4) containing protease inhibitors (1
µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin, 1 mM sodium
orthovanadate, 1 mM sodium fluoride and 1 mM
phenylmethanesulfonylfluoride) on ice for 30 min. The extracts were
centrifuged at 12,000 × g for 10 min at 4°C, and the supernatants
were used for western blot analysis. Protein concentration was
measured using the Bicinchoninic Acid protein assay kit. Total
protein (30 µg) was electrophoresed using a 10–15% acrylamide gel
and transferred to PVDF transfer membranes (EMD Millipore). The
membranes were blocked with 1% bovine serum albumin (BSA; GenDEPOT)
in TBST (5 mM Tris and 20 mM sodium chloride; pH 7.4; 0.1%
Tween-20) and incubated with primary antibodies (dilution, 1:1,000)
in 1% BSA-TBST with gentle agitation at 4°C overnight. The
membranes were washed twice for 15 min each in TBST, incubated with
the corresponding horseradish peroxidase (HRP)-conjugated secondary
antibody (dilution, 1:10,000) for 2 h at room temperature, and then
washed. Immunoreactive bands were detected using an enhanced
chemiluminescence substrate (Advansta Inc.) and visualized using
the GeneSys imaging system (SynGene; Synoptics Ltd.). Differences
in protein levels were determined by semi-quantifying western blot
band densities using ImageJ software (version IJ.146r; National
Institutes of Health). The primary antibodies used in the present
study are as follows: Anti-catalase (CAT; cat. no. OAAB05216;
rabbit), anti-superoxide dismutase 1 (cat. no. OASE00355; rabbit),
anti-superoxide dismutase 2 (SOD2; cat. no. OASE00357; rabbit;
Aviva Systems Biology Corporation), anti-heme oxygenase 1 (HO1;
cat. no. sc-1796; goat), anti-quinone oxidoreductase 1 (NQO1; cat.
no. sc-16464; goat), anti-nuclear factor, erythroid 2 like 2 (Nrf2;
cat. no. sc-722; rabbit), anti-phosphorylated-(p-)JNK (cat. no.
sc-6254; mouse), anti-JNK (cat. no. sc-7345; mouse), anti-p- p38
MAP kinase (p38; cat. no. sc-7973; mouse), anti-p38 (cat. no.
sc-7149; rabbit), anti-p-glycogen synthase kinase 3β (GSK3β; cat.
no. sc-81496; mouse), anti-GSK3β (cat. no. sc-7291; mouse),
anti-p-AMP-activated protein kinase (AMPK; cat. no. sc-33524;
rabbit), anti-AMPK (cat. no. sc-74461; mouse), anti-Bcl-2 (cat. no.
sc-492; rabbit), anti-Bcl-xL (cat. no. sc-7195; rabbit), anti-BH3
interacting domain death agonist (Bid; cat. no. sc-11423; rabbit),
anti- poly (ADP-ribose) polymerase 1 (PARP; cat. no. sc-7150;
rabbit), anti-caspase-9 (cat. no. sc-7885; rabbit), anti-caspase-3
(cat. no. sc-7148; rabbit), anti-Bad (cat. no. sc-7869; rabbit),
anti-Bax (cat. no. sc-493; rabbit), anti-insulin-like growth factor
1 receptor (IGF-IR; cat. no. sc-390130; mouse), anti-epidermal
growth factor receptor (EGFR; cat. no. sc-03; goat), anti-erb-b2
receptor tyrosine kinase 2 (ErbB2; cat. no. sc-284; rabbit),
anti-erb-b2 receptor tyrosine kinase 3 (ErbB3; cat. no. sc-285;
rabbit), anti-insulin receptor substrate 1 (IRS-1; cat. no. sc-560;
rabbit), anti-PI3K (cat. no. sc-374534; mouse), anti-PTEN (cat. no.
sc-7974; mouse), anti-pyruvate dehydrogenase kinase 1 (cat. no.
sc-28783; rabbit), anti-p-Akt (cat. no. sc-7985; rabbit), anti-Akt
(cat. no. sc-8312; rabbit), anti-p-mTOR (cat. no. sc-293132;
mouse), anti-mTOR (cat. no. sc-8319; rabbit), anti- p70S6 kinase
(p70S6K; cat. no. sc-8418; mouse), anti-eukaryotic translation
initiation factor 4E (elF4E; cat. no. sc-514875; mouse), anti-SHC
adaptor protein 1 (SHC; cat. no. sc-967; mouse), anti-growth factor
receptor bound protein 2 (GRB2; cat. no. sc-255; rabbit), anti-SOS
Ras/Rac guanine nucleotide exchange factor 1 (SOS; cat. no. sc-259;
rabbit), anti-Ras (cat. no. sc-520; rabbit), anti-Raf (cat. no.
sc-227; rabbit), anti-p-mitogen-activated protein kinase kinase
(MEK; cat. no. sc-81503; mouse), anti-MEK (cat. no. sc-81504;
mouse), anti-p-ERK (cat. no. sc-7383; mouse), anti-ERK (sc-292838;
rabbit, Santa Cruz Biotechnology, Inc.). The anti-β-actin (cat. no.
sc-47778; mouse) antibody was used as a control. The secondary
antibodies were HRP-conjugated anti-mouse IgG (cat. no. 32430),
anti-rabbit IgG (cat. no. 31460) and anti-goat IgG (cat. no. 31400;
Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse
transcription-semi-quantitative PCR
Total RNA was extracted from HepG2 cells using a
QIAzol Lysis Reagent kit (Qiagen Sciences, Inc.). Reverse
transcription was performed using AccuPower RT PreMix (Bioneer
Corporation) according to the manufacturer's protocol. PCR
amplification was performed using the template cDNA (1 ng). The
reverse transcribed cDNA was amplified using a PCR premix kit (dNTP
mix, nTaq Buffer and nTaq; Enzynomics), and the
following specific primer pairs (Cosmogenetech Co., Ltd.) were
used: IGF-IR forward, 5′-ACAACTACGCCCTGGTCATC-3′ and reverse,
5′-TGGCAGCACTCATTGTTCTC-3′; EGFR forward,
5′-TGGATTCATCAGCATTTGGA-3′ and reverse, 5′-GCACCTGTAAAATGCCCTGT-3′;
ErbB2 forward, 5′-CTACGGCAGAGAACCCAGAG-3′ and reverse,
5′-ACACCATTGCTGTTCCTTCC-3′; ErbB3 forward,
5′-GCGGCACTTTTCTCTACTGG-3′ and reverse, 5′-GGTCAGCCACACCAAAATCT-3′;
and β-actin forward, 5′-AAATCTGGCACCACACCTTC-3′ and reverse,
5′-AGCACTGTGTTGGCGTACAG-3′. Reaction mixtures were subjected to
initial denaturation at 95°C for 3 min, followed by 34 cycles of
95°C for 30 sec, 55–60°C for 30 sec and 72°C for 60 sec, then a
final extension of 72°C for 5 min. The products were normalized to
β-actin as an internal control and separated by electrophoresis
using a 1% agarose gel and stained with 0.5 µg/ml ethidium bromide
for detection. Signal intensities were examined using a bio-imaging
system (MiniBis Pro; DNR Bio-Imaging Systems, Ltd.). The software
GeneTools version 4.03 (Syngene Europe) was used for densitometric
analysis.
Statistical analysis
Results are presented as the mean ± SD of three
independent experiments. The significance of differences among
multiple means was assessed by one-way or two-way ANOVA followed by
Bonferroni's multiple comparison test using GraphPad Prism software
(version 7; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
PYP1-4 protects against APAP-induced
toxicity in HepG2 cells
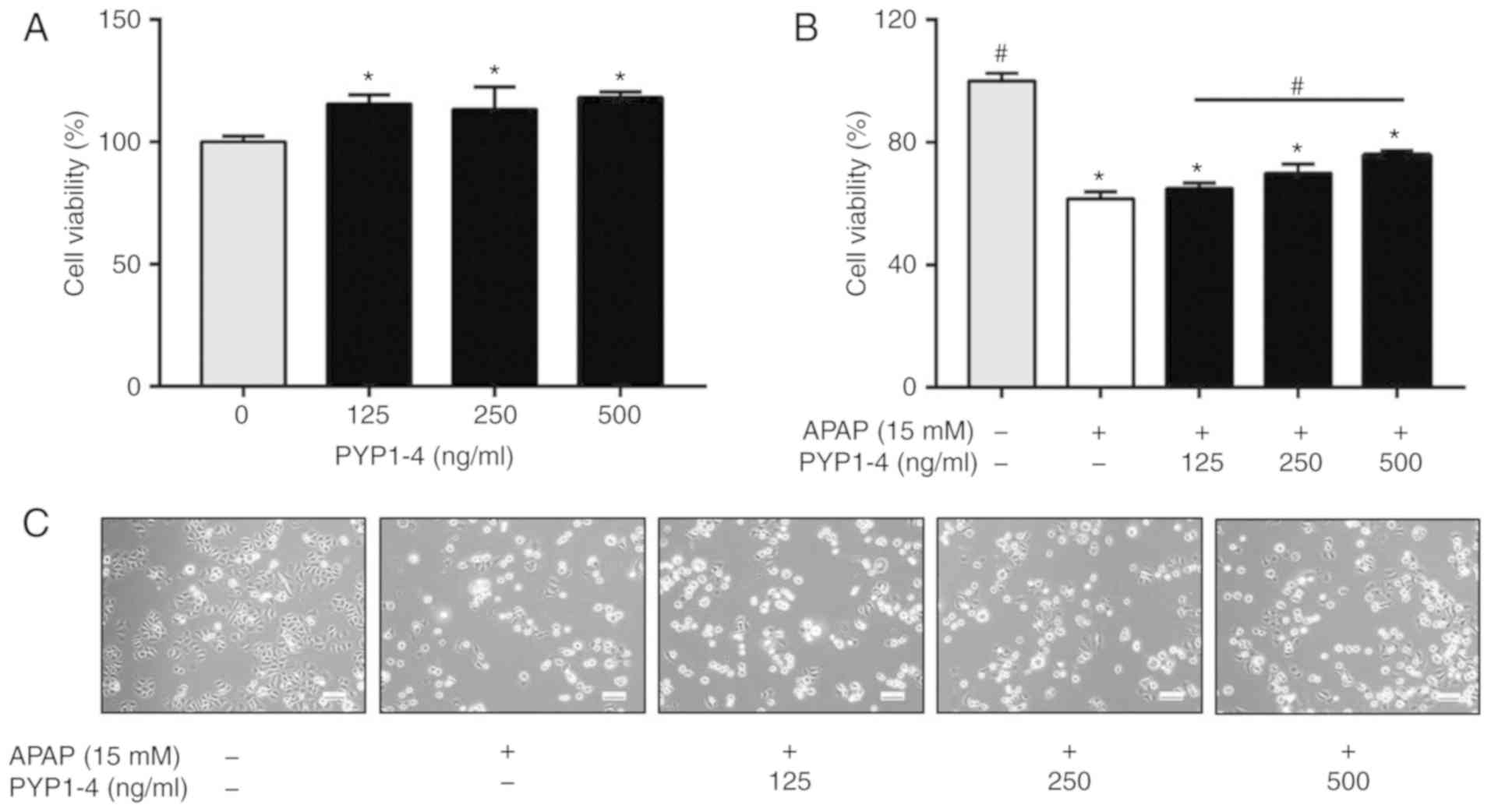
The present study assessed the effect of 0–500 ng/ml
PYP1-4 on HepG2 cell viability. Treatment with PYP1-4 alone
appeared to have significantly increased cell viability, but no
significance differences between concentrations were observed
(Fig. 1A). Subsequently, a survival
rate of 60% was selected following treatment with 15 mM APAP for 18
h. The cell viability of the APAP overdose group was 61.5±2.4%
compared with the control (Fig. 1B).
Treatment with 15 mM APAP and 125, 250 and 500 ng/ml PYP1-4
significantly restored cell viability to 64.9±1.8, 69.9±3.0 and
75.9±1.4%, respectively, compared with the control. Microscopic
observations confirmed these results (Fig. 1C).
PYP1-4 decreases APAP-induced
oxidative stress in HepG2 cells
Oxidative stress is involved in APAP-induced liver
failure, and liver tissue is damaged by various cytokines and high
levels of NO following an APAP overdose (35). Griess reagent was used to investigate
NO production in HepG2 cells treated with PYP1-4 and APAP overdose.
The APAP overdose group exhibited a significantly higher NO level
(130.5±9.9%) than the control group, whereas co-treatment with
PYP1-4 significantly suppressed the NO level in a
concentration-dependent manner (Fig.
2A). In the 500 ng/ml PYP1-4 group, NO production was reduced
to 100.2±11.8% compared with the APAP overdose group
(130.5±9.9%).
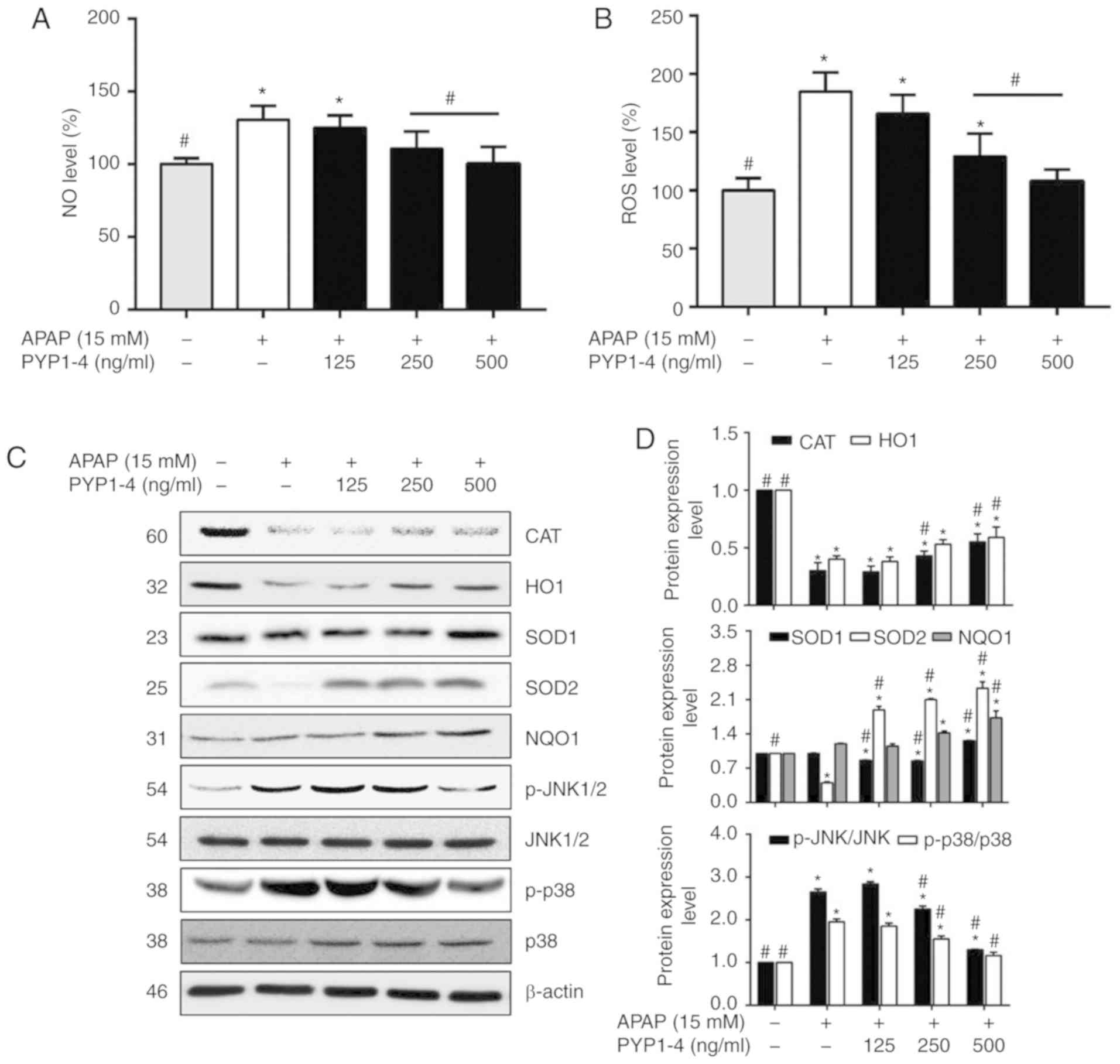 | Figure 2.PYP1-4 treatment restores
APAP-induced oxidative stress in APAP-induced HepG2 cells. (A)
HepG2 cells were incubated with 15 mM APAP with or without various
concentrations of PYP1-4 for 18 h. NO levels were analyzed using a
Griess assay. (B) ROS levels were analyzed by
2′,7′-dichlorofluorescein diacetate assay. (C) Levels of oxidative
stress-associated proteins (CAT, HO1, SOD1, SOD2, NQO1, JNK and
p38) were determined by western blot analysis. (D) Bands were
normalized to β-actin as an internal control, and the
phosphorylated vs. total protein ratio was graphed. Data are
presented as the mean ± SD of three independent experiments and
were subjected to two-way ANOVA. *P<0.05 vs. the control group;
#P<0.05 vs. the 15 mM APAP group. APAP,
acetaminophen; NO, nitric oxide; PYP1-4, P. yezoensis
peptide; ROS, reactive oxygen species; CAT, catalase; HO1, heme
oxygenase 1; SOD1, superoxide dismutase 1; SOD2, superoxide
dismutase 2; NQO1, quinone oxidoreductase 1; p-, phosphorylated;
p38, p38 MAP kinase. |
APAP-induced toxicity increases ROS levels and
promotes oxidative stress (36–38). The
present study investigated ROS levels in HepG2 cells using the
fluorescent dye DCF-DA following treatment with PYP1-4 and APAP
overdose. The ROS level was significantly higher in the APAP
overdose group (184.6±16.6%) than in the control group (Fig. 2B). However, PYP1-4 co-treatment
reduced the ROS level compared with that in the APAP overdose
group, in a concentration-dependent manner (165.9±16.1, 129.1±19.8
and 107.9±10.1% for 125, 250 and 500 ng/ml PYP1-4,
respectively).
Subsequently, the levels of antioxidant enzymes,
including CAT, HO1, SOD and NQO1 were investigated by western
blotting. The APAP overdose group exhibited lower protein levels of
CAT, HO1 and SOD2 than the control group, whereas PYP1-4
co-treatment significantly increased the CAT, HO1, SOD2 and NQO1
levels in a concentration-dependent manner (Fig. 2C and D).
To investigate the role of PYP1-4 in the modulation
of MAPK signaling in APAP-induced cells, the phosphorylation levels
of JNK and p38 were determined by western blotting. The
phosphorylation levels were 2.7-fold (JNK) and 2.0-fold (p38)
higher in the APAP overdose group than in the control group,
whereas co-treatment with PYP1-4 significantly inhibited the
phosphorylation of JNK and p38 compared APAP overdose group
(Fig. 2C and D). p-JNK/JNK
phosphorylation was significantly decreased in the PYP1-4
co-treatment groups compared with in the APAP group in a
concentration-dependent manner (2.8-, 2.3- and 1.3-fold,
respectively). Similarly, p-p38/p38 was significantly decreased
(1.9-, 1.6- and 1.2-fold, respectively).
PYP1-4 increases Nrf2 expression and
phosphorylation of GSK3β, Akt and AMPK in APAP-induced HepG2
cells
AMPK increases the inhibitory phosphorylation of
GSK3β upstream of Akt (39) and
phosphorylation of GSK3β stimulates Nrf2 (40,41). To
identify the upstream effector of activation of Nrf2 by PYP1-4, the
role of AMPK in PYP1-4-induced Akt/GSK3β phosphorylation and Nrf2
nuclear translocation was investigated in the present study. The
APAP overdose group exhibited reduced HO1 and Nrf2 levels, as well
as reduced ratios of p-GSK3β/GSK3β and p-AMPK/AMPK, compared with
the control group (Fig. 3). However,
PYP1-4 co-treatment groups significantly induced the expression and
nuclear translocation of Nrf2, as well as the phosphorylation of
GSK3β, Akt and AMPK in HepG2 cells.
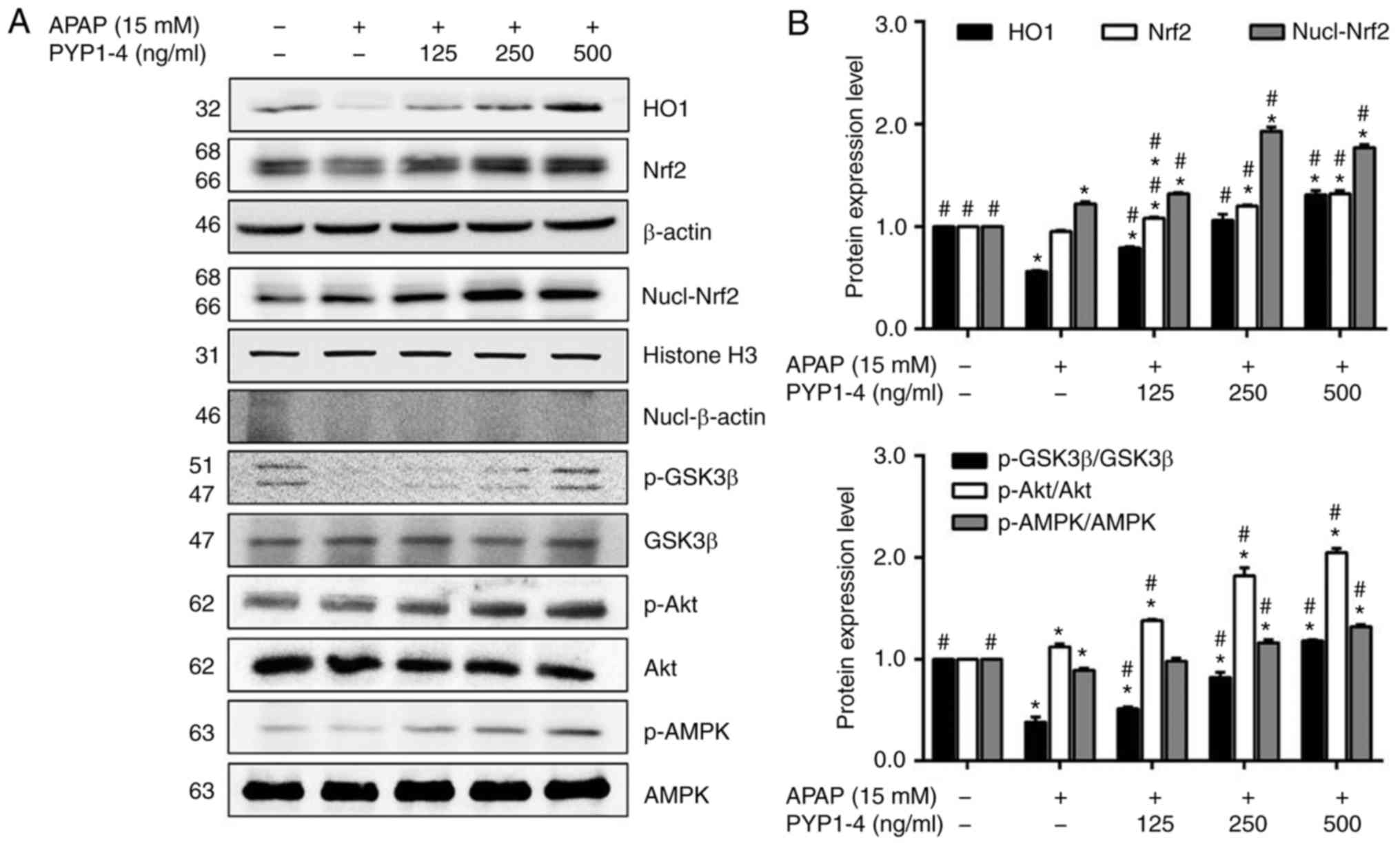 | Figure 3.Effect of PYP1-4 on AMPK activation
is required for Akt/GSK3β-mediated Nrf2 activity. (A) HepG2 cells
were incubated with 15 mM APAP with or without various
concentrations of PYP1-4 for 18 h. Levels of Nrf2-associated
proteins (HO1, Nrf2, GSK3β, Akt and AMPK) were determined by
western blot analysis. (B) Bands were normalized to β-actin and
histone H3 as internal controls, and the phosphorylated vs. total
protein ratio was graphed. Data are presented as the mean ± SD of
three independent experiments and were subjected to two-way ANOVA.
*P<0.05 vs. control group; #P<0.05 vs. 15 mM APAP
group. AMPK, protein kinase AMP-activated catalytic subunit α2;
APAP, acetaminophen; GSK3β, glycogen synthase kinase 3β; HO1, heme
oxygenase 1; Nrf2, nuclear factor, erythroid 2 like 2; p-,
phosphorylated-; PYP1-4, P. yezoensis peptide. |
PYP1-4 inhibits APAP-induced
apoptosis
Toxic APAP doses reportedly induce apoptosis of
murine hepatocytes (42,43). Therefore, the induction of apoptosis
by APAP was investigated using FITC Annexin V assays in the present
study. The apoptosis ratio was significantly higher in the APAP
overdose group (43.2%) compared with the control group (4.77%;
Fig. 4A). However, the cell survival
rate was increased and the apoptosis ratio was significantly
decreased in the PYP1-4 co-treatment groups compared with in the
APAP group in a concentration-dependent manner (34.2, 26.6 and
20.2%, respectively).
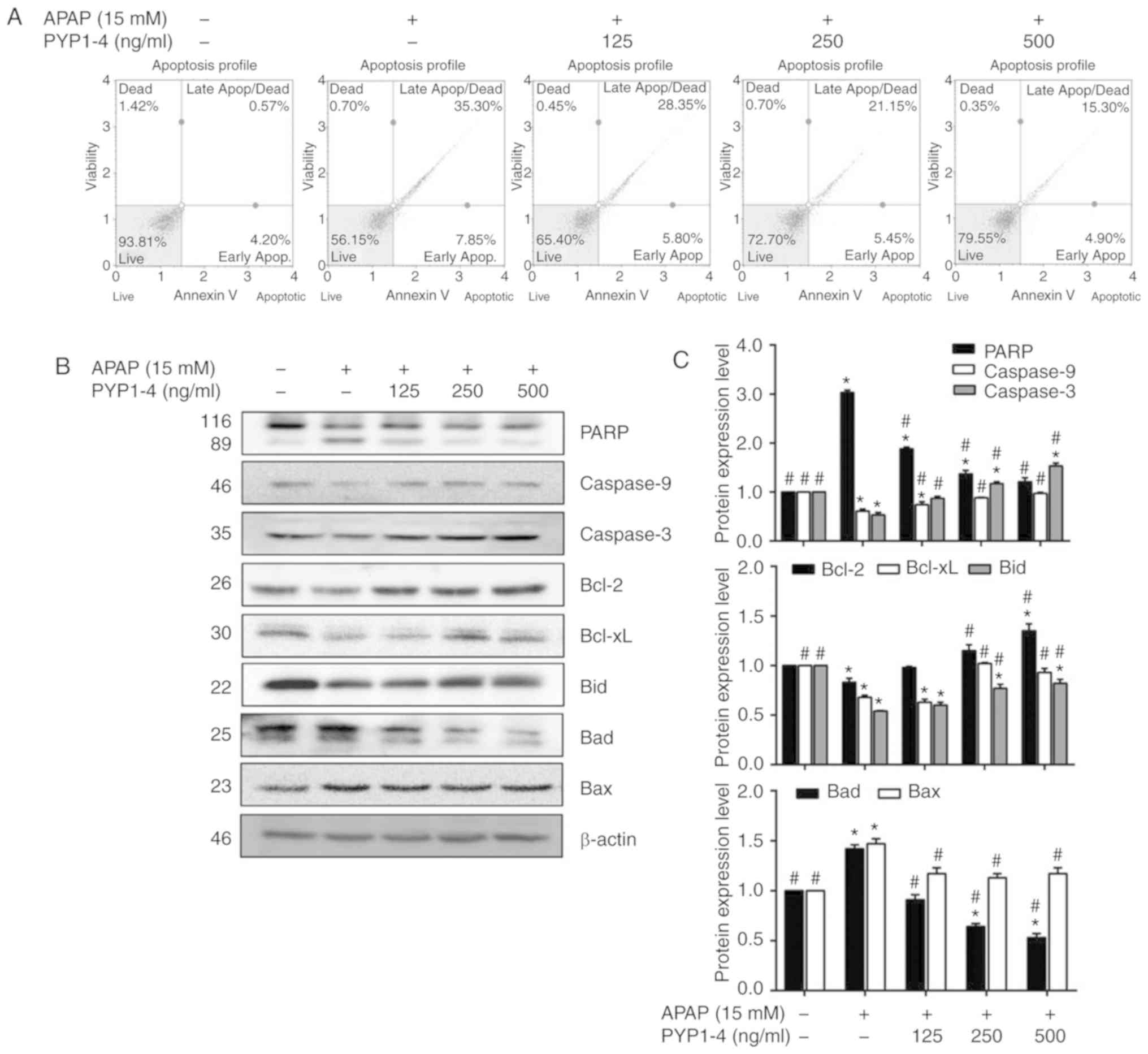 | Figure 4.PYP1-4 treatment suppresses
APAP-induced apoptosis of HepG2 cells. (A) HepG2 cells were
incubated with 15 mM APAP with or without various concentrations of
PYP1-4 for 18 h. FITC Annexin V flow cytometry was employed to
determine the percentages of apoptotic and necrotic cells. (B)
Levels of apoptosis-associated proteins (PARP, caspase-9,
caspase-3, Bcl-2, Bcl-xL, Bid, Bad and Bax) were determined by
western blot analysis. (C) Bands were normalized to β-actin as an
internal control, and protein levels were graphed. Data are
presented as the mean ± SD of three independent experiments, and
were subjected to two-way ANOVA. *P<0.05 vs. control group;
#P<0.05 vs. 15 mM APAP group. APAP, acetaminophen;
Apop, apoptosis; Bid, BH3 interacting domain death agonist; PARP,
poly(ADP-ribose) polymerase 1; PYP1-4, P. yezoensis
peptide. |
To investigate the molecular mechanism by which
PYP1-4 suppresses apoptosis, the present study examined its effect
on the expression levels of Bcl-2-family proteins, which regulate
apoptosis by controlling mitochondrial membrane permeability and
cytochrome c release (44),
in APAP-induced HepG2 cells. The levels of pro-apoptotic (Bad and
Bax) and anti-apoptotic (Bcl-2, Bcl-xL and Bid) Bcl-2-family
proteins were examined. The PYP1-4 co-treatment groups exhibited
lower Bad levels and higher Bcl-2 and Bid levels than the APAP
overdose group, in a concentration-dependent manner (Fig. 4B and C).
Additionally, the present study demonstrated that
PYP1-4 activated caspases. The PYP1-4 co-treatment groups exhibited
increased expression levels of caspase-9 and caspase-3 compared
with the APAP group in a concentration-dependent manner.
Additionally, PARP cleavage in the PYP1-4 co-treatment groups was
significantly decreased compared with that in the APAP group, in a
concentration-dependent manner (Fig. 4B
and C).
PYP1-4 reverses the effects of
overdose on the levels of growth-associated receptors
PYP1-4 co-treatment reversed the effects of APAP
overdose on apoptosis and survival. The present study assessed the
protein and RNA levels of IGF-IR, EGFR, ErbB2 and ErbB3, based on
the assumption that the increased cell survival was associated with
effects on growth-associated receptors. PYP1-4 co-treatment groups
increased the protein levels of IGF-IR and EGFR compared with APAP
overdose (Fig. 5). However, the RNA
levels of these receptors were unaffected, with the exception of
ErbB3.
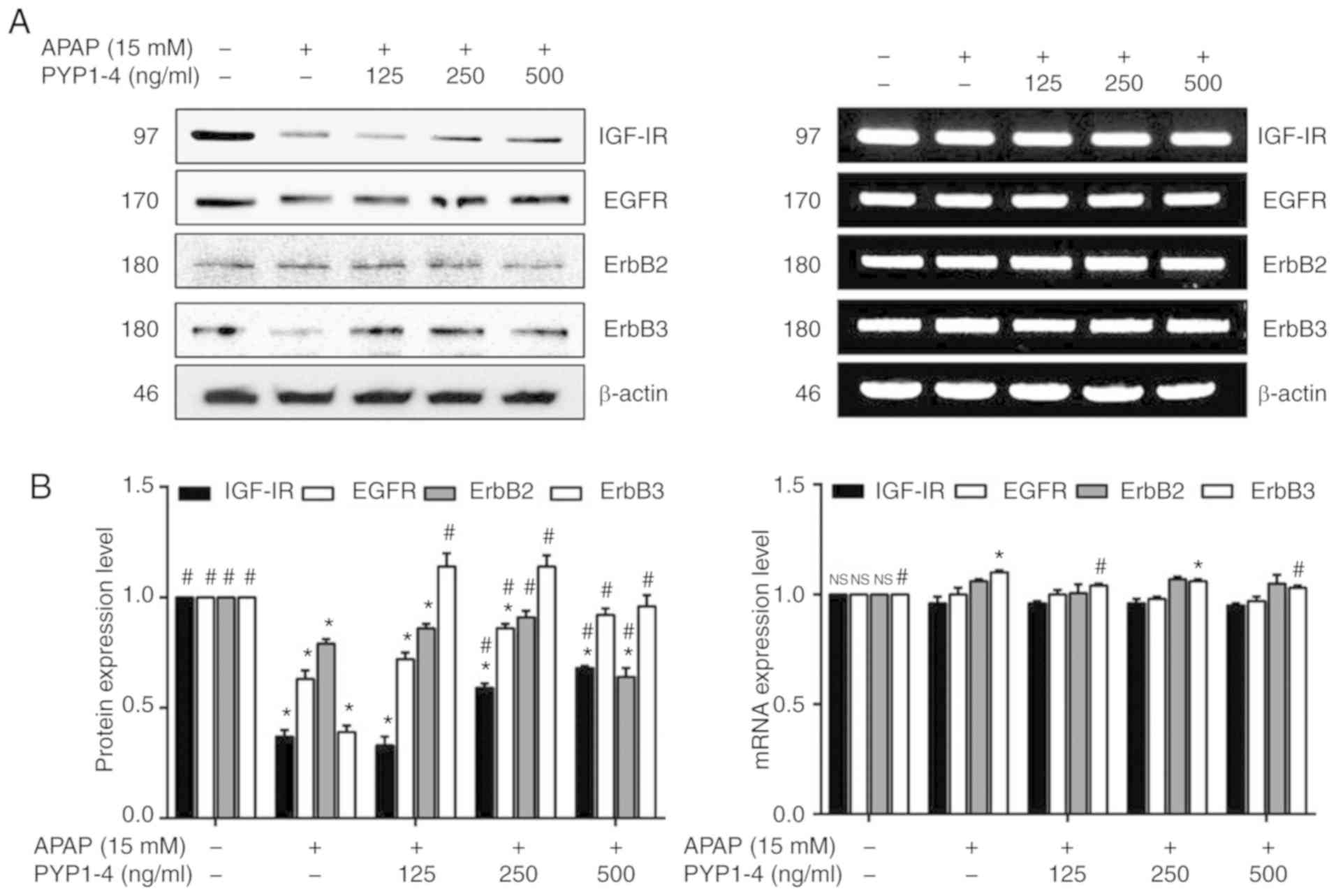 | Figure 5.PYP1-4 treatment restores the levels
of growth-associated factors in APAP-induced HepG2 cells. (A) HepG2
cells were incubated with 15 mM APAP with or without various
concentrations of PYP1-4 for 18 h. The protein and RNA levels of
growth-associated factors (IGF-IR, EGFR ErbB2, and ErbB3) were
determined by western blot analysis and reverse transcription PCR.
(B) Bands were normalized to β-actin as an internal control, and
protein and RNA levels were graphed. Data are presented as the mean
± SD of three independent experiments, and were subjected to
two-way ANOVA. *P<0.05 vs. the control group;
#P<0.05 vs. the 15 mM APAP group. APAP,
acetaminophen; EGFR, epidermal growth factor receptor; ErbB2,
erb-b2 receptor tyrosine kinase 2; ErbB3, erb-b2 receptor tyrosine
kinase 3; IGF-IR, insulin-like growth factor 1 receptor; PYP1-4,
P. yezoensis peptide. |
PYP1-4 increases the levels of
IRS-1/PI3K/Akt signaling pathway-associated proteins in
APAP-induced cells
IGF signaling affects cell survival, and the IGF-I
protein level is elevated in HepG2 cells (45). PYP1-4 co-treatment restored
APAP-induced apoptosis and the levels of growth factor receptors
(Figs. 4 and 5). Subsequently, the levels of proteins
associated with the IRS-1/PI3K/Akt signaling pathway, one of the
two major downstream IGF-IR signaling pathways (46), were assessed. PYP1-4 co-treatment
groups significantly increased the protein levels of IGF-IR, IRS-1,
PI3Kp85, PTEN, p70S6K and eIF4E in a concentration-dependent manner
compared with the levels in the APAP overdose group (Fig. 6). In addition, PYP1-4 co-treatment
groups significantly increased ratios of p-Akt/Akt and p-mTOR/mTOR
compared with the ratios in the APAP overdose group.
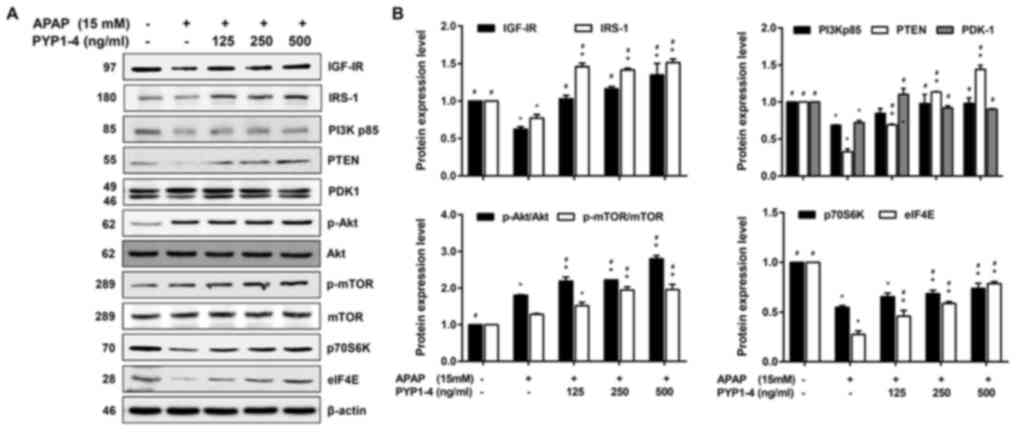 | Figure 6.PYP1-4 restores the levels of
IRS-1/PI3K/Akt signaling pathway proteins in APAP-induced HepG2
cells. (A) HepG2 cells were incubated with 15 mM APAP with or
without various concentrations of PYP1-4 for 18 h. The levels of
IRS-1/PI3K/Akt signaling pathway proteins (IGF-IR, IRS-1, PI3K,
PTEN, PDK1, Akt, mTOR, p70S6K and elF4E) were determined by western
blot analysis. (B) Bands were normalized to β-actin as an internal
control, and the phosphorylated vs. total protein ratios were
graphed. Data are the means ± SD of three independent experiments
and were subjected to two-way analysis of variance. *P<0.05 vs.
control group; #P<0.05 vs. 15 mM APAP group. APAP,
acetaminophen; elF4E, eukaryotic translation initiation factor 4E;
IGF-IR, insulin-like growth factor 1 receptor; IRS-1, insulin
receptor substrate 1; p-, phosphorylated-; p70S6K, p70S6 kinase;
PDK1, pyruvate dehydrogenase kinase 1; PYP1-4, P. yezoensis
peptide. |
PYP1-4 increases the levels of
Ras/Raf/ERK signaling pathway-associated proteins in APAP-induced
cells
Subsequently, the present study investigated the
levels of proteins associated with the Ras/Raf/ERK signaling
pathway, which is the other major IGF-IR downstream signaling
pathway (47). PYP1-4 co-treatment
groups significantly increased the protein levels of IGF-IR, SHC,
SOS and GRB2 in a concentration-dependent manner compared with the
APAP group (Fig. 7). In addition,
PYP1-4 co-treatment groups significantly increased the levels of
p-MEK/MEK and p-ERK/ERK compared with those in the APAP group.
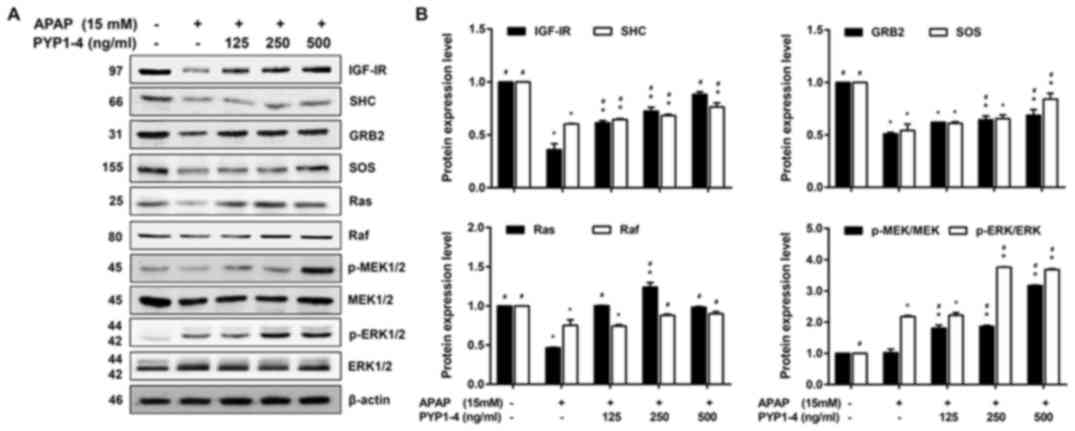 | Figure 7.PYP1-4 restores the levels of
Ras/Raf/ERK signaling pathway proteins in APAP-induced HepG2 cells.
(A) HepG2 cells were incubated with 15 mM APAP with or without
various concentrations of PYP1-4 for 18 h. The levels of
Ras/Raf/ERK signaling pathway proteins (IGF-IR, SHC, GRB2, SOS,
Ras, Raf, MEK and ERK) were determined by western blot analysis.
(B) Bands were normalized to β-actin as an internal control, and
the phosphorylated vs. total protein ratios were graphed. Data are
presented as the mean ± SD of three independent experiments, and
were subjected to two-way ANOVA. *P<0.05 vs. control group;
#P<0.05 vs. 15 mM APAP group. APAP, acetaminophen;
GRB2, growth factor receptor bound protein 2; IGF-IR, insulin-like
growth factor 1 receptor; MEK, mitogen-activated protein kinase
kinase; p-, phosphorylated; PYP1-4, P. yezoensis peptide;
SHC, SHC adaptor protein 1; SOS, SOS Ras/Rac guanine nucleotide
exchange factor 1. |
Discussion
Seaweeds have attracted attention from researchers
due to their abundance of polysaccharides, proteins, vitamins,
minerals and polyphenols (48,49).
Seaweeds, including brown, green and red algae, possess
anti-obesogenic, anticancer, antioxidant and anti-inflammatory
activities due to various bioactive compounds (50). The red alga P. yezoensis is of
increasing interest due to its rich sugars and protein content
(51). Although numerous studies on
have been investigated the polysaccharide and polyphenol
constituents of P. yezoensis (22–24,26,28,31,32,52),
studies on the proteins contained in this alga remain lacking
(25,27,29,30,33,34).
APAP is safe at therapeutic doses; however,
excessive doses cause serious hepatotoxicity in laboratory animals
and humans, and are a major cause of liver and kidney failure
(3,7,8).
Therefore, methods to reduce the hepatotoxicity of APAP overdose
are required.
Studies on seaweeds and APAP-induced hepatotoxicity
have focused on Sargassum species (Hizikia
fusiformis, syn. Sargassum fusiforme), red algae (P.
yezoensis), green algae (Ulva reticulata and
Chlorella sorokiniana) and sulfated polysaccharides
(fucoidan) (33,53–56). In
a previous study, prevention of APAP-induced hepatotoxicity is
associated with a 14-kDa protein (PYP) of P. yezoensis
(33). PYP may inhibit APAP-induced
GSH depletion in rats. APAP also increases caspase-3 activity
during apoptosis, DNA fragmentation and serum glutamic oxaloacetic
transaminase/glutamic pyruvic transaminase levels, which are
indicators of hepatic damage (33).
Additionally, co-treatment with PYP and APAP reversed these effects
to the levels in the control (33).
Therefore, although further studies are required, there is evidence
to support that PYP inhibits APAP-induced hepatotoxicity.
Based on these results, PYP has been purified from
the 14-kDa protein using SDS-PAGE, automated protein sequencing and
matrix assisted laser desorption/ionization quadrupole ion
trap-time-of-flight mass spectrometry (34). The PYP fraction contains two
proteins, PYP1 (10 kDa; an SDS-resistant dimer) and PYP2 (10 kDa)
(34). Based on these results, the
synthetic peptide PYP1 (1–20) corresponding to the N-terminal 20
residues of PYP1 (ALEGGKSSGGGEATRDPEPT) has been obtained (34). PYP1 (1–20)
protects against APAP-induced apoptosis in HeLa (Chang Liver)
cells, and has been determined to be the active fraction of PYP
(34).
The present study investigated the protective
effects of P. yezoensis peptides on APAP-induced
hepatotoxicity. In a previous study, a total of 13 peptides were
obtained by treating PYP1 (1–20) with
trypsin, chymotrypsin and pepsin (34). These peptides were finally selected
for PYP1-4 based on the cell viability assay results. The present
study revealed that PYP1-4 at 0–500 ng/ml was non-toxic in HepG2
cells and reversed the effects of APAP-induced hepatotoxicity.
Activation of the Nrf2 signaling pathway serves an
essential role in APAP-induced acute liver failure (57). Nrf2 is a redox-sensitive
transcription factor and regulates the transcription of genes
associated with protection against oxidative stress (58). In the cytoplasm, Nrf2 is typically
present in the Nrf2-Kelch-like ECH-associated protein 1 (Keap1)
complex (59). In response to
oxidative stress, Nrf2 dissociates from Keap1 and translocates to
the nucleus to induce the expression of genes encoding antioxidant
enzymes (NQO1, glutathione S-transferase and HO1) by binding to the
antioxidant response element in their promoters (60). Intracellular ROS accumulation
disrupts the Nrf2-Keap1 interaction; oxidized Keap1 binds to the
adapter protein GAP-associated tyrosine phosphoprotein p62 and
releases Nrf2, which translocates to the nucleus and activates
transcription of genes encoding antioxidant and detoxifying enzymes
(61). Therefore, Nrf2 has potential
as a therapeutic target for liver diseases, including APAP-induced
hepatotoxicity (62). In present
study, the antioxidant activity of PYP1-4 contributes to its
protective effect against APAP-induced hepatotoxicity, and this
protective effect is associated with the activation of the
Nrf2/HO1/SOD2 signaling pathway.
Additionally, Nrf2 can be activated by
post-transcriptional modification by kinases, including protein
kinase C, PI3K and MAPK (63,64).
AMPK activates the PI3K/Akt signaling pathway, and Akt activation
is essential for the phosphorylation of GSK3β and may modulate
oxidative stress (65). A
heterotrimeric serine/threonine kinase, AMPK, senses the cellular
energy status and regulates cell survival and death under oxidative
stress (66). GSK3β is a
constitutively activated Ser/Thr protein kinase that regulates
glycogen metabolism, gene expression and cell death (67). Previously, based on evidence that
GSK3β is a novel regulator of Nrf2, Nrf2 has been suggested to
function in combination with the AMPK/Akt/GSK3β signaling network
(40,41). Therefore, regulation of the Nrf2
signaling pathway by PYP1-4 may ameliorate APAP-induced acute liver
failure by modulating the AMPK/Akt/GSK3β signaling pathway. In the
present study, PYP1-4 increased Akt activity by phosphorylating
GSK3β, and PYP1-4-induced Akt activation stimulated Nrf2 activity.
In addition, the increased GSK3β phosphorylation caused by
activation of AMPK protected against oxidative stress.
JNK phosphorylation and mitochondrial translocation
increase mitochondrial dysfunction, and AMPK activation serves a
crucial role in protecting mitochondria (68). In the present study, treatment with
PYP1-4 activated AMPK, and inhibited the APAP-induced
phosphorylation of JNK. These results suggested that PYP1-4
treatment protected against APAP-induced hepatotoxicity by
inhibiting JNK phosphorylation. Resveratrol has been reported to
protect mitochondria against oxidative stress by increasing
phosphorylation of GSK3β by activating AMPK (68). In addition, esculentoside A regulates
Nrf2 activation via the AMPK/Akt/GSK3β signaling pathway (69). These results suggest that PYP1-4
treatment exhibits an antioxidant effect by activating Nrf2 via the
AMPK/Akt/GSK3β pathway, thus protecting against APAP-induced
hepatotoxicity (Fig. 3).
APAP-induced cell death remains controversial. The
signal transduction pathways involved in apoptosis and necrosis
exhibit a degree of overlap (70).
In a previous study using ICR mice, 95% of APAP-damaged hepatocytes
died due to necrosis in vivo (71); however, another study reported that
APAP-induced hepatocyte (HuH7 cells) apoptosis serves a crucial
role in liver failure (72).
APAP-induced cell death has been hypothesized to be caused by
necroptosis, which is characterized by features of necrosis and
apoptosis (73). In the present
study, APAP overdose increased apoptosis, whereas co-treatment with
PYP1-4 resulted in a dose-dependent decrease in apoptosis.
Apoptosis can be initiated by intrinsic and/or
extrinsic signaling pathways (73).
Apoptosis of mammalian cells is regulated by Bcl-2 family proteins
(44), which modulate mitochondrial
membrane permeability and cytochrome c release. APAP induces
metastasis of Bcl-2 family proteins (70), leading to the release of cytochrome
c. Activation of apoptosis via the exogenous signaling
pathway is mediated by the binding of an apoptotic ligand to a
death receptor (74). These death
receptors have intracellular domains that function as protein
binding modules. Following recruitment and signaling of adapter
molecules, cleavage and activation of pro-caspase-8, −9, −10 and
−12 occur (75). This leads to the
activation of caspase-3, −6 and −7, as well as the effector
caspase, resulting in DNA fragmentation (75). In addition, APAP-induced
hepatotoxicity occurs via matrix metallopeptidase degradation of
cytochrome c and activation of caspase-8, −9 and −3
(76). Cleaved PARP is a marker of
apoptosis; PARP is activated in cells undergoing stress and/or DNA
damage, and is inactivated by cleavage of caspase-3 during
programmed cell death (76).
Therefore, the results of the present study suggested that PYP1-4
inhibits APAP-induced apoptosis via intrinsic (endogenous) and
extrinsic (exogenous) signaling pathways (Fig. 4).
Several studies have investigated the mechanism by
which IGF-IR protects against apoptosis (45,77,78).
During apoptosis, the binding of wild-type IGF-IR suppresses cell
death. A previous study has demonstrated that APAP-induced HeLa
(Chang Liver) cells were restored to apoptosis following treatment
with IGF-I (79). The present study
demonstrated that the IGF-IR signaling pathway was affected by
PYP1-4.
IGFs are synthesized in and secreted by adult and
fetal hepatocytes, widely expressed in a number of cell types,
essential for normal growth, development and differentiation, and
mediate signals for apoptosis inhibition, mitogenesis and
immobilization-independent growth (45,80).
IGF-IR-associated signaling pathways comprise the
IRS-1/PI3K/Akt and Ras/Raf/ERK signaling pathways (46,47).
IGF-IR is autophosphorylated by intrinsic tyrosine kinase activity
and promotes activation of downstream signaling molecules. The
binding of activated lGF-IR and phosphorylated adaptor proteins
such as IRS-1 then activate the PI3K/Akt signaling pathway
(81,82). IRS-1/PI3K/Akt along with mTOR/p70S6K
signaling activates translation initiation factors and inactivates
regulatory factors (83). This
signaling pathway is also involved in the crosstalk with the
Ras/Raf/ERK signaling pathways (84). In addition, Ras signaling is enhanced
by upstream events such as the activation of IGF-1R (85). Ras continuously stimulates the
MAPK-activating serine/threonine kinase Raf and induces cell growth
through transcriptional activation of multiple targets (86).
MAPKs, including ERK, JNK and p38, are part of the
IGF-IR signaling pathway and convert extracellular stimuli into a
wide range of cellular responses. These proteins serve important
roles in cell proliferation, differentiation, metabolism, survival
and death (87,88), as well as in oxidative damage
(77,78). JNK is primarily involved apoptosis
and is activated by oxidative damage, whereas ERK regulates cell
growth and differentiation, is activated by oxidative damage, and
acts as a cell death suppression signal to maintain homeostasis
(89). Akt is a downstream target of
the PI3K/Akt signaling pathway and serves an important role in the
inhibition of PI3K-mediated cell proliferation (90). In the present study, PYP1-4 treatment
of APAP-induced HepG2 cells induced growth and reduced oxidative
damage and apoptosis in a dose-dependent manner on the
IRS-1/PI3K/Akt and Ras/Raf/ERK signaling pathways.
In conclusion, the present study revealed that
PYP1-4 decreased APAP-induced oxidative damage, growth inhibition
and apoptosis in HepG2 cells. Additionally, the IGF-IR signaling
pathway contributed to the suppression of apoptosis and necrosis.
These observations suggested that PYP1-4 exerts a hepatoprotective
effect against APAP-induced oxidative damage and apoptosis.
However, further research on the structure of PYP1-4 and on the
signal transduction pathways involved in APAP-induced
hepatotoxicity is required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
2012R1A6A1028677).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IHK and TJN designed the experiments. IHK and JWC
performed the experiments, interpreted the experimental results and
drafted the manuscript. TJN and JWC performed revising the
manuscript critically for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cuzzolin L, Antonucci R and Fanos V:
Paracetamol (acetaminophen) efficacy and safety in the newborn.
Curr Drug Metab. 14:178–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klotz U: Paracetamol (acetaminophen) - a
popular and widely used nonopioid analgesic. Arzneimittelforschung.
62:355–359. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mazaleuskaya LL, Sangkuhl K, Thorn CF,
FitzGerald GA, Altman RB and Klein TE: PharmGKB summary: Pathways
of acetaminophen metabolism at the therapeutic versus toxic doses.
Pharmacogenet Genomics. 25:416–426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prescott LF: Hepatotoxicity of mild
analgesics. Br J Clin Pharmacol. 2 (Suppl 10):373S–379S. 1980.
View Article : Google Scholar
|
|
5
|
Litovitz TL, Klein-Schwartz W, Rodgers GC
Jr, Cobaugh DJ, Youniss J, Omslaer JC, May ME, Woolf AD and Benson
BE: 2001 annual report of the american association of poison
control centers toxic exposure surveillance system. Am J Emerg Med.
20:391–452. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blieden M, Paramore LC, Shah D and
Ben-Joseph R: A perspective on the epidemiology of acetaminophen
exposure and toxicity in the United States. Expert Rev Clin
Pharmacol. 7:341–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Simkin S, Hawton K, Kapur N and Gunnell D:
What can be done to reduce mortality from paracetamol overdoses? A
patient interview study. QJM. 105:41–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Craig DG, Ford AC, Hayes PC and Simpson
KJ: Systematic review: Prognostic tests of paracetamol-induced
acute liver failure. Aliment Pharmacol Ther. 31:1064–1076.
2010.PubMed/NCBI
|
|
9
|
Lee WM: Acetaminophen and the U.S. Acute
Liver Failure Study Group: Lowering the risks of hepatic failure.
Hepatology. 40:6–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Knight TR, Fariss MW, Farhood A and
Jaeschke H: Role of lipid peroxidation as a mechanism of liver
injury after acetaminophen overdose in mice. Toxicol Sci.
76:229–236. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hinson JA, Roberts DW and James LP:
Mechanisms of acetaminophen-induced liver necrosis. Handb Exp
Pharmacol. 196:369–405. 2010. View Article : Google Scholar
|
|
12
|
Yoon E, Babar A, Choudhary M, Kutner M and
Pyrsopoulos N: Acetaminophen-induced hepatotoxicity: A
comprehensive update. J Clin Transl Hepatol. 4:131–142.
2016.PubMed/NCBI
|
|
13
|
James LP, Mayeux PR and Hinson JA:
Acetaminophen-induced hepatotoxicity. Drug Metab Dispos.
31:1499–1506. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao X, Cong X, Zheng L, Xu L, Yin L and
Peng J: Dioscin, a natural steroid saponin, shows remarkable
protective effect against acetaminophen-induced liver damage in
vitro and in vivo. Toxicol Lett. 214:69–80. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang YL, Zhang ZH, Liu XJ, Liu XQ, Tao L,
Zhang YF, Wang H, Zhang C, Chen X and Xu DX: Melatonin protects
against apoptosis-inducing factor (AIF)-dependent cell death during
acetaminophen-induced acute liver failure. PLoS One. 7:e519112012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Slitt AM, Dominick PK, Roberts JC and
Cohen SD: Effect of ribose cysteine pretreatment on hepatic and
renal acetaminophen metabolite formation and glutathione depletion.
Basic Clin Pharmacol Toxicol. 96:487–494. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yousef MI, Omar SA, El-Guendi MI and
Abdelmegid LA: Potential protective effects of quercetin and
curcumin on paracetamol-induced histological changes, oxidative
stress, impaired liver and kidney functions and haematotoxicity in
rat. Food Chem Toxicol. 48:3246–3261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yan M, Huo Y, Yin S and Hu H: Mechanisms
of acetaminophen-induced liver injury and its implications for
therapeutic interventions. Redox Biol. 17:274–283. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Corcoran GB, Todd EL, Racz WJ, Hughes H,
Smith CV and Mitchell JR: Effects of N-acetylcysteine on the
disposition and metabolism of acetaminophen in mice. J Pharmacol
Exp Ther. 232:857–863. 1985.PubMed/NCBI
|
|
20
|
Shahidi F and Rahman J: Bioactive in
seaweeds, algae, and fungi and their role in health promotion. J
Food Bioact. 2:58–81. 2018. View Article : Google Scholar
|
|
21
|
Niwa K: Genetic analysis of artificial
green and red mutants of Porphyra yezoensis Ueda (Bangiales,
Rhodophyta). Aquaculture. 308:6–12. 2010. View Article : Google Scholar
|
|
22
|
Kim S, You DH, Han T and Choi EM:
Modulation of viability and apoptosis of UVB-exposed human
keratinocyte HaCaT cells by aqueous methanol extract of laver
(Porphyra yezoensis). J Photochem Photobiol B. 141:301–307.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryu J, Park SJ, Kim IH, Choi YH and Nam
TJ: Protective effect of porphyra-334 on UVA-induced photoaging in
human skin fibroblasts. Int J Mol Med. 34:796–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma XT, Sun XY, Yu K, Gui BS, Gui Q and
Ouyang JM: Effect of content of sulfate groups in seaweed
polysaccharides on antioxidant activity and repair effect of
subcellular organelles in injured HK-2 cells. Oxid Med Cell Longev.
2017:25429502017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toyosaki T and Iwabuchi M: New antioxidant
protein in seaweed (Porphyra yezoensis Ueda). Int J Food Sci
Nutr. 60 (Suppl 2):46–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu X, Zhou C, Yang H, Huang X, Ma H, Qin X
and Hu J: Effect of ultrasonic treatment on the degradation and
inhibition cancer cell lines of polysaccharides from Porphyra
yezoensis. Carbohydr Polym. 117:650–656. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Activation of the mTOR signaling pathway in breast cancer MCF-7
cells by a peptide derived from Porphyra yezoensis. Oncol
Rep. 33:19–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yanagido A, Ueno M, Jiang Z, Cho K,
Yamaguchi K, Kim D and Oda T: Increase in anti-inflammatory
activities of radical-degraded porphyrans isolated from discolored
nori (Pyropia yezoensis). Int J Biol Macromol. 117:78–86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee HA, Kim IH and Nam TJ: Bioactive
peptide from Pyropia yezoensis and its anti-inflammatory
activities. Int J Mol Med. 36:1701–1706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oh JH, Kim EY and Nam TJ:
Phycoerythrin-derived tryptic peptide of a red alga Pyropia
yezoensis attenuates glutamate-induced ER stress and neuronal
senescence in primary rat hippocampal neurons. Mol Nutr Food Res.
62:e17004692018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mohibbullah M, Bhuiyan MM, Hannan MA,
Getachew P, Hong YK, Choi JS, Choi IS and Moon IS: The edible red
alga Porphyra yezoensis promotes neuronal survival and
cytoarchitecture in primary hippocampal neurons. Cell Mol
Neurobiol. 36:669–682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi JW, Kim IH, Kim YM, Lee MK and Nam
TJ: Pyropia yezoensis glycoprotein regulates antioxidant
status and prevents hepatotoxicity in a rat model of
D-galactosamine/lipopolysaccharide-induced acute liver failure. Mol
Med Rep. 13:3110–3114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hwang HJ, Kwon MJ, Kim IH and Nam TJ:
Chemoprotective effects of a protein from the red algae Porphyra
yezoensis on acetaminophen-induced liver injury in rats.
Phytother Res. 22:1149–1153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi YH, Yamaguchi K, Oda T and Nam TJ:
Chemical and mass spectrometry characterization of the red alga
Pyropia yezoensis chemoprotective protein (PYP): Protective
activity of the N-terminal fragment of PYP1 against
acetaminophen-induced cell death in chang liver cells. Int J Mol
Med. 35:271–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wallace JL: Acetaminophen hepatotoxicity:
NO to the rescue. Br J Pharmacol. 143:1–2. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu WX, Jia FL, He YY and Zhang BX:
Protective effects of 5-methoxypsoralen against
acetaminophen-induced hepatotoxicity in mice. World J
Gastroenterol. 18:2197–2202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Olaleye MT and Rocha BT:
Acetaminophen-induced liver damage in mice: Effects of some
medicinal plants on the oxidative defense system. Exp Toxicol
Pathol. 59:319–327. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee KJ, You HJ, Park SJ, Kim YS, Chung YC,
Jeong TC and Jeong HG: Hepatoprotective effects of Platycodon
grandiflorum on acetaminophen-induced liver damage in mice. Cancer
Lett. 174:73–81. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng T, Yang X, Wu D, Xing S, Bian F, Li
W, Chi J, Bai X, Wu G, Chen X, et al: Salidroside ameliorates
insulin resistance through activation of a mitochondria-associated
AMPK/PI3K/Akt/GSK3 β pathway. Br J Pharmacol. 172:3284–3301. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mathur A, Rizvi F and Kakkar P: PHLPP2
down regulation influences nuclear Nrf2 stability via
Akt-1/Gsk3β/Fyn kinase axis in acetaminophen induced oxidative
renal toxicity: Protection accorded by morin. Food Chem Toxicol.
89:19–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xing HY, Cai YQ, Wang XF, Wang LL, Li P,
Wang GY and Chen JH: The cytoprotective effect of hyperoside
against oxidative stress is mediated by the Nrf2-ARE signaling
pathway through GSK-3β inactivation. PLoS One. 10:e01451832015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song E, Fu J, Xia X, Su C and Song Y:
Bazhen decoction protects against acetaminophen induced acute liver
injury by inhibiting oxidative stress, inflammation and apoptosis
in mice. PLoS One. 9:e1074052014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sharma S, Singh RL and Kakkar P:
Modulation of Bax/Bcl-2 and caspases by probiotics during
acetaminophen induced apoptosis in primary hepatocytes. Food Chem
Toxicol. 49:770–779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yao NH, Yao DF, Dong ZZ, Yan XD, Chen J,
Yao M, Wang L and Yan MJ: Effects of inhibited IGF-IR expression on
proliferation and apoptosis of human hepatocellular carcinoma cell
lines. Zhonghua Gan Zang Bing Za Zhi. 21:376–380. 2013.(In
Chinese). PubMed/NCBI
|
|
46
|
Kulik G, Klippel A and Weber MJ:
Antiapoptotic signalling by the insulin-like growth factor I
receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol.
17:1595–1606. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yu H and Rohan T: Role of the insulin-like
growth factor family in cancer development and progression. J Natl
Cancer Inst. 92:1472–1489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
MacArtain P, Gill CI, Brooks M, Campbell R
and Rowland IR: Nutritional value of edible seaweeds. Nutr Rev.
65:535–543. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dawczynski CH, Schubert R and Jahreis G:
Amino acids, fatty acids, and dietary fiber in edible seaweed
products. Food Chemistry. 103:891–899. 2007. View Article : Google Scholar
|
|
50
|
Lee JC, Hou MF, Huang HW, Chang FR, Yeh
CC, Tang JY and Chang HW: Marine algal natural products with
anti-oxidative, anti-inflammatory, and anti-cancer properties.
Cancer Cell Int. 13:552013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Qu W, Ma H, Pan Z, Luo L, Wang Z and He R:
Preparation and antihypertensive activity of peptides from
Porphyra yezoensis. Food Chem. 123:14–20. 2010. View Article : Google Scholar
|
|
52
|
Ueno M, Cho K, Isaka S, Nishiguchi T,
Yamaguchi K, Kim D and Oda T: Inhibitory effect of sulphated
polysaccharide porphyran (isolated from Porphyra yezoensis)
on RANKL-induced differentiation of RAW264.7 cells into
osteoclasts. Phytother Res. 32:452–458. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hira K, Sultana V, Ara J and Haque SE:
Protective role of Sargassum species in liver and kidney
dysfunctions and associated disorders in rats intoxicated with
carbon tetrachloride and acetaminophen. Pak J Pharm Sci.
30:721–728. 2017.PubMed/NCBI
|
|
54
|
Balaji Raghavendra Rao H, Sathivel A and
Devaki T: Antihepatotoxic nature of Ulva reticulata
(Chlorophyceae) on acetaminophen-induced hepatoxicity in
experimental rats. J Med Food. 7:495–497. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Escapa C, Coimbra RN, Paniagua S, García
AI and Otero M: Paracetamol and salicylic acid removal from
contaminated water by microalgae. J Environ Manage. 203:799–806.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hong SW, Lee HS, Jung KH, Lee H and Hong
SS: Protective effect of fucoidan against acetaminophen-induced
liver injury. Arch Pharm Res. 35:1099–1105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Itoh K, Mimura J and Yamamoto M: Discovery
of the negative regulator of Nrf2, Keap1: A historical overview.
Antioxid Redox Signal. 13:1665–1678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Inoue H, Maeda-Yamamoto M, Nesumi A and
Murakami A: Delphinidin-3-O-galactoside protects mouse hepatocytes
from (−)-epigallocatechin-3-gallate-induced cytotoxicity via
up-regulation of heme oxygenase-1 and heat shock protein 70. Nutr
Res. 32:357–364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kensler TW, Wakabayash N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jiang ZY, Xu LL, Lu MC, Chen ZY, Yuan ZW,
Xu XL, Guo XK, Zhang XJ, Sun HP and You QD: Structure-activity and
structure-property relationship and exploratory in vivo evaluation
of the nanomolar Keap1-Nrf2 protein-protein interaction inhibitor.
J Med Chem. 58:6410–6421. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Bataille AM and Manautou JE: Nrf2: A
potential target for new therapeutics in liver disease. Clin
Pharmacol Ther. 92:340–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kong AN, Owuor E, Yu R, Hebbar V, Chen C,
Hu R and Mandlekar S: Induction of xenobiotic enzymes by the MAP
kinase pathway and the antioxidant or electrophile response element
(ARE/EpRE). Drug Metab Rev. 33:255–271. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Nakaso K, Yano H, Fukuhara Y, Takeshima T,
Wada-Isoe K and Nakashima K: PI3K is a key molecule in the
Nrf2-mediated regulation of antioxidative proteins by hemin in
human neuroblastoma cells. FEBS Lett. 546:181–184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Horike N, Sakoda H, Kushiyama A, Ono H,
Fujishiro M, Kamata H, Nishiyama K, Uchijima Y, Kurihara Y,
Kurihara H and Asano T: AMP-activated protein kinase activation
increases phosphorylation of glycogen synthase kinase 3beta and
thereby reduces cAMP-responsive element transcriptional activity
and phosphoenolpyruvate carboxykinase C gene expression in the
liver. J Biol Chem. 283:33902–33910. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Konrad D, Rudich A, Bilan PJ, Patel N,
Richardson C, Witters LA and Klip A: Troglitazone causes acute
mitochondrial membrane depolarisation and an AMPK-mediated increase
in glucose phosphorylation in muscle cells. Diabetologia.
48:954–966. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Luo J: The role of Glycogen synthase
kinase 3β (GSK3β) in tumorigenesis and cancer chemotherapy. Cancer
Lett. 273:194–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shin SM, Cho IJ and Kim SG: Resveratrol
protects mitochondria against oxidative stress through
AMP-activated protein kinase-mediated glycogen synthase
kinase-3beta inhibition downstream of
poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 76:884–895.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wang L, Zhang S, Cheng H, Lv H, Cheng G
and Ci X: Nrf2-mediated liver protection by esculentoside A against
acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free
Radic Biol Med. 101:401–412. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jaeschke H and Bajt ML: Intracellular
signaling mechanisms of acetaminophen-induced liver cell death.
Toxicol Sci. 89:31–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ray SD, Mumaw VR, Raje RR and Fariss MW:
Protection of acetaminophen-induced hepatocellular apoptosis and
necrosis by cholesteryl hemisuccinate pretreatment. J Pharmacol Exp
Ther. 279:1470–1483. 1996.PubMed/NCBI
|
|
72
|
Kass GE, Macanas-Pirard P, Lee PC and
Hinton RH: The role of apoptosis in acetaminophen-induced injury.
Ann NY Acad Sci. 1010:557–559. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Malhi H, Gores GJ and Lemasters JJ:
Apoptosis and necrosis in the liver: A tale of two deaths?
Hepatology. 43 (Suppl 1):S31–S44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Bürkle A, Brabeck C, Diefenbach J and
Beneke S: The emerging role of poly(ADP-ribose) polymerase-1 in
longevity. Int J Biochem Cell Biol. 37:1043–1053. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW,
Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, et al:
Poly(ADP-ribose) (PAR) polymer is a death signal. Proc Natl Acad
Sci USA. 103:18308–18313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Isabelle M, Moreel X, Gagné JP, Rouleau M,
Ethier C, Gagné P, Hendzel MJ and Poirier GG: Investigation of
PARP-1, PARP-2, and PARG interactomes by affinity-purification mass
spectrometry. Proteome Sci. 8:222010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Alexia C, Fallot G, Lasfer M,
Schweizer-Groyer G and Groyer A: An evaluation of the role of
insulin-like growth factors (IGF) and of type-I IGF receptor
signalling in hepatocarcinogenesis and in the resistance of
hepatocarcinoma cells against drug-induced apoptosis. Biochem
Pharmacol. 68:1003–1015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yanai R, Yamada N, Inui M and Nishida T:
Correlation of proliferative and anti-apoptotic effects of HGF,
insulin, IGF-1, IGF-2, and EGF in SV40-transformed human corneal
epithelial cells. Exp Eye Res. 83:76–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hwang HJ, Kwon MJ and Nam TJ:
Chemoprotective effect of insulin-like growth factor I against
acetaminophen-induced cell death in chang liver cells via ERK1/2
activation. Toxicology. 230:76–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Brodt P, Samani A and Navab R: Inhibition
of the type I insulin-like growth factor receptor expression and
signaling: Novel strategies for antimetastatic therapy. Biochem
Pharmacol. 60:1101–1107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Segrelles C, Moral M, Lara MF, Ruiz S,
Santos M, Leis H, García-Escudero R, Martínez-Cruz AB,
Martínez-Palacio J, Hernández P, et al: Molecular determinants of
Akt-induced keratinocyte transformation. Oncogene. 25:1174–1185.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lee ER, Kim JY, Kang YJ, Ahn JY, Kim JH,
Kim BW, Choi HY, Jeong MY and Cho SG: Interplay between PI3K/Akt
and MAPK signaling pathways in DNA-damaging drug-induced apoptosis.
Biochim Biophys Acta. 1763:958–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bhandari BK, Feliers D, Duraisamy S,
Stewart JL, Gingras AC, Abboud HE, Choudhury GG, Sonenberg N and
Kasinath BS: Insulin regulation of protein translation repressor
4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney
Int. 59:866–875. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
O'Reilly KE, Rojo F, She QB, Solit D,
Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al:
mTOR inhibition induces upstream receptor tyrosine kinase signaling
and activates Akt. Cancer Res. 66:1500–1508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Treisman R: Regulation of transcription by
MAP kinase cascades. Curr Opin Cell Biol. 8:205–215. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Gunawan BK, Liu ZX, Han D, Hanawa N,
Gaarde WA and Kaplowitz N: c-Jun N-terminal kinase plays a major
role in murine acetaminophen hepatotoxicity. Gastroenterology.
131:165–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Wang KP, Bai Y, Wang J and Zhang JZ:
Inhibitory effects of Schisandra chinensis on
acetaminophen-induced hepatotoxicity. Mol Med Rep. 9:1813–1819.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dews M, Prisco M, Peruzzi F, Romano G,
Morrione A and Baserga R: Domains of the insulin-like growth factor
I receptor required for the activation of extracellular
signal-regulated kinases. Endocrinology. 141:1289–1300. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Prasad R, Vaid M and Katiyar SK: Grape
proanthocyanidin inhibit pancreatic cancer cell growth in vitro and
in vivo through induction of apoptosis and by targeting the
PI3K/Akt pathway. PLoS One. 7:e430642012. View Article : Google Scholar : PubMed/NCBI
|