Introduction
Morbidity of children with hemangioma has gradually
increased, ranging from 3 to 8%. Premature delivery, low birth
weight, placental dysfunction, elderly parturient women,
amniocentesis and fetal hypoxia are risk factors for the occurrence
of hemangioma in children, with obvious family medical history
(1,2). It usually occurs at birth or within 3
to 6 months after birth, often in the head, face and neck.
Hemangioma, as one of the most common benign tumors in children,
has different treatment methods due to its different locations,
including laser and medical surgical treatment (3). The medical treatment includes oral
propranolol and local injection of pingyangmycin. Propranolol is a
non-selective β-adrenoceptor blocker, which can achieve obvious
efficacy 6 months after continuous use, but has the risk of causing
serious complications such as bradycardia (4), while pingyangmycin is a kind of
cytotoxic glycopeptide antitumor antibiotic, and local injection
can shorten the degeneration process of hemangioma (5). These two drugs are used as the basic
drugs for hemangioma in children, and both have certain adverse
reactions in the process of drug use alone. Pulsed dye laser (PDL)
is a liquid laser with fluorescent organic dye as medium, which can
promote coagulation degeneration of hemoglobin, necrosis of
vascular endothelial cells and vascularization embolism, thus
selectively damaging microvessels, and can treat skin lesions more
effectively than other types of lasers (6). Furthermore, it has been proven that
miRNAs can affect angiogenesis. miRNA-4295, a new oncogenic miRNA,
is highly expressed in many tumor tissues (7). Some studies showed that propranolol
promoted apoptosis by inhibiting the expression of miR-4295 during
the treatment of hemangioma (8). In
this study, pulsed dye laser and two drugs, respectively, were
combined to treat hemangioma in children, to analyze the efficacy,
and compare the two treatment methods to establish the one more
suitable for clinical use, and to further observe the effect of
miR-4295 in the two treatment methods.
Patients and methods
Clinical data
From January 2017 to January 2019, 120 children with
hemangioma admitted to the 3rd Affiliated Hospital of Shenzhen
University (Shenzhen, China) were selected. Among them, 60 received
propranolol combined with pulsed dye laser therapy (group A), the
other 60 received pingyangmycin combined with pulsed dye laser
therapy (group B), and 60 normal children who underwent physical
examination at the same time were selected as control group (group
C). The study was approved by the Ethics Committee of the 3rd
Affiliated Hospital of Shenzhen University. Signed informed
consents were obtained from the parents of the child patients.
Inclusion and exclusion criteria
The age of children involved in this study was 1–6
months. All the children were first time hospitalized. Hemangioma
was definitely diagnosed by clinical examination, B-ultrasound, CT
plain scan, MRI or X-ray examination before treatment, and all
occurred on the body surface. Exclusion criteria were as follows:
i) Children with other tumor history; ii) children who had received
other treatments before this study; iii) children with scar
constitution; iv) children with allergic history; v) children with
cardiovascular diseases such as sinus bradycardia and
atrioventricular block; vi) children with skin ulcer or infection
at the treatment site that were not suitable for laser treatment;
vii) children with dysphoria and coordination difficulties.
Methods
Altogether 120 children were treated with 595 nm
pulsed dye laser (model: Vbeam595nm; Candela Company). First, the
children were locally given 2% lidocaine for surface anesthesia.
The laser wavelength was adjusted to 595 nm and the pulse width was
0.45–20 msec. Appropriate treatment parameters were selected
according to the location of hemangioma. After irradiation, they
were ice compressed for 30 min and zinc oxide cream was used until
the petechiae caused by laser disappeared, with an interval of 1
month (9). At the time of laser
treatment, children in group A were treated with propranolol
[Ruiyang Pharmaceutical (Shanghai) Co., Ltd.; SFDA approval no.
H31021347, specification: 10 mg], they were orally administered 3
times a day, 0.5 mg/kg each time, if there were no adverse
reactions, the dose was doubled to 1 mg/kg/day after 3 days and was
changed to 2 mg/kg/day to maintain (10) 3 days after further treatment. By
contrast, children in group B were given pingyangmycin (Hanhui
Pharmaceutical Co., Ltd.; SFDA approval no. H20059039,
specification: 8 mg) 8 mg mixed with 2 ml normal saline for local
injection of lesions, twice/15 days.
Blood samples were collected from children in the
three groups before and after treatment. Real-time fluorescence
quantitative PCR (RT-PCR) was used to detect the expression of
miR-4295 (the kit was purchased from Beijing Kangjiahongyuan
Biotechnology Co., Ltd.). Total RNA was extracted in strict
accordance with the instructions of TRIzol kit (Anhui Jingke
Biology Co., Ltd.; art. no. WLA088a). RNA was reverse transcribed
into cDNA according to the instruction manual of reverse
transcription kit (Beijing Protein Innovation; art. no. BPI01030),
and then amplification was carried out using amplification kit
(Shenzhen Ziker Biotechnology Co., Ltd.; art. no. TQ2104-01). The
primer sequence was synthesized by Shanghai Rebiosci Biotechnology
Co., Ltd. Details are shown in Table
I. First, they were pre-denatured at 94°C for 30 sec, denatured
at 94°C for 5 sec, annealed and extended at 60°C for 30 sec, with
40 cycles. The experiment was carried out three times, U6 was used
for internal reference and data were analyzed by
2−ΔCT.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
|
| miR-4295 | U6 |
|---|
| Reverse |
5′-CAGTGCAATGTTTTCCTTGCC-3′ |
5′-ATTGGAACGATACAGAGAAGATT-3′ |
| Forward |
5′-GTAGGAACAGTCTAGTTTCTTAGCC-3′ |
5′-GGAACGCTTCACGAATTTG-3′ |
Criterion for observation indicators
and efficacy
After 6 months of treatment, the efficacy and
adverse reactions of children in the two groups were observed and
compared. According to Achauer grading evaluation standard
(11), hemangioma is divided into
four levels: Level I: Hemangioma tumor volume shrinks or tumor
color fades <25%; level II: Hemangioma tumor volume shrinks or
tumor color fades by 25–50%; level III: Hemangioma tumor volume
shrinks or tumor color fades by 51–75%; level IV: Hemangioma tumor
volume shrinks or tumor color fades 76–100%. Level I is defined as
no obvious efficacy, level II–III as effective, and level IV as
markedly effective. Remission rate = markedly effective percentage
+ effective percentage.
Statistical methods
All the data used in this study were analyzed by
SPSS 24.0. Measurement data were expressed as mean ± standard
deviation, the enumeration data were expressed as percentage, the
comparison of the enumeration data was performed by Chi-square
test, the comparison between the two groups was performed by
t-test, the comparison between multiple groups was performed by
variance analysis, and the diagnostic value was performed by ROC
curve analysis. P<0.05 was considered statistically significant
between the two groups.
Results
Comparison of clinical data
There was no significant difference in gestational
age, age (month), sex, body weight, white blood cells, location,
course of disease, delivery mode and tumor type between group A,
group B (research group) and group C (control group) (P>0.05),
proving that children in the three groups were comparable, as shown
in Table II.
 | Table II.Comparison of clinical data [n
(%)]. |
Table II.
Comparison of clinical data [n
(%)].
| Features | Group A (n=60) | Group B (n=60) | Group C (n=60) | χ2 or
F | P-value |
|---|
| Gestational age
(weeks) | 33.51±5.84 | 34.17±6.22 | 34.53±5.79 | 0.453 | 0.636 |
| Age (month) |
4.26±1.72 |
3.83±2.13 |
4.19±1.56 | 0.965 | 0.383 |
| Sex |
|
|
|
|
|
| Male | 21 (35.00) | 23 (38.33) | 28 (46.67) |
|
|
|
Female | 39 (65.00) | 37 (61.67) | 32 (53.33) |
|
|
| Weight (kg) | 5.37±2.21 | 5.31±2.02 | 5.41±1.98 | 0.035 | 0.965 |
| White blood cells
(109/l) | 7.12±2.37 | 7.24±2.08 | 7.18±1.64 | 0.051 | 0.950 |
| Location |
|
|
|
|
|
|
Maxillofacial region | 22 (36.67) | 24 (40.00) |
|
|
|
|
Trunk | 15 (25.00) | 10 (16.67) |
|
|
|
|
Limbs | 17 (28.33) | 18 (30.00) |
|
|
|
| Multiple
parts of the body | 6
(10.00) | 8
(13.33) |
|
|
|
| Course of disease
(month) | 3.41±2.26 | 3.19±2.14 |
| 0.548 | 0.585 |
| Delivery mode |
|
|
|
|
|
|
Eutocia | 42 (70.00) | 38 (63.33) | 46 (76.67) |
|
|
| Cesarean
section | 18 (30.00) | 22 (36.67) | 14 (23.33) |
|
|
| Tumor
classification |
|
|
|
|
|
| Capillary
hemangioma | 41 (68.33) | 44 (73.33) |
|
|
|
| Cavernous
hemangioma | 12 (20.00) | 10 (16.67) |
|
|
|
| Plexiform
hemangioma | 7
(11.67) | 6
(10.00) |
|
|
|
Comparison of the total remission rate
of children in the two groups 6 months after treatment
Through analysis, we found that children in the two
groups had obvious efficacy after drug combination, respectively,
and there was no obvious difference in efficacy between the two
groups (P<0.05) (Table
III).
 | Table III.Comparison of efficacy between group A
and group B. |
Table III.
Comparison of efficacy between group A
and group B.
| Group | Number of cases | Markedly
effective | Effective | Ineffective | Total remission
rate |
|---|
| Group A | 60 | 43 (71.67) | 15 (25.00) | 2 (3.33) | 58 (96.67) |
| Group B | 60 | 40 (66.67) | 17 (28.33) | 3 (5.00) | 57 (95.00) |
| χ2
value |
|
|
|
| 0.209 |
| P-value |
|
|
|
| 0.648 |
Comparison of the incidence rate of
adverse reactions between children in the two groups
The adverse reactions such as decreased heart rate,
fever, gastrointestinal symptoms and scar formation were compared
between the two groups. The incidence rate of adverse reactions in
group A was 13.33%, while that in group B was 31.67%, with
statistically significant difference (P<0.05) (Table IV).
 | Table IV.Comparison of adverse reactions n
(%). |
Table IV.
Comparison of adverse reactions n
(%).
| Group | Number of
cases | Decreased heart
rate [n (%)] | Fever [n (%)] | Gastrointestinal
symptoms [n (%)] | Scar formation [n
(%)] | Occurrence of
adverse reactions [n (%)] |
|---|
| Group A | 60 | 2 (3.33) | 2 (3.33) | 3 (5.00) | 1 (1.67) | 8
(13.33) |
| Group B | 60 | 3 (5.00) | 6 (10.00) | 8 (13.33) | 2 (3.33) | 19 (31.67) |
| χ2
value |
|
|
|
|
| 5.783 |
| P-value |
|
|
|
|
| 0.016 |
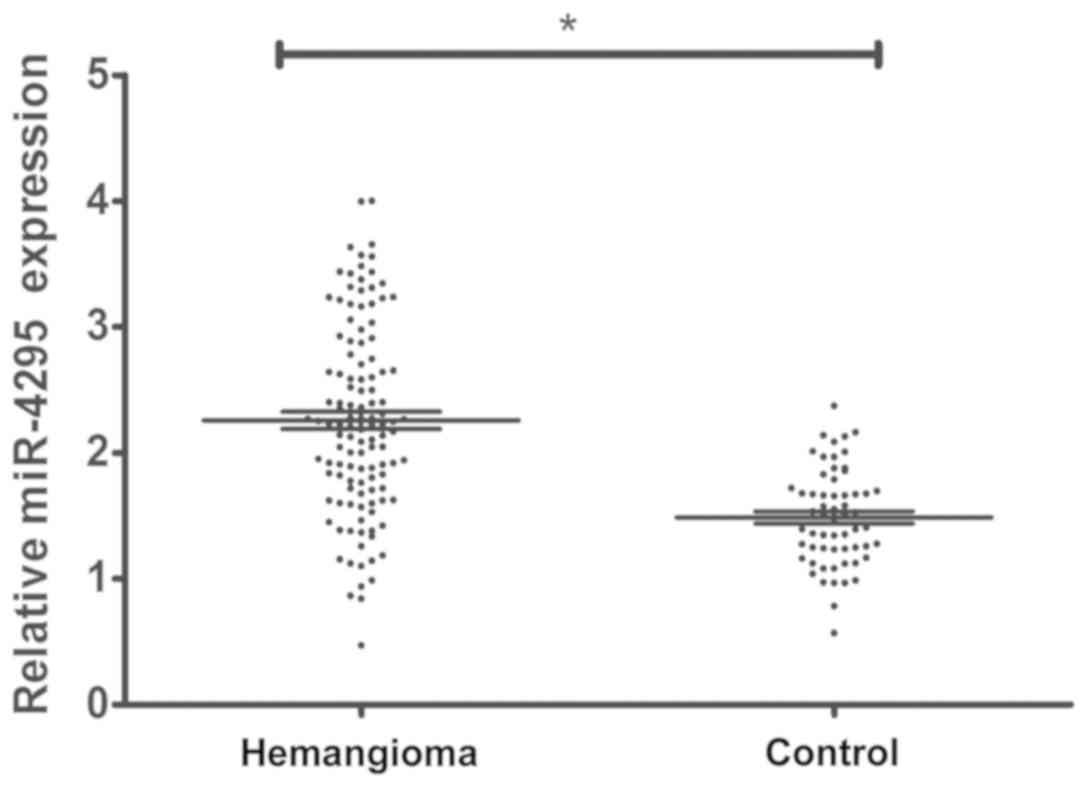
Detection of the expression level of
miR-4295 in hemangioma tissues of children
Blood samples of 120 children with hemangioma and 60
normal children were collected before treatment. The expression
level of miR-4295 was detected by real-time fluorescence
quantitative PCR, and the results showed that the expression level
of miR-4295 in children with hemangioma was significantly higher
than that in normal samples (P<0.0001) (Fig. 1).
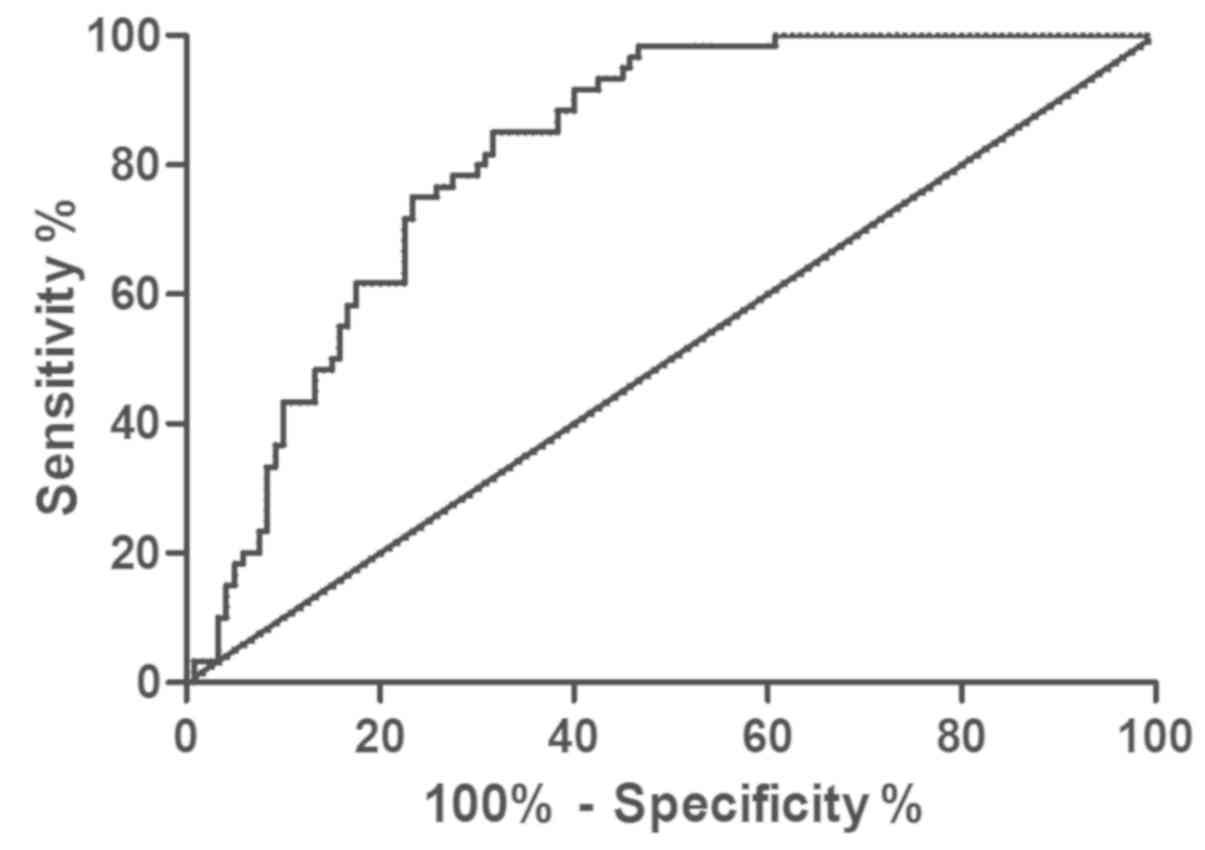
Prediction of hemangioma through
miR-4295
Real-time fluorescence quantitative PCR (RT-PCR) was
used to detect the expression level of miR-4295 in blood of
children with hemangioma, and ROC curve analysis was drawn. Area
under miR-4295 curve was 0.817, sensitivity was 85.00%, specificity
was 68.33%, cut-off value was 1.88, and 95% CI was 59.22–76.52%
(Fig. 2).
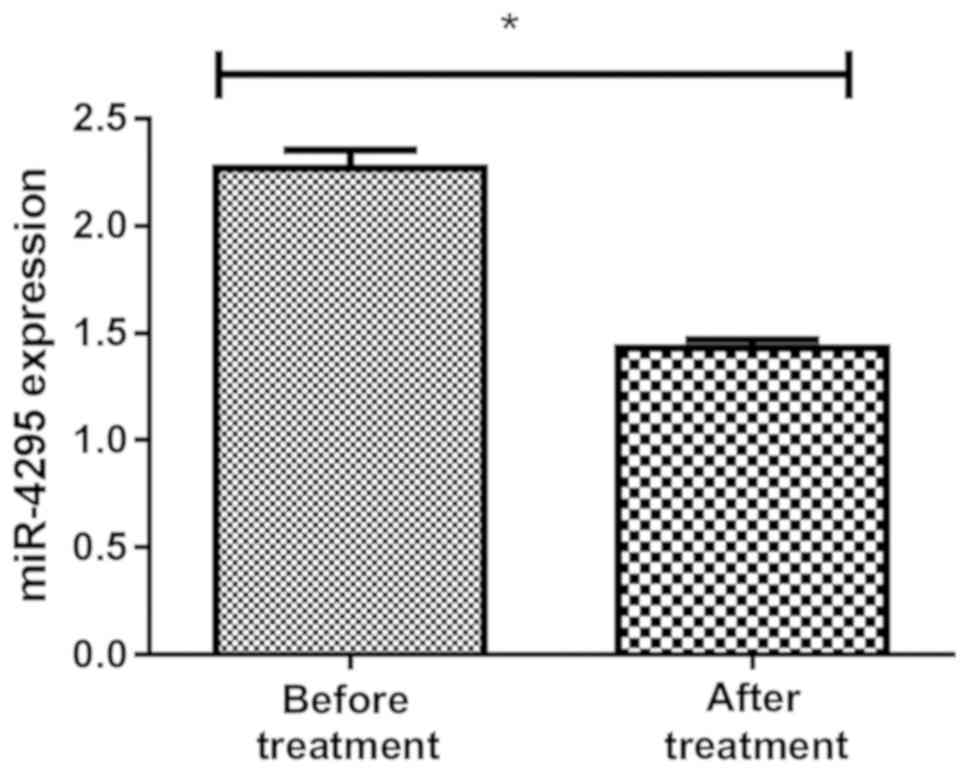
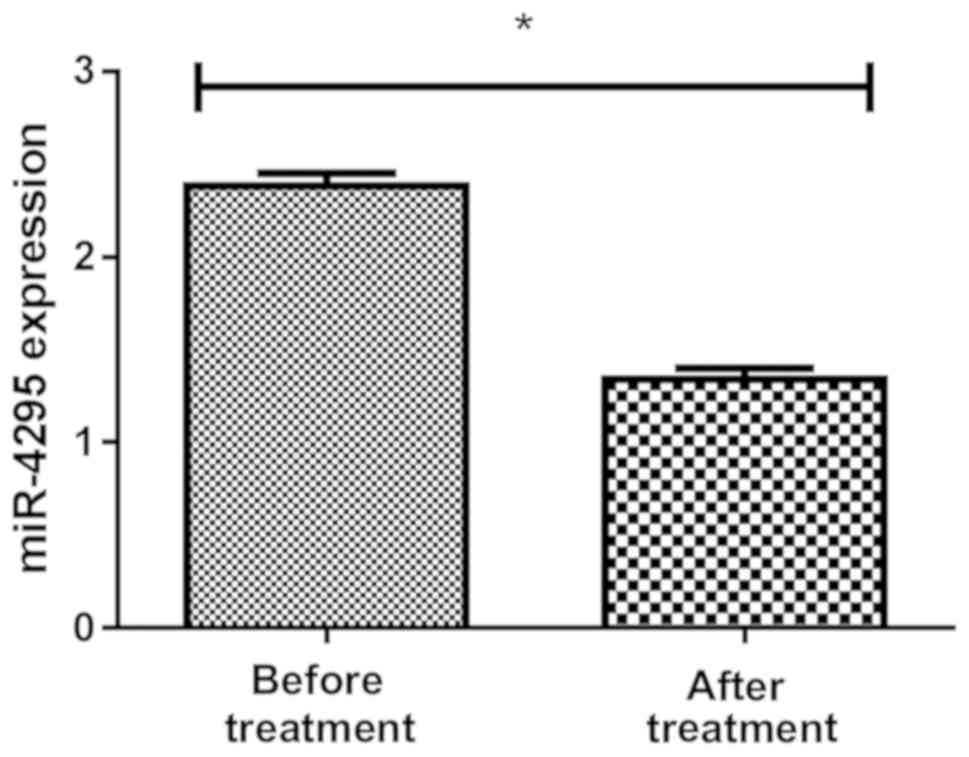
Detection of the expression level of
miR-4295 before and after treatment in groups A and B
The expression of miR-4295 in blood samples of
children in group A was 2.29±0.77 before treatment and 1.49±0.36
after treatment, and the expression of miR-4295 in blood samples of
children in group B was 2.33±0.73 before treatment and 1.39±0.47
after treatment, with statistically significant difference
(P<0.01) (Figs. 3 and 4).
As shown in Fig. 1,
the expression level of miR-4295 in children with hemangioma was
significantly higher than that in normal children, and the
expression level of miR-4295 significantly reduced after the
combination of propranolol and pingyangmycin with PDL,
respectively.
Correlation analysis of the expression
level of miR-4295 and clinical pathology of children with
hemangioma in the two groups
Correlation between miR-4295 and clinical
characteristics of the children in the research groups was
analyzed. The results of variance analysis showed that the
expression of miR-4295 in different tumor types was significantly
different (P<0.01), and the comparison of LSD showed that the
expression of miR-4295 in cavernous hemangioma was significantly
higher than that in capillary hemangioma (P<0.05). The analysis
revealed that the course of disease, gestational age and tumor type
were related to the expression of miR-4295 (P<0.05), while sex
and location of occurrence were not significantly related to the
expression of miR-4295 (P>0.05) (Table V).
 | Table V.Correlation analysis of the
expression level of miR-4295 and clinical pathology of children
with hemangioma in the two groups. |
Table V.
Correlation analysis of the
expression level of miR-4295 and clinical pathology of children
with hemangioma in the two groups.
| Features | N | miR-4295 level | t or F | P-value |
|---|
| Course of disease
(month) |
| 3.090 | 0.003 |
|
|
<3 | 49 | 2.42±0.54 |
|
|
| ≥3 | 71 | 2.71±0.48 |
|
|
| Gestational age
(weeks) |
|
| 3.100 | 0.002 |
|
32.83±4.16 | 89 | 2.58±0.56 |
|
|
|
39.51±2.27 | 31 | 2.24±0.41 |
|
|
| Sex |
|
| 0.209 | 0.835 |
|
Male | 21 | 2.37±0.74 |
|
|
|
Female | 39 | 2.33±0.69 |
|
|
| Location |
|
| 0.172 | 0.915 |
|
Maxillofacial region | 46 | 2.29±0.81 |
|
|
|
Trunk | 25 | 2.41±0.52 |
|
|
|
Limbs | 35 | 2.34±0.58 |
|
|
|
Multiple parts of the
body | 14 | 2.33±0.64 |
|
|
| Tumor
classification |
|
| 7.944 | P<0.01 |
|
Capillary hemangioma | 85 | 2.18±0.42 |
|
|
|
Cavernous hemangioma | 22 | 2.63±0.57 |
|
|
|
Plexiform hemangioma | 13 | 2.25±0.61 |
|
|
Discussion
Hemangioma in children is a benign vascular tumor
caused by abnormal proliferation of endothelial cells and
peripheral cells. It is the most common tumor in infants (12), especially those aged under one year.
Tumors show unique life cycle and can be divided into three stages:
proliferation stage, stable stage and dissipation stage (13). It is characterized by rapid
proliferation in early postnatal period and spontaneous and slow
regression afterwards. Different from other tumors, they have
unique regression ability after diffusion, which often leads
primary health care providers to think that they will be solved
without intervention. Unfortunately, severe hemangiomas will
rapidly develop complications, resulting in pain, functional damage
or permanent deformity (14).
Hemangioma is a self-limiting tumor, and the tumors that usually
need to be treated include periorbital region, central face,
airway, skin fold and anal region, as well as the high-risk parts
of ulcer, dysfunction or deformity, which will affect the normal
growth and development of children, and will have serious impact on
the appearance and psychology of children.
Propranolol is a non-selective β receptor blocker
commonly used clinically. In recent years, a large number of
studies on the successful treatment of hemangioma with oral
propranolol have been reported, making propranolol the first-line
drug for the treatment of hemangioma (15–17).
Pingyangmycin, as an antitumor antibiotic, forms thrombus in tumor
blood sinus and causes fibrosis of the tumor body when injected
into a tumor, which is a reliable and effective treatment method
for children with hemangioma (18–20).
With the rapid development of laser technology, it has been
gradually applied to the treatment of clinical diseases. Through
pulsed dye laser technology, hemoglobin is destroyed under laser
selective photothermolysis. Early use can accelerate the transition
from hemangioma to plateau or degeneration stage to prevent further
growth of hemangioma, and has obvious efficacy on superficial
hemangioma (21,22). In this study, propranolol and
pingyangmycin were, respectively, combined with pulsed dye laser to
treat hemangioma in children. The results revealed that the two
treatment methods had obvious efficacy, but the adverse reactions
of propranolol combined with PDL in the treatment of hemangioma
were less than those of pingyangmycin. In the later stage of
treatment, the tumor volume of hemangioma in children significantly
reduced and there was no other discomfort. The method is simple and
convenient to operate in clinical application and is worth
promoting. The expression level of miR-4295 before and after
treatment was detected, and the expression level of miR-4295 in
hemangioma specimens before treatment was significantly higher than
that in normal specimens. After intervention by two methods, the
expression level of miR-4295 in tumor tissues was found to be
significantly lower than that before (P<0.05). This finding
suggested that the two treatment methods might be mediated by
miR-4295 to produce relevant effects. Through the detection of
miR-4295, it was found that the expression of miR-4295
significantly increased in children and premature infants with
different tumor types and course of disease over three months
(P<0.05), while sex was not correlated with the expression of
miR-4295 (P>0.05). In addition, the regulatory networks
identified in previous studies included microRNA miR-9, miR-939 and
let-7 families; these microRNA and deregulated genes had the most
interaction in children with hemangioma, suggesting that they might
play an important role in the molecular mechanism of disease
(23). However, according to the
results of this study, the expression of miR-4295 in hemangiomas
obviously increased. Therefore, whether miR-4295 also had a
mechanism of action in hemangiomas and played a part in the
occurrence and development remained to be further studied. If
miR-4295 is confirmed to be a new potential biomarker, it can be
helpful for early screening and prognosis of hemangiomas in
clinical practice.
The limitations to this study are that no basic
research was carried out, thus, it is impossible to further
understand how miR-4295 is related to hemangiomas, and at present
impossible to judge which treatment method is more closely related
to miR-4295. Further research is needed as well as relevant
follow-up.
In conclusion, the clinical efficacy of propranolol
and pingyangmycin combined with pulsed dye laser respectively in
the treatment of children with hemangioma is ideal, and the adverse
reactions of propranolol group are less and its safety is higher,
and the process of clinical application should be comprehensively
analyzed based on the actual situation. However, miR-4295 is highly
expressed in children with hemangioma, and its expression level is
reduced after two methods of treatment. The mechanism of miR-4295
on hemangiomas needs further study, to make miR-4295 a promising
indicator for future diagnosis and treatment of hemangiomas.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZH, NZ and HC conceived and designed the study, and
drafted the manuscript. ZH, NZ, HC and KL collected, analyzed and
interpreted the experimental data. ZH revised the manuscript for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the 3rd Affiliated Hospital of Shenzhen University (Shenzhen,
China). Signed informed consents were obtained from the parents of
the child patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Castrén E, Salminen P, Vikkula M,
Pitkäranta A and Klockars T: Inheritance patterns of infantile
hemangioma. Pediatrics. 138:e201616232016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Jong S, Itinteang T, Withers AH, Davis
PF and Tan ST: Does hypoxia play a role in infantile hemangioma?
Arch Dermatol Res. 308:219–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Darrow DH, Greene AK, Mancini AJ and
Nopper AJ; Section on dermatology, section on otolaryngology-head
and neck surgery, section on plastic surgery, : Diagnosis and
management of infantile hemangioma: Executive summary. Pediatrics.
136:786–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Léauté-Labrèze C, Hoeger P,
Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, Phillips RJ,
Caceres H, Lopez Gutierrez JC, Ballona R, et al: A randomized,
controlled trial of oral propranolol in infantile hemangioma. N
Engl J Med. 372:735–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang L, Yuan WE and Zheng JW:
Pharmacological therapies for infantile hemangiomas: A clinical
study in 853 consecutive patients using a standard treatment
algorithm. Sci Rep. 6:216702016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chinnadurai S, Sathe NA and Surawicz T:
Laser treatment of infantile hemangioma: A systematic review.
Lasers Surg Med. 48:221–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nan YH, Wang J, Wang Y, Sun PH, Han YP,
Fan L, Wang KC, Shen FJ and Wang WH: MiR-4295 promotes cell growth
in bladder cancer by targeting BTG1. Am J Transl Res. 8:4892–4901.
2016.PubMed/NCBI
|
|
8
|
Zhao F, Yang X, Xu G, Bi J, Lv R and Huo
R: Propranolol suppresses HUVEC viability, migration, VEGF
expression, and promotes apoptosis by downregulation of miR-4295. J
Cell Biochem. 120:6614–6623. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asilian A, Mokhtari F, Kamali AS,
Abtahi-Naeini B, Nilforoushzadeh MA and Mostafaie S: Pulsed dye
laser and topical timolol gel versus pulse dye laser in treatment
of infantile hemangioma: A double-blind randomized controlled
trial. Adv Biomed Res. 4:2572015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hogeling M, Adams S and Wargon O: A
randomized controlled trial of propranolol for infantile
hemangiomas. Pediatrics. 128:e259–e266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Achauer BM, Chang CJ and Vander Kam VM:
Management of hemangioma of infancy: Review of 245 patients. Plast
Reconstr Surg. 99:1301–1308. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji Y, Chen S, Li K, Li L, Xu C and Xiang
B: Signaling pathways in the development of infantile hemangioma. J
Hematol Oncol. 7:132014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Greenberger S: Infantile
Hemangioma[M]//Sex and Dermatology. Springer Cham; New York, NY:
pp. 215–225. 2018
|
|
14
|
Darrow DH, Greene AK, Mancini AJ and
Nopper AJ; Section on dermatology, section on otolaryngology-head
and neck surgery, section on plastic surgery, : Diagnosis and
management of infantile hemangioma. Pediatrics. 136:e1060–e1104.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chinnadurai S, Fonnesbeck C, Snyder KM,
Sathe NA, Morad A, Likis FE and McPheeters ML: Pharmacologic
interventions for infantile hemangioma: A meta-analysis.
Pediatrics. 137:e201538962016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Léaute-Labrèze C, Boccara O,
Degrugillier-Chopinet C, Mazereeuw-Hautier J, Prey S, Lebbé G,
Gautier S, Ortis V, Lafon M, Montagne A, et al: Safety of oral
propranolol for the treatment of infantile hemangioma: A systematic
review. Pediatrics. 138:e201603532016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ng M, Knuth C, Weisbrod C and Murthy A:
Propranolol therapy for problematic infantile hemangioma. Ann Plast
Surg. 76:306–310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou J, Wang M, Tang H, Wang Y and Huang H:
Pingyangmycin sclerotherapy for infantile hemangiomas in oral and
maxillofacial regions: An evaluation of 66 consecutive patients.
Int J Oral Maxillofac Surg. 40:1246–1251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Li P, Xia S, Zhuo Y and Wu L:
Proapoptotic effect and the mechanism of action of pingyangmycin on
cavernous hemangiomas. Exp Ther Med. 7:473–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tu JB, Li QY, Jiang F, Hu XY, Ma RZ, Dong
Q, Zhang H, Pattar P and Li SX: Pingyangmycin stimulates apoptosis
in human hemangioma-derived endothelial cells through activation of
the p53 pathway. Mol Med Rep. 10:301–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwon SH, Choi JW, Byun SY, Kim BR, Park
KC, Youn SW, Huh CH and Na JI: Effect of early long-pulse pulsed
dye laser treatment in infantile hemangiomas. Dermatol Surg.
40:405–411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kessels JP, Hamers ET and Ostertag JU:
Superficial hemangioma: Pulsed dye laser versus wait-and-see.
Dermatol Surg. 39:414–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bertoni N, Pereira LM, Severino FE, Moura
R, Yoshida WB and Reis PP: Integrative meta-analysis identifies
microRNA-regulated networks in infantile hemangioma. BMC Med Genet.
17:42016. View Article : Google Scholar : PubMed/NCBI
|