Introduction
Sepsis is defined as infection resulting in multiple
organ dysfunction (1). Acute kidney
injury (AKI) is a common early complication of sepsis, with an
incidence ranging from 11–42% (1).
In addition, the occurrence of AKI in patients with sepsis
indicates poor prognosis and significantly increased mortality
(1,2). The elderly are the primary age group
affected by AKI (3). Therefore,
early detection of sepsis-induced AKI and timely treatment may
improve the clinical prognosis of elderly patients with sepsis.
The mechanism by which sepsis induces AKI is yet to
be elucidated. However, the inflammatory response to sepsis is
considered to serve a role in kidney injury. Inflammatory responses
in sepsis initiates the release of pro-inflammatory cytokines into
the circulation, which results in systemic inflammatory responses
(2). Multiple studies have
demonstrated the associations between inflammatory factors and
kidney oxygenation, metabolism, inflammation and function, where
these factors promote leukocyte aggregation, which may result in
renal inflammation (4–7). In addition, clinical studies have also
suggested that novel biomarkers are required in order to detect AKI
prior to creatinine and urine volume assessments (8), which with high susceptibility, is also
a predictor of prognosis (8).
ATIII serves a vital role in anticoagulation and
influences anti-inflammatory effects (8–10).
Studies in animal models have indicated that ATIII exhibits a
protective effect on the kidney, primarily via its
anti-inflammatory activity (9–11).
Furthermore, endogenous ATIII dysfunction exacerbates renal injury
in animal models. In clinical studies, low ATIII levels increase
the incidence of AKI in a number of patients (12,13).
However, further studies are needed to validate the protective
effects of ATIII in sepsis-induced AKI. Although Cr and urine
output can be used to diagnose AKI development, these assessments
are often performed too late to identify AKI. The present study
hypothesized that the occurrence of AKI in infectious elderly
patients may be predicted by serum ATIII levels or a combination of
ATIII and creatinine.
Materials and methods
Study population
In the current study, patients age ≥60 years who
presented with sepsis according to ‘Sepsis 3.0’ clinical criteria
were included (14). The included
data was collected from Shanghai General Hospital (Shanghai, China)
between October 2015 and March 2018. The medical records of
patients with sepsis who had been admitted to the intensive care
unit (ICU) of Shanghai General Hospital were reviewed. The present
study was approved by the institutional review board of the
Shanghai General Hospital. Since the data published was
retrospective and anonymized, the need for informed consent was
waived.
Inclusion and exclusion criteria
In the present study, ‘elderly’ was defined as
individuals ages ≥60 years old, according to Chinese laws. AKI was
defined as an increase in serum creatinine or decrease in urine
output following the Kidney Disease Improving Global Outcomes
(KDIGO) criteria (15). Patients
were receiving no previous or ongoing treatment that may have
effected the results of the current study. The inclusion criterion
were: i) Patients of ‘ICU’ admission aged between 60 and 85 years
that had ii) presented with sepsis, which was diagnosed according
to the ‘Survival Sepsis Campaign 2012’ (14). The exclusion criteria were patients
with: i) Stages 4 or 5 chronic kidney disease (creatinine clearance
<30 ml/min); ii) a kidney transplant; iii) an ICU stay <24 h;
and iv) a previous admission to the ICU with AKI. Patients with
end-stage diseases (malignant tumour, leukaemia, cirrhosis, NYHA
class IV, and severe COPD), a written ‘do not resuscitate’ form or
a history of allergies to antibiotics or other drugs, were also
excluded.
Baseline glomerular filtration rate was estimated
using the Modification of Diet in Renal Disease study equation
(16). Baseline creatinine was
defined as the lowest serum creatinine value detected in the 6
months prior to AKI onset, or for those without this measurement,
the lowest value achieved during hospitalization (in the absence of
dialysis) (17). Complete data
regarding the inclusion and exclusion processes are presented in
Fig. 1.
Outcome
The primary outcome measured was AKI, and the
secondary outcomes included use of continuous renal replacement
therapy (CRRT), 28-day mortality, 90-day mortality, incidence of
multiple organ dysfunction syndrome, shock, length of hospital stay
(days), duration of mechanical ventilation (days) and use of a
vasoactive agent.
Data collection
All data were retrospectively collected. Baseline
data, including demographics, medical history and disease severity,
were collected retrospectively for each patient by reviewing
patients' medical records. Laboratory data were collected from the
first day in the ICU. Vital signs, haemodynamic and laboratory data
were collected each day during the ICU stay. Renal function was
assessed daily according to creatinine levels and urine output.
Serial Acute Physiology and Chronic Health Evaluation (APACHE) II
score and Sequential Organ Failure Assessment (SOFA) score were
calculated on the first day of admission to the ICU. AKI was
categorized according to the KDIGO staging system 7 days after AKI
diagnosis.
Blood samples were collected and tested for ATIII
and Cr levels within the first 48 h of treatment of sepsis
diagnosis in the ICU following admission. The samples were
centrifuged (3,000 × g, 37°C and 10 min of centrifugation) and
stored at −80°C prior to subsequent analysis. ATIII was detected
using human antithrombin III ELISA kit (cat. no. SY-01862; Shanghai
win-win biotechnology company), and ATIII activity was measured via
colorimetry. Following plasma incubation (37°C; 10 min) with
heparin and thrombin in excess, residual thrombin was measured
according to its amidolytic activity on the chromogenic substrate
CBS 3447. Serum Cr was measured using an enzymatic colorimetric
test based on the Jaffé reaction (alkaline picrate) (18).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
(SPSS Inc.). Quantitative data are expressed as the mean ± standard
deviation. For data with a normal distribution, an independent
sample t-test was used to compare the differences between two sets
of data. For data that were not normally distributed,
non-parametric tests were used to compare differences.
χ2 test or Fisher's test were used for analysis of
nominal data. A logistical regression analysis was used to evaluate
the outcomes based on risk factors selected using univariate
analysis. The diagnostic accuracy of ATIII levels in predicting AKI
and mortality were assessed by calculating the area under the
receiver operating characteristic curve (AUC-ROC). AUC-ROC analysis
was performed by comparing patients with AKI to those without, and
by comparing patient survival. The optimal cut-off values were
defined as the highest values of sensitivity and specificity
calculated via the AUC-ROC analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient demographics and baseline
characteristics
Study enrolment was undertaken between October 2015
and March 2018. Throughout this period, a total of 130 patients
were selected for the present study. A total of 23 patients were
excluded upon admission to the ICU, in accordance with the
pre-defined exclusion criteria. Therefore, a total of 107 (82.3%)
patients were included for further analysis (Fig. 1).
Patient demographics and baseline characteristics
are presented in Table I. In total,
65 patients were male (60.7%), and 42 (39.3%) patients were female.
All 107 patients were included in the final data analysis (Fig. 1). The mean age was 69.76±4.773 years,
and the majority of patients had comorbidities (87.8%).
Hypertension, cardiovascular disease and diabetes mellitus were the
most common comorbidities. The APACHE II score was 17.42±2.28 and
the SOFA score was 6.757±3.10. The requirement for mechanical
ventilation and noradrenaline use in the first 24 h after admission
to ICU was 63.5 and 51.4%, respectively. The primary source of
infection was lung infection (57.0%), followed by abdominal cavity
(43.0%) and urinary tract infection (24.0%). A total of 29 patients
(27.1%) developed AKI, with the majority classified as KDIGO 3
(63.0%). KDIGO 1 occurred in 11.1% and KDIGO 2 in 33.3% of
patients. ‘Need for dialysis’ was indicated in 17.8%, and the
mortality rate was 37.3%.
 | Table I.Patient demographics and clinical
characteristics (n=107) based on AKI. |
Table I.
Patient demographics and clinical
characteristics (n=107) based on AKI.
| Clinicopathological
characteristics | Non-AKI (n=78) | AKI (n=29) | P-value | χ2
value | Total (n=107) |
|---|
| Sex, % male | 42 (53.8) | 23 (79.3) | 0.025a | 5.719 | 65 (60.7) |
| Age, years | 69.70±5.90 | 69.90±5.83 | 0.881 | 0.150 | 69.76±4.773 |
| Comorbidities, n
(%) |
|
|
|
|
|
| Hypertension | 36 (46.1) | 22 (75.9) | 0.008a | 7.516 | 58 (54.2) |
|
Diabetes | 21 (26.9) | 8 (27.6) | 1.000 | 0.005 | 29 (27.1) |
| Immune
diseases | 7 (8.97) | 6 (20.7) | 0.179 | 2.718 | 13 (12.1) |
|
Cardiovascular disease | 40 (51.3) | 24 (82.7) | 0.004a | 8.714 | 64 (59.8) |
| Liver
disease | 3 (3.85) | 2 (6.90) | 0.611 | 0.022 | 5 (4.67) |
| COPD | 16 (20.5) | 3 (10.3) | 0.268 | 0.881 | 19 (17.7) |
| Noradrenaline use,
% | 32 (41.0) | 23 (79.3) | ≤0.001a | 12.404 | 55 (51.4) |
| Mechanical
ventilation, n (%) | 45 (57.7) | 23 (79.3) | 0.044a | 4.265 | 68 (63.5) |
| SOFA | 5.756±2.960 | 9.448±3.611 | ≤0.001a | 5.395 | 6.757±3.10 |
| APACHE II | 15.83±5.86 | 21.69±7.04 | ≤0.001a | 4.344 | 17.42±2.28 |
| Mortality | 24 (30.8) | 16 (89.6) | 0.026a | 5.378 | 40 (37.4) |
| Need for dialysis,
n (%) | 2 (2.56) | 17 (58.6) | ≤0.001a | 45.488 | 19 (17.8) |
The characteristics of the non-AKI (n=78) and AKI
(n=29) groups were similar with regards to age, diabetic status and
comorbidity with immune disease, liver disease and COPD. Whereas
patients who were male or had hypertension or cardiovascular
disease were higher in patients with AKI, and mortality was higher
in patients with AKI (89.6 vs. 30.8%; P=0.026). Table I indicates the clinical and
laboratory characteristics of the population based on AKI. A
significantly higher proportion of patients with AKI required
mechanical ventilation (79.3 vs. 57.7%, P=0.044), noradrenaline use
(79.3 vs. 41%; P<0.0001), APACHE II score (21.69±7.04 vs.
15.83±5.86; P<0.0001) and SOFA score (9.448±3.611 vs.
5.756±2.960; P<0.0001), compared with patients without AKI
(Table I).
The characteristics of the non-survivor (n=40) and
survivor group (n=67) were similar with regards to age,
hypertension, diabetes, cardiovascular disease, immune disease,
liver disease and COPD. AKI was more prevalent in non-survivors (40
vs. 19.4%; P=0.02), and mortality was higher in AKI patients (55.17
vs. 30.77.9%; P=0.02). Table I
reveals the clinicopathological characteristics of the population
based on hospital outcome. Compared with those without AKI,
patients with AKI had a significantly higher need for mechanical
ventilation (82.5 vs. 52.2%; P=0.002) and noradrenaline use (92.5
vs. 26.9%; P<0.0001), and a higher APACHE II (21.97±6.28 vs.
14.70±5.36; P<0.0001) and SOFA score (9.15±3.05 vs. 5.328±3.01;
P<0.0001), all of which were associated with poorer survival
rates (Table II).
 | Table II.Patient demographics and clinical
characteristics (n=107) based on outcome. |
Table II.
Patient demographics and clinical
characteristics (n=107) based on outcome.
| Clinicopathological
characteristics | Non-Survivors
(n=40) | Survivors
(n=67) | P-value | χ2
value | Total (n=107) |
|---|
| Sex, n (%
male) | 24 (67.5) | 41 (61.2) | 0.903 | 0.015 | 65 (60.7) |
| Age, years | 70.47±6.214 | 69.33±5.634 | 0.329 | 0.980 | 69.76±4.773 |
| Comorbidities, n
(%) |
|
|
|
|
|
|
Hypertension | 22 (55) | 36 (53.7) | 0.899 | 0.016 | 58 (54.2) |
|
Diabetes | 12 (30) | 17 (25.4) | 0.602 | 0.217 | 29 (27.1) |
| Immune
diseases | 7 (17.5) | 6 (8.95) | 0.191 | 1.713 | 13 (12.1) |
|
Cardiovascular disease | 26 (65) | 38 (56.7) | 0.398 | 0.715 | 64 (59.8) |
| Liver
disease | 3 (7.5) | 2 (2.99) | 0.360 | 0.357 | 5 (4.67) |
|
COPD | 8 (20) | 11 (16.4) | 0.639 | 0.220 | 19 (17.7) |
| Noradrenaline use,
n (%) | 37 (92.5) | 18 (26.9) | ≤0.001a | 43.193 | 55 (51.4) |
| Mechanical
ventilation, n (%) | 33 (82.5) | 35 (52.2) | 0.002a | 9.902 | 68 (63.5) |
| SOFA | 9.15±3.051 | 5.328±3.012 | ≤0.001a | 6.319 | 6.757±3.10 |
| APACHE II | 21.97±6.282 | 14.70±5.359 | ≤0.001a | 6.364 | 17.42±2.28 |
| AKI | 16 (40) | 13 (19.4) | 0.020a | 5.378 | 29 (27.1) |
| KDIGO, n (%): |
|
|
|
|
|
| I | 2 (5) | 1 (1.49) | 0.554 | 0.131 | 32.80 |
| II | 5 (12.5) | 4 (5.97) | 0.290 | 0.668 | 9 (8.41) |
|
III | 10 (25) | 7 (10.4) | 0.046a | 3.969 | 17 (15.9) |
| Need for dialysis,
n (%) | 13 (32.5) | 6 (8.95) | 0.002a | 9.508 | 19 (17.8) |
Multivariate analysis of AKI and
mortality risk
Being male (OR, 0.140; 95% CI, 0.030–0.643;
P=0.011), exhibiting hypertension (OR, 4.74; 95% CI, 1.21–18.64;
P=0.026), cardiovascular disease (OR, 5.36; 95% CI, 1.23–23.31;
P=0.025) and ATIII (OR, 0.961; 95% CI 0.93–0.99; P=0.005) were
identified as independent risk factors for AKI in multivariate
regression analysis (Table
III).
 | Table III.Multivariate analysis of AKI and
mortality risk (n=107). |
Table III.
Multivariate analysis of AKI and
mortality risk (n=107).
| AKI | OR | 95% CI | P-value |
|---|
| Male sex | 0.140 | 0.030–0.643 | 0.011a |
| Hypertension | 4.744 | 1.207–18.643 | 0.026a |
| Cardiovascular
disease | 5.364 | 1.234–23.311 | 0.025a |
| ATIII | 0.961 | 0.935–0.988 | 0.005a |
| Risk of
mortality |
|
|
|
|
Noradrenaline use | 1.129 | 1.00–1.275 | 0.05 |
|
Mechanical ventilation | 0.983 | 0.917–1.054 | 0.633 |
|
AKI | 2.406 | 0.977–5.926 | 0.056 |
|
ATIII | 0.980 | 0.958–1.002 | 0.079 |
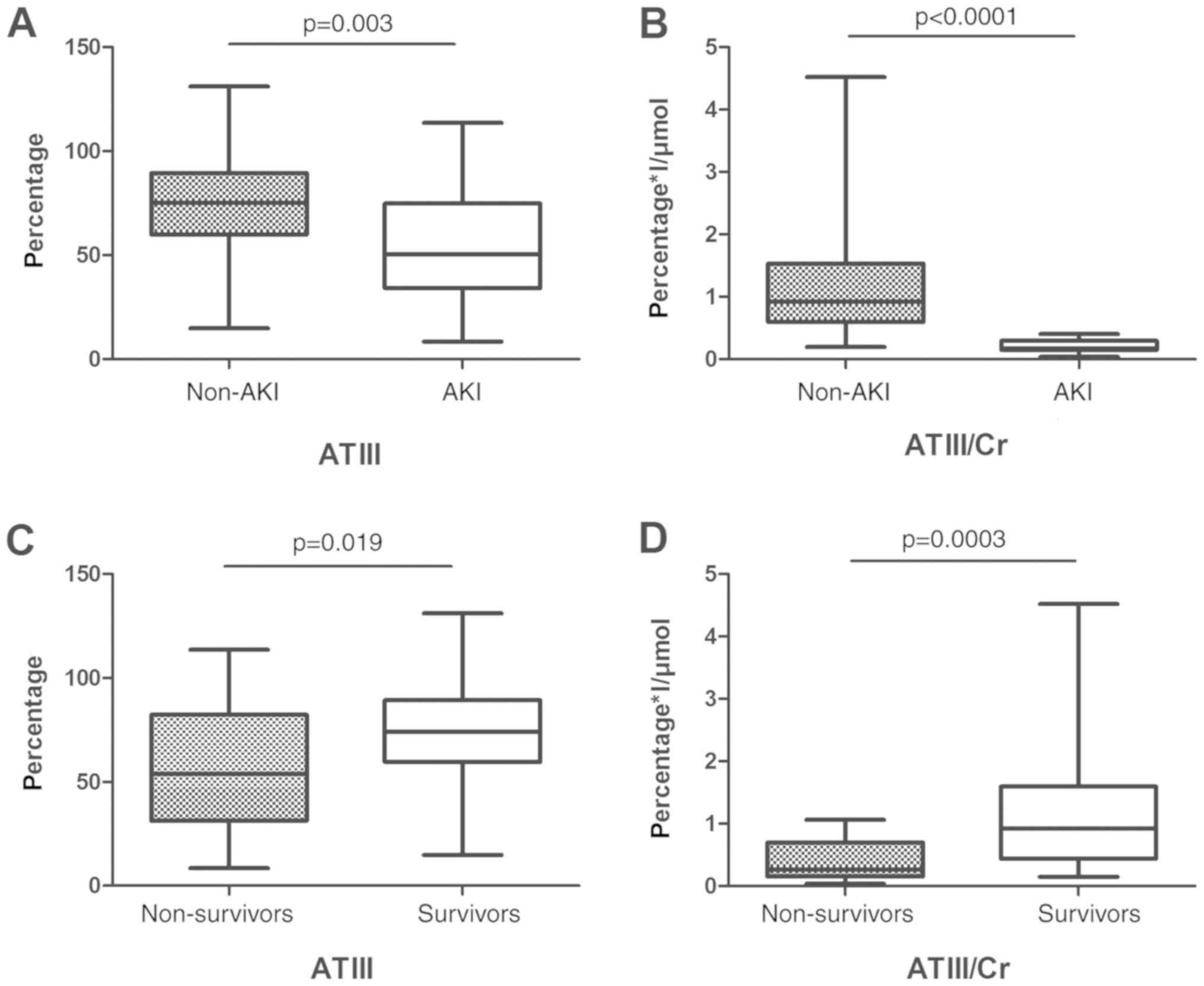
ATIII values based on development of
AKI and patient outcome
ATIII and ATIII/Cr were both higher in the non-AKI
group compared with the AKI group: ATIII (73.43±23.42 vs.
54.01±25.46 g/l; P=0.003), ATIII/Cr (1.10±0.75 vs. 0.21±0.10
g/µmol; P<0.0001). Similarly, ATIII and ATIII/Cr were higher in
the survival group, compared with the non-survival group: ATIII
(73.14±22.55 vs. 56.99±27.87 g/l; P=0.019), ATIII/Cr (1.06±0.81 vs.
0.40±0.30 g/µmol; P=0.0003; Fig.
2).
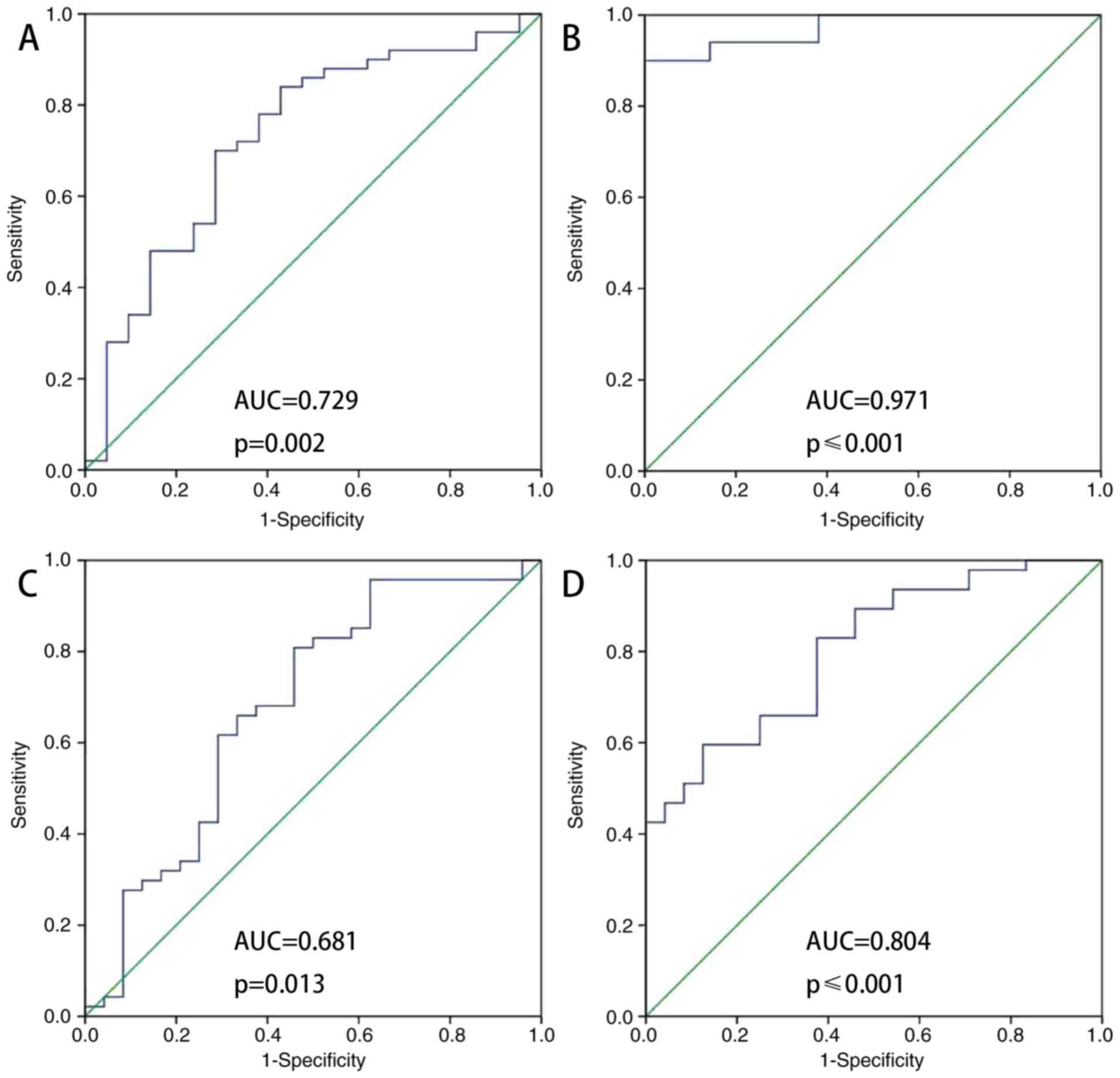
ROC analysis of ATIII in elderly
patients with sepsis with AKI vs. non-AKI
Fig. 3 indicates that
ATIII was a predictor of AKI nondevelopment (AUC-ROC, 0.729;
sensitivity 0.700; specificity, 0.714). The ATIII/Cr ratio was also
a predictor of AKI nondevelopment (AUC-ROC, 0.971; sensitivity,
0.900; specificity, 1). The accuracy of ATIII (AUC/ROC, 0.681;
sensitivity, 0.802; specificity, 0.542) and ATIII/Cr (AUC/ROC,
0.804; sensitivity, 0.596; specificity, 0.875) as predictors of
survival was intermediate. ATIII and ATIII/Cr were accurate
predictors of AKI nondevelopment at 48 h following admission to the
ICU.
Regarding ATIII as a predictor of survival, the
areas under the curve for ATIII and ATIII/Cr were 0.681 and 0.804,
respectively (Fig. 3C and D). The
optimal cut-off value of each one had a sensitivity between 0.802
and 0.596, and specificity between 0.542 and 0.875 (Table IV).
 | Table IV.ATIII sensitivity and specificity in
predicting AKI nondevelopment in elderly septic patients
(n=107). |
Table IV.
ATIII sensitivity and specificity in
predicting AKI nondevelopment in elderly septic patients
(n=107).
|
| AUC-ROC | P-value | Cut-off value | Sensitivity | Specificity | 95% CI |
|---|
| ATIII | 0.729 | 0.002a | 66.95 | 0.700 | 0.714 | 0.596–0.861 |
| ATIII/Cr | 0.971 | ≤0.001a | 0.421 | 0.900 | 1 | 0.940–1 |
Patient outcome data with ATIII
cut-off
The ATIII cut-off value in predicting AKI
non-development in elderly patients with sepsis was 66.95 g/l
(Table IV). The ATIII cut-off used
for predicting survival in elderly patients with sepsis was 55.70
g/l (Table V). When patients were
divided into a low ATIII group and high ATIII group using 66.95 or
55.70 as the cut-off value, the ICU stay was significantly lower in
the high ATIII group (P=0.020 and 0.049, respectively), and off
mechanical ventilation time, off CRRT time, and survival time were
significantly higher in the high ATIII group (P=0.049, 0.048, and
0.014 using 66.95 as the cut-off and 0.041, 0.036, and 0.021 using
55.70 as the cut-off value; Tables
VI and VII).
 | Table V.ATIII sensitivity and specificity in
predicting survival of elderly septic patients (n=107). |
Table V.
ATIII sensitivity and specificity in
predicting survival of elderly septic patients (n=107).
|
| AUC-ROC | P-value | Cut-off value | Sensitivity | Specificity | 95% CI |
|---|
| ATIII | 0.681 | 0.013a | 55.7 | 0.802 | 0.542 | 0.541–0.821 |
|
ATIII/Creatinine | 0.804 | ≤0.001a | 0.758 | 0.596 | 0.875 | 0.702–0.906 |
 | Table VI.Important clinical events of patients
with ATIII. |
Table VI.
Important clinical events of patients
with ATIII.
| Days | ATIII ≥66.95%
n=41 | ATIII <66.95%
n=28 | P-value |
χ2-value |
|---|
| HLOS | 14.87±10.62 | 18.40±15.14 | 0.361 | −0.922 |
| ICU hospital
stay | 9.81±5.78 | 20.80±15.89 | 0.020a | −2.599 |
| Duration of no
vasoactive drug use | 26.36±4.48 | 16.87±13.29 | 0.088 | 1.940 |
| No mechanical
ventilation time | 25.09±4.61 | 13.12±14.12 | 0.049a | 2.310 |
| No CRRT time | 26.46 ±5.13 | 15.50±12.76 | 0.048a | 2.297 |
| Survival time | 24.73±6.51 | 18.78±10.99 | 0.014a | 2.572 |
 | Table VII.Important clinical events of patients
with ATIII. |
Table VII.
Important clinical events of patients
with ATIII.
| Days | ATIII ≥55.7%
n=47 | ATIII <55.7%
n=22 | P-value |
χ2-value |
|---|
| HLOS | 15.33±10.12 | 18.18±17.85 | 0.623 | −0.505 |
| ICU hospital
stay | 10.44±6.08 | 22.73±18.00 | 0.049a | −2.225 |
| Duration of no
vasoactive drug use | 26.50±4.30 | 15.28±13.52 | 0.072 | 2.134 |
| No mechanical
ventilation time | 25.17±4.41 | 11.28±14.17 | 0.041a | 2.521 |
| No CRRT time | 26.58±4.91 | 13.71±12.66 | 0.036a | 2.579 |
| Survival time | 24.36±6.97 | 17.95±11.32 | 0.021a | 2.446 |
Discussion
The present retrospective study aimed to evaluate
the association between ATIII expression and certain clinical
outcomes in elderly patients with sepsis, and revealed an
association between decreased serum ATIII levels and AKI, mortality
and other morbidities. It has been previously demonstrated that
morbidity and mortality in sepsis is associated with decreased
ATIII levels. A systemic review and meta-analysis of ATIII
prospective randomized trials indicated no overall effect on
mortality (19). However, the
current study revealed that ATIII was associated with prediction of
AKI in elderly patients with sepsis. Patients with a high
expression of ATIII were indicated to have a shorter ICU stay,
longer time without mechanical ventilation, longer time without
CRRT and longer overall survival time.
It was discovered that 27.1% of elderly patients
with sepsis developed AKI, and the majority developed AKI stage 3.
This is consistent with a previous study, which reported that the
incidence of AKI among geriatric patients ranged between 11 and 42%
(1). The in-ICU mortality of
geriatric patients with AKI (37.4%) calculated in the present
study, was similar to that reported in other studies (16–40%)
(3). Notably, being male, exhibiting
hypertension, cardiovascular disease and having high serum ATIII
level were all identified as predictors of AKI using logistic
regression analysis. ATIII level was a predictor of
AKI-nondevelopment (AUC-ROC=0.729; sensitivity 0.700; specificity,
0.714). The ATIII/Cr ratio was a predictor of AKI-nondevelopment
(AUC-ROC=0.971; sensitivity, 0.900; specificity, 1). The accuracy
of ATIII and ATIII/Cr as predictors of survival was intermediate
(AUC-ROC, 0.681 and 0.804; sensitivity of 0.802 and 0.596, and
specificity 0.542 and 0.875, respectively). In the setting of the
ICU, ATIII demonstrated accurate predictive value for
AKI-nondevelopment in elderly patients with sepsis. The ATIII/Cr
ratio was used because it makes more sense to measure both
parameters simultaneously. This is because, decreased creatinine
and increased ATIII predict the improvement of renal function
injury; increased creatinine and decreased ATIII predict
aggravation of severe renal damage; an increase in both creatinine
and ATIII suggests that renal injury may not be caused by
inflammation; and decreased creatinine suggests possible
coagulation abnormalities (4,9,10). This may also be the reason that the
ATIII/Cr ratio exhibited higher significance (and a lower P-value)
than ATIII.
To the best of our knowledge, this is the largest
dataset analysed to determine the predictive potential of serum
ATIII level on AKI development in patients with sepsis. There are
articles have described the protective effect of ATIII on the
kidneys, but these were studies conducted on animals (12,20).
Katayama et al (21) revealed
that the AUC-ROC of AT III was 0.618 (0.564–0.670); however, in the
present study the AUC-ROC of ATIII was calculated to be 0.729
(0.596–0.861), close to sTM 0.758 (0.677–0.825). Furthermore, the
AUC-ROC of ATIII/Cr was 0.971 (0.940–1). To the best of our
knowledge, the prognostic value of ATIII level in the prediction of
AKI in elderly patients with sepsis has not previously been
elucidated. ATIII has been revealed as a promising single,
universal predictive methodology for sepsis with an AKI diagnosis.
In addition, further studies on ATIII will be further studied in
the future and may help to improve evaluation of prognosis in
patients with sepsis-associated renal injury, in addition to
helping clinicians diagnose AKI more quickly and accurately. ATIII
is gradually being introduced into clinical work in the setting of
ICUs, however, there are also multiple challenges for its use,
including a lack of universal standards for results analysis or
guidelines for results interpretation. The early detection of AKI
in sepsis is still challenging, requiring a combination of clinical
judgement and other objective workups.
In the present study, ATIII exhibited a predictive
effect on kidney injury caused by sepsis. Low ATIII levels are
associated with an increased risk of AKI in patients with sepsis.
In rat models, ATIII improves kidney injury prognosis primarily via
the reduction of inflammation, apoptosis and oxidative stress. In
addition, ATIII may serve an anti-inflammatory role by inhibiting
the release of both inflammatory cytokines, and chemical
kinase-mediated infiltration of immune cells (12). Therefore, ATIII may serve a
predictive, preventative and protective role in sepsis-induced AKI.
ATIII serine protease inhibitors not only represent major coagulant
factor inhibitors in vivo, but also exhibit numerous
clinical anti-inflammatory properties, including in disseminated
intravascular coagulation and sepsis (9,22). In
addition to inhibiting inflammatory cytokines produced by
endothelial cells (23), the
mechanisms underlying the ATIII anti-inflammatory effect include
increased synthesis and secretion of prostaglandin, inhibition of
neutrophil roll and adhesion (24)
and prevention of platelet activity (9).
ATIII does not appear to have a significant
advantage over other biomarkers (including NGAL, KIM-1 and cystatin
C) already used in the clinic. However, the limitations of the
current study may have contributed to this; the retrospective
design, small population size poor follow-up of long-term survival
mean further studies with a larger sample size and improved study
design may help further validate the findings. Nevertheless, ATIII
represents a promising biomarker for renal injury secondary to
sepsis, especially in hospitals where NGAL, KIM-1 and cystatin C
cannot be detected.
The results of the present study indicated that
ATIII can predict AKI in septic elderly patients, guide clinical
management and reduce mortality. Clinics that adopt new methods,
including ATIII assessment for patients with sepsis to assess the
aim of personalized and precision therapy, may be able to utilize
ATIII to predict AKI quickly and guide treatment. As a promising
biomarker, ATIII may change conventional management of patients
with sepsis.
In conclusion, the present study indicated that
ATIII predicts AKI in elderly patients with sepsis. Therefore,
lower serum ATIII levels may predict a poorer prognosis. The
findings of the present study can lead to new ideas on ATIII and
AKI prevention. Further studies are needed to confirm the role of
ATIII administration in predicting AKI and increasing survival in
patients with sepsis.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Important and Weak Subject Construction Project of Shanghai Health
and Family Planning System (grant nos. 2016ZB0205 and 2016ZB0205,
respectively), Shanghai Science and Technology Committee Scientific
and Technological Support Project (grant nos. 18411950600 and
18411950602, respectively), Clinical Research Innovation Plan of
Shanghai General Hospital (grant no. CTCCR-2016B01) and Wu Jieping
Medical Foundation (grant no. 320.6750.18546).
Authors' contributions
YX, RT, WJ, HX, JD, ZZ and RW contributed to the
study conception and design, analysis and interpretation of data;
drafting of the article and critical revision for important
intellectual content. All the authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from Shanghai General
Hospital Institutional Review Board [approval no. (2018)KY004].
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Department of Critical Care Medicine, Shanghai
General Hospital, Shanghai Jiao Tong University School of Medicine,
650 New Songjiang Road, Songjiang, Shanghai, 201600, China.
Glossary
Abbreviations
Abbreviations:
|
APACHE II
|
Acute Physiology and Chronic Health
Evaluation II score
|
|
SOFA
|
Sepsis-related Organ Failure
Assessment
|
|
COPD
|
chronic obstructive pulmonary
disease
|
|
ICU
|
intensive care unit
|
|
AKI
|
acute kidney injury
|
|
CRRT
|
continuous renal replacement
therapy
|
|
ATIII
|
Antithrombin III
|
|
KDIGO
|
Kidney Disease Improving Global
Outcomes
|
References
|
1
|
Godin M, Murray P and Mehta RL: Clinical
Approach to the patient with AKI and Sepsis. Semin Nephrol.
35:12–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gomez H, Ince C, De Backer D, Pickkers P,
Payen D, Hotchkiss J and Kellum JA: A unified theory of
sepsis-induced acute kidney injury: Inflammation, microcirculatory
dysfunction, bioenergetics, and the tubular cell adaptation to
injury. Shock. 41:3–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cao Y and Wang RH: Associations among
metabolism, circadian rhythm and age-associated diseases. Aging
Dis. 8:314–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wen X, Peng Z and Kellum JA: Pathogenesis
of acute kidney injury: Effects of remote tissue damage on the
kidney. Contrib Nephrol. 174:129–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Post EH, Kellum JA, Bellomo R and Vincent
JL: Renal perfusion in sepsis: From macro- to microcirculation.
Kidney Int. 91:45–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Regueira T, Andresen M, Mercado M and
Downey P: Physiopathology of acute renal failure during sepsis. Med
Intensiva. 35:424–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaifei W, Sheling X, Kun X, Peng Y, Wanxue
H and Lixin X: Biomarkers of sepsis-induced acute kidney injury.
BioMed Res International. 2018:1–7. 2018.
|
|
8
|
Lentini P, de Cal M, Clementi A, D'Angelo
A and Ronco C: Sepsis and AKI in ICU patients: The role of plasma
biomarkers. Crit Care Res Pract. 2012:8564012012.PubMed/NCBI
|
|
9
|
Levy JH, Sniecinski RM, Welsby IJ and Levi
M: Antithrombin: Anti-inflammatory properties and clinical
applications. Thromb Haemost. 115:712–728. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Z, Cheng D, Yin J, Wu R, Zhang G, Zhao
Q, Wang N, Wang F and Liang M: Antithrombin III protects against
contrast-induced nephropathy. EBioMedicine. 17:101–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Q, Yin J, Lu Z, Kong Y, Zhang G, Zhao
B and Wang F: Sulodexide protects contrast-induced nephropathy in
Sprague-Dawley rats. Cell Physiol Biochem. 40:621–632. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang F, Zhang G, Lu Z, Geurts AM, Usa K,
Jacob HJ, Cowley AW, Wang N and Liang M: Antithrombin III/SerpinC1
insufficiency exacerbates renal ischemia/reperfusion injury. Kidney
Int. 88:796–803. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu Z, Wang F and Liang M:
SerpinC1/Antithrombin III in kidney-related diseases. Clin Sci
(Lond). 131:823–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seymour CW, Liu VX, Iwashyna TJ,
Brunkhorst FM, Rea TD, Scherag A, Rubenfeld G, Kahn JM,
Shankar-Hari M, Singer M, et al: Assessment of clinical criteria
for sepsis: For the third international consensus definitions for
sepsis and septic shock (Sepsis-3). JAMA. 351:762–774. 2016.
View Article : Google Scholar
|
|
15
|
Abdel-Rahman EM and Okusa MD: Effects of
aging on renal function and regenerative capacity. Nephron Clin
Prac. 127:15–20. 2014. View Article : Google Scholar
|
|
16
|
Levey AS, Bosch JP, Lewis JB, Greene T,
Rogers N and Roth D: A more accurate method to estimate glomerular
filtration rate from serum creatinine: A new prediction Equation.
Modification of Diet in Renal Disease Study Group. Ann Intern Med.
130:461–470. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Siew ED, Matheny ME, Ikizler TA, Lewis JB,
Miller RA, Waitman LR, Go AS, Parikh CR and Peterson JF: Commonly
used surrogates for baseline renal function affect the
classification and prognosis of acute kidney injury. Kidney Int.
77:536–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu WS, Chung YT, Yang CY, Lin CC, Tsai
KH, Yang WC, Chen TW, Lai YT, Li SY and Liu TY: Serum creatinine
determined by Jaffe, Enzymatic method, and isotope dilution-liquid
chromatography-mass spectrometry in patients under hemodialysis. J
Clin Lab Anal. 26:206–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Allingstrup M, Wetterslev J, Ravn FB,
Møller AM and Afshari A: Antithrombin III for critically ill
patients: A systematic review with meta-analysis and trial
sequential analysis. Intensive Care Med. 42:505–520. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong Y, Yin J, Cheng D, Lu Z, Wang N, Wang
F and Liang M: Antithrombin III Attenuates AKI following acute
severe pancreatitis. Shock. 49:572–579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Katayama S, Nunomiya S, Koyama K, Wada M,
Koinuma T, Goto Y, Tonai K and Shima J: Markers of acute kidney
injury in patients with sepsis: The role of soluble thrombomodulin.
Crit Care. 21:2292017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Peng C, Zhang G, Zhao Q, Xuan C,
Wei M and Wang N: Delayed kidney injury following coronary
angiography. Exp Ther Med. 12:530–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Souter PJ, Thomas S, Hubbard AR, Poole S,
Römisch J and Gray E: Antithrombin inhibits
lipopolysaccharide-induced tissue factor and interleukin-6
production by mononuclear cells, human umbilical vein endothelial
cells, and whole blood. Crit Care Med. 29:134–139. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ostrovsky L, Woodman RC, Payne D, Teoh D
and Kubes P: Antithrombin III prevents and rapidly reverses
leukocyte recruitment in ischemia/reperfusion. Circulation.
96:2302–2310. 1997. View Article : Google Scholar : PubMed/NCBI
|