Introduction
Mild head injury (MHI), mostly presenting as brain
concussion, is a common accident and usually does not result in
severe complications (1). However,
in some cases, MHI may lead to basal ganglia-internal capsule
(BGIC) infarction and cause severe neurological deficits. BGIC
infarction after MHI has rarely been described in children, and the
morbidity of the disease is only 2–3% in all pediatric
craniocerebral trauma (2,3).
Despite a high incidence, reports of this entity are
limited to case reports or small case series (4,5).
Currently, the frequency, cause, imaging changes and influence on
mortality of BGIC infarction are not well defined (6). As such, limited information is
available on BGIC. Hence, a literature search was conducted using
the PubMed database and relevant search terms to review the
research on BGIC infarction after MHI. The present review was
organized following the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) guidelines and was established
as a systematic review (7). In this
review, except for an illustrative case, the risk factors of
infarction, pathophysiology, clinical and radiological features,
diagnosis, treatment and prognosis were analyzed and delineated for
BGIC infarction in children after MHI.
Illustrative case
A 1-year-old male boy was admitted in May 2018 to
The First Hospital of Jilin University due to an inability to walk.
He tripped over a baseball and lost his balance at kindergarten.
The accident was a deceleration injury. Although he suffered an
abrasion, he showed no signs of abnormal behavior. The child did
not lose consciousness following the accident, but 5 h later
developed left-sided weakness, involving the leg and arm. At
admission, upon physical examination, the patient showed clear
consciousness and could answer common questions. The pupils and
relevant reflexes were normal. The power in the left lower limbs
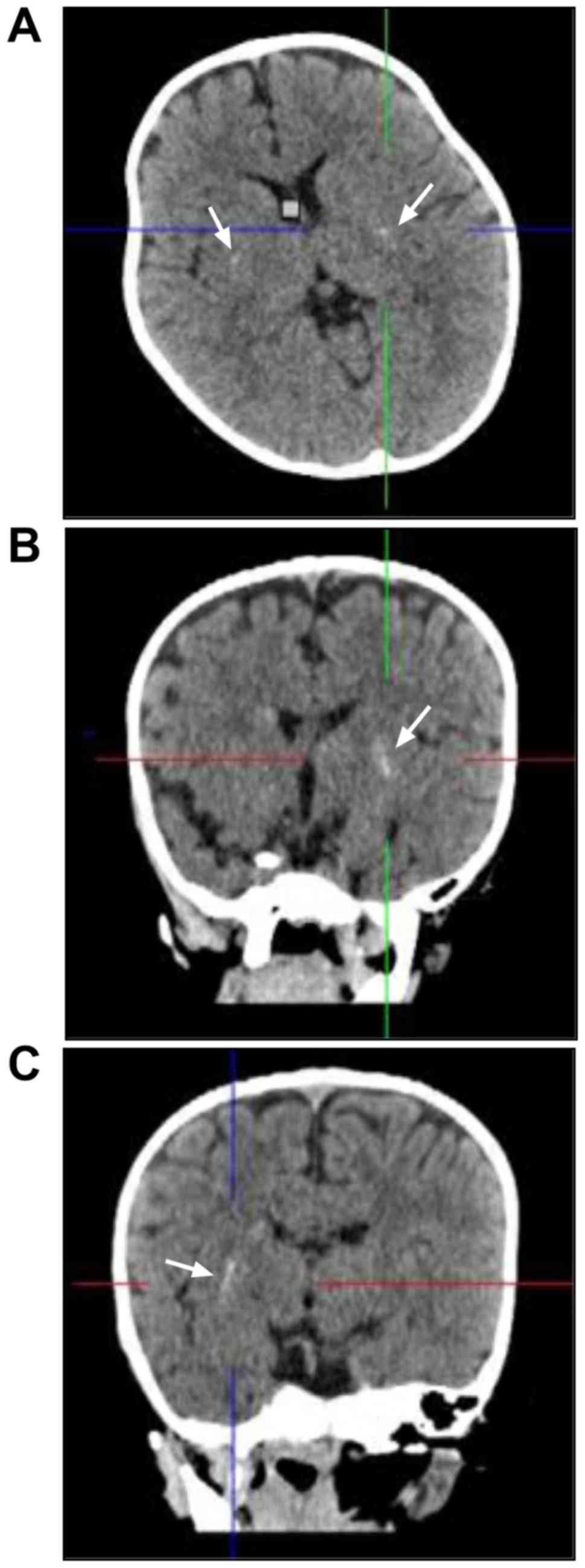
according to Medical Research Council grading was 3/5 (8). Head computed tomography (CT) scans at 6
h showed scattered calcification in the bilateral basal ganglia.
Coronal and sagittal reconstruction showed linear calcification
perpendicular to lateral fissure (Fig.
1). Magnetic resonance imaging (MRI) T2 showed bilateral basal
ganglia infarction 7 h after trauma (Fig. 2A). No abnormalities were found in the
internal carotid artery system by magnetic resonance angiogram
(MRA; Fig. 2B). The male boy was
diagnosed with post-traumatic BGIC. He was given rehabilitative
treatment and a full recovery was made within 1 month. At the
6-month follow-up, his conditions had improved markedly and he had
regained a power of 5/5 in the affected limbs. Outcome assessed
according to The Glasgow Outcome Scale was 5 (9).
Literature search and processing
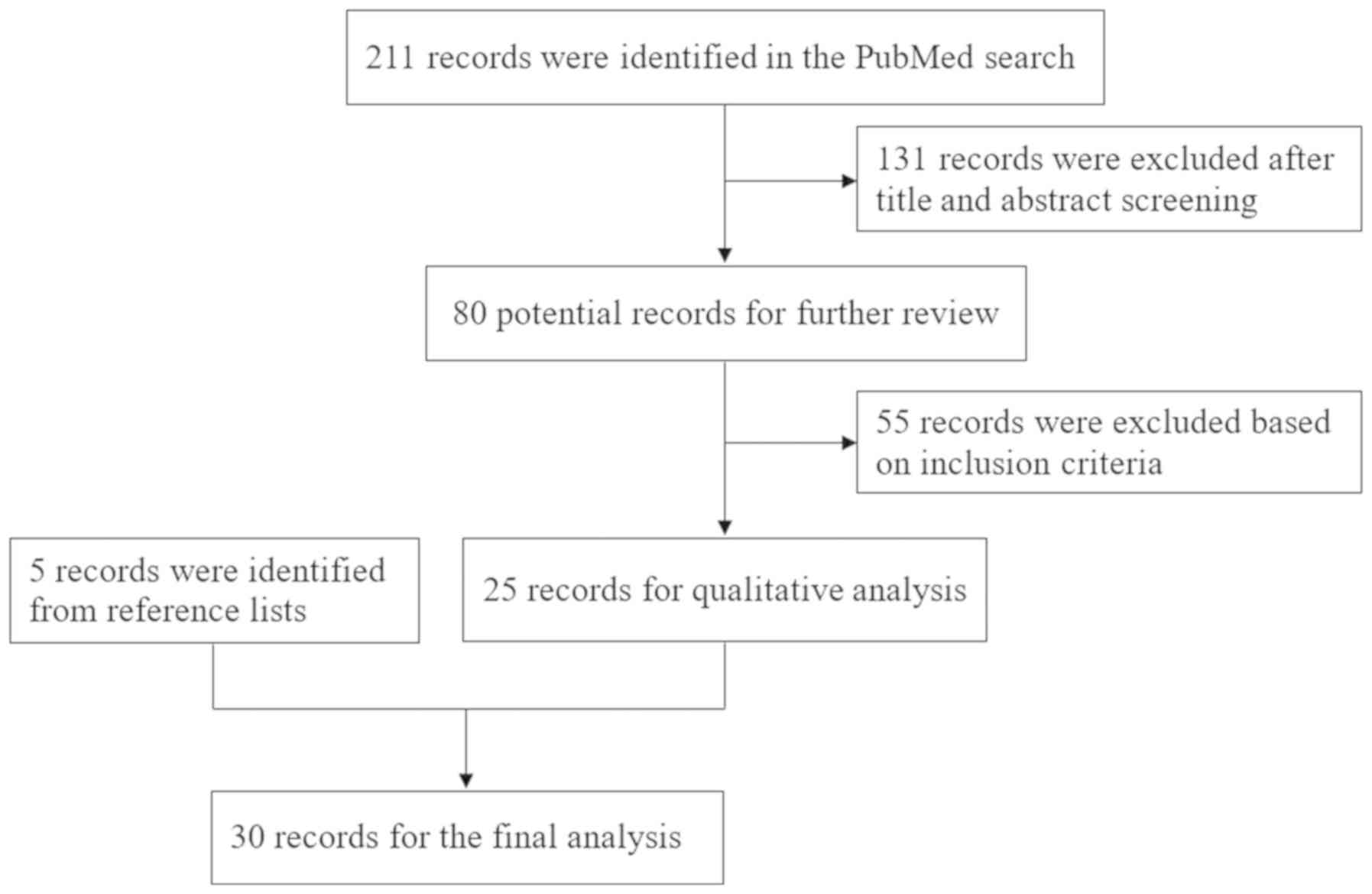
The present systematic review was conducted in
accordance with the PRISMA guidelines (7). Eligible English language articles (case
reports, case series and studies considering BGIC infarction after
MHI) were identified through searches of PubMed publications (the
last search date was May 2019). The search algorithm used the terms
‘basal ganglia-internal capsule,’ ‘mild head injury,’ ‘infarction’
and ‘children’ as key words in relevant combinations. The reference
lists of the identified articles were also manually searched for
additional studies. The resulting flowchart is depicted in Fig. 3.
The inclusion criteria were as follows: i) Full text
was available; ii) clinical data were complete; and iii) all of the
cases in the articles were BGIC infarction after MHI. The studies
without sufficient descriptions of BGIC infarction after MHI were
excluded. After a review of the obtained literature, the current
status of BGIC infarction after MHI was summarized in terms of risk
factors, pathophysiology and pathology, clinical features,
radiological features, treatment and prognosis.
Risk factors
The underlying mechanism leading to the increased
incidence of BGIC infarction in pediatric patients has not been
well described. The incidence of trauma in adults is higher than
that in children, but the morbidity of BGIC is much lower in adults
than in children (5). An increasing
number of studies have focused on the peculiarity of the
craniocerebral anatomy and the pathophysiology of the cerebral
artery with traumatic basal ganglia region apoplexy in children
(1,4,5,10).
Course of the middle cerebral artery
(MCA) and lenticulostriate arteries (LAs)
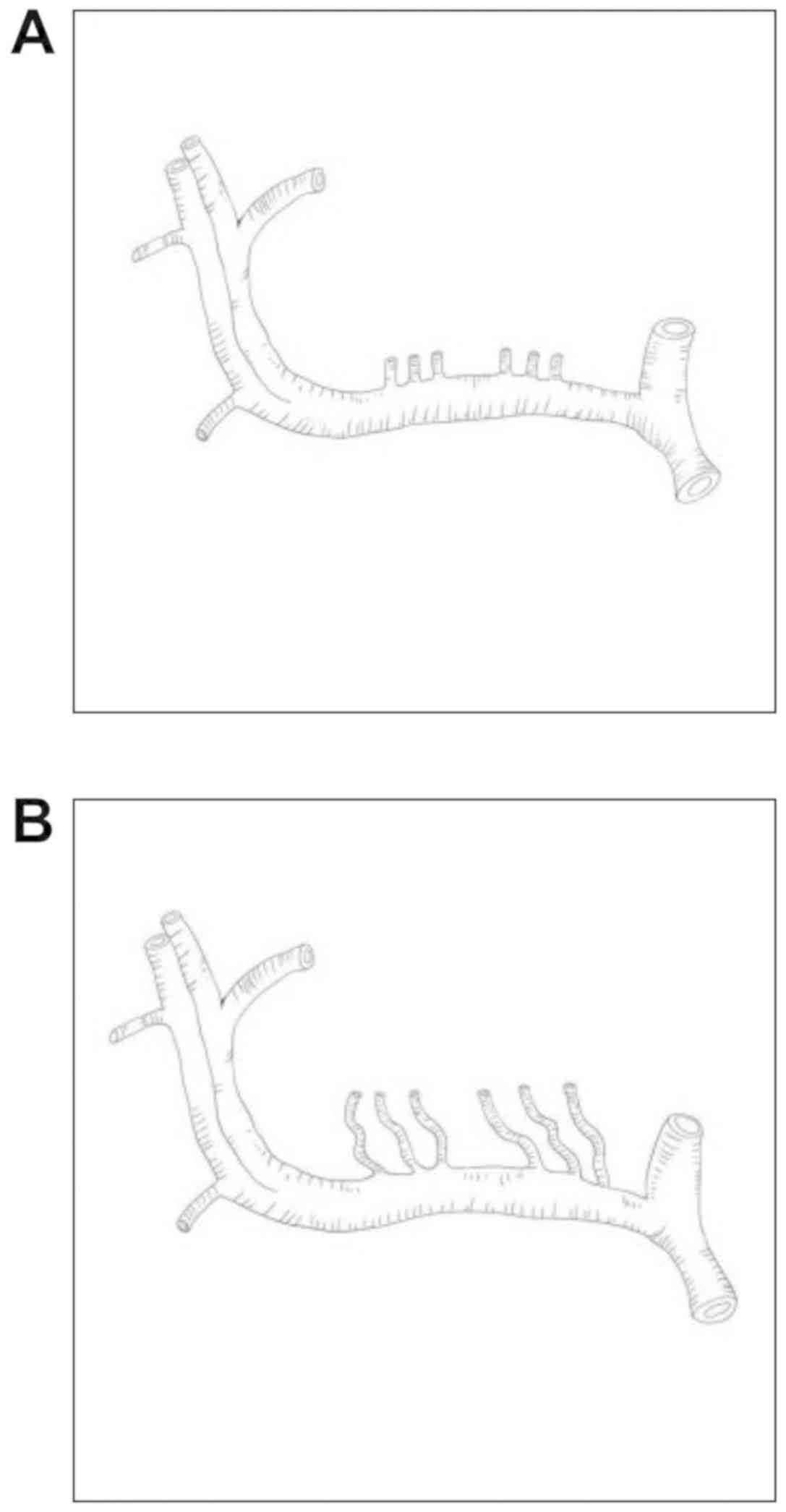
An aged-related anatomical peculiarity of the
cerebral artery may be a possible explanation for BGIC infarction
in children, although the precise pathogenesis remains unclear
(5). The BGIC is supplied by the LAs
of the MCA; the LAs originating from the MCA can be divided into
two segments: The subarachnoid space [extracerebral segments (ES)]
and intracerebral segments (11).
The mobile subarachnoid space segment, the ES, is between two fixed
ends (proximal to the MCA and distal to the brain parenchyma) and
is vulnerable to any sudden movement-induced stretching, inflicting
trauma on the intima, resulting in vasospasm and/or thrombosis
(1,12). In normal conditions, the anatomical
relationship between the LAs and the MCA trunk changes from fetal
life through to childhood and adulthood (13,14). In
infancy, there is an acute angle between the MCA and the LAs. The
lateral perforators are more acute than the medial ones, and this
sharp angle becomes more obtuse during a person's lifetime
(11). The length of these ES also
tends to be shorter in younger individuals (15).
Based on the short ES and acute angle
characteristics of the pediatric LA, the subarachnoid space segment
of the LA in a child is more tensely stretched at an acute angle
compared with that of an adult, and these arteries are functional
end arteries; they are thus mechanically vulnerable to ischemia,
even after MHI (16). The LA
differences between children and adults are presented in Fig. 4.
Unmatured brain and skull
The development of brain tissue in children is not
yet mature, and the subarachnoid space is relatively larger than
adults', which allows relatively violent and rapid displacement
between brain tissue and the skull base during traumatic impact,
resulting in shearing injury of the LAs (17,18). In
addition, the young pediatric brain has greater mobility than the
skull base because the sphenoid bone is underdeveloped and does not
cover the temporal lobes completely, facilitating stretching of the
LAs during MHI (19). Furthermore,
due to the elasticity of the unmatured pediatric skull, the
shearing forces are stronger (4).
Viral infection
The association between cytomegalovirus infection
and stroke has been increasingly emphasized (20–22). For
instance, varicella zoster infection causes vasculopathy and
susceptibility to the development of thrombosis or vasospasm after
MHI (14). It is possible that this
viral infection leads to an increase in the brittleness of the LAs,
which are most likely to cause BGIC infarction in the presence of
external force (22). In addition,
it is possible that viral infection, such as cytomegalovirus
infection, could damage vascular endothelial cells and increase
their susceptibility to developing arterial thrombosis or spasm
following MHI (16).
Genetic factors
It was previously demonstrated that some children
with BGIC have mutations in the calcium voltage-gated channel
subunit α1 A gene, suggesting that vulnerability to adverse
neurological sequelae following MHI may be genetically determined
in some individuals (23). It is
therefore possible that the children described in a previous study
may have an underlying genetic susceptibility to vasospasm or
intimal disruption following MHI (24).
Mineralization and vasculopathy of the
LAs
Basal ganglia mineralization is also a major risk
factor for cerebral infarction identified after MHI in children
(5,25,26).
When mineralization exists, LAs are particularly vulnerable to
transforming, stretching and distorting forces, which can be
imposed even by MHI, making it easier to develop vasospasm and/or
thrombosis (4,27). Further research of the underlying
cause of the mineralization of LAs is needed (28–31). The
genetic factors or viral infection may be the cause, but further
research is needed to explore the mechanisms underlying
mineralizing angiopathy.
Previous studies have found idiopathic lesions in
the arteria lenticularis, termed lenticulostriate vasculopathy
(LSV), which could be detected by cranial ultrasound (32,33).
This is known to occur in 0.4% of all live-born neonates and in
1.9–5.8% of ill neonates (33). It
has been reported to occur in association with a variety of
congenital and acquired disorders and is known to regress over time
(34). The pathology of LSV may
involve mineralization of the hypercellular arterial wall; however,
the etiology is obscure (33).
Cantey and Sisman (31) reported
that, in infants initially identified with congenital infection,
LSV was associated with a variety of infectious and noninfectious
conditions. The congenital and acquired factors may lead to
endothelial dysfunction and vascular inflammation, as well as
vascular smooth muscle cell proliferation (30). Therefore, it was hypothesized that
congenital or acquired damage, for example viral infection, may
cause LSV, and lenticulostriate calcification may be the end stage
of LSV. The etiology needs to be further clarified. The risk
factors are presented in Table
I.
 | Table I.Possible risk factors in the
post-traumatic basal ganglia-internal capsule infarction. |
Table I.
Possible risk factors in the
post-traumatic basal ganglia-internal capsule infarction.
| Type of factor | Possible risk
factors |
|---|
| Anatomic | Course of
lenticulostriate, unmatured brain and skull, unmatured skull |
| Pathological | Viral infection,
genetic factors, mineralization of lenticulostriate artery,
idiopathic lenticulostriate vasculopathy |
Pathogenesis
The occlusion of the perforating vessels of the MCA
led to small infarctions in the BGIC (26,27,35). The
pathogenesis of post-traumatic occlusions of the MCA can be divided
into four different types of lesions: Emboli from the cervical
portion of the carotid artery, vasospasm, traumatic dissection and
post-traumatic thrombosis (36).
Some cases of BGIC infarction after MHI have a reversible nature,
and it was hypothesized that mechanical spasm or thrombosis of the
perforating vessels might play a role in the injury (16,17,37–39).
The theory of vasoconstriction due to physical
stimulation by direct stretching or mechanical irritation has been
supported, and local inflammation inducing arterial narrowing has
been demonstrated (40). Based on
the aforementioned risk factors of BGIC, stretching or mechanically
altering a vessel causes vasospasm and the thrombosis response,
which may result in BGIC infarction (41).
Clinical features
History of trauma
In children with BGIC infarction, most suffer from
minor injuries. The mechanism of trauma is different from that of
high-speed injury, such as a motor vehicle crash, high-altitude
crash injuries or abusive head trauma. It is noteworthy that most
injuries are low-speed injuries. Most had a definite history of a
fall from a low-altitude height, either from the lap of a mother, a
chair, a bed, a table, fences, stairs or the child tripped while
running (17,33,35,42).
Age
Most children with BGIC infarction after MHI were
<2 years of age (4). In a
previous study by Yang et al (5), there was an association between BGIC
infarction in children <18 months and recent MHI reported.
However, BGIC infarction after MHI can occasionally occur in older
children. For instance, in a previous study by Erbayraktar et
al (43), the oldest child was a
12-year-old male.
Timing between onset and MHI
Most of the symptoms appeared between a few minutes
and 6 h, and there were also reports of symptoms appearing 7 days
after MHI (2,26). For example, in a previous study by
Jain et al (4) in 2015, the
median time was 2 h, with all children developing symptoms within
24 h after MHI.
Neurological defects
All children with BGIC had Glasgow Coma Scale scores
(44) ranging from 13 to 15. Most of
the patients had MHI, often without loss of consciousness (17). After infarction occurred,
contralateral hemiparesis presented with hemiplegic and facial
paralysis, and some children appeared to have epileptic seizures
(5). Otherwise, the associated
findings of dysarthria, athetosis, and cognate and behavioral
abnormalities are rarely reported (28). Occasionally, BGIC infarctions can
occur on bilateral sides, and the weakness was only on one side
(6). This observation is the same as
that presented in the aforementioned illustrative case.
Radiological features
CT
Early CT scans showed no hypodense lesions, and late
CT showed an infarct in the BGIC. In a CT scan, mineralization in a
basal ganglion can be found; the calcification, remade by 3-D
technology, shows linear pointing to the sylvian fissure (5).
Magnetic resonance (MR)
MRI could find an infarction signal in a few hours
after MHI (32); in some cases,
bilateral infarcts were observed. In a MRA, the internal carotid
artery and vertebral artery system are often normal. MR fiber
tracking was helpful, which demonstrated that the severity of motor
deficit depends on the extent of the infarct in the upper part of
the internal capsule (45,46).
Ultrasound
Children <2 years old with open fontanelle are
scanned through the fontanel as a ‘sound window’. Intracranial
ultrasound often suggest hyperechogenicity that is consistent with
the line of the LA (1). Ivanov et
al (33) identified that coronal
and parasagittal cerebral sonogram examinations demonstrated linear
hyperechogenic LAs, which corresponded to the calcifications
observed on CT. Lenticulostriate vasculopathy refers to increased
echogenicity of the penetrating vessels that supply the basal
ganglia and segments of the internal capsule seen on a cranial
ultrasound (1,33,35).
Diagnosis
In summary, the diagnostic criteria are as follows:
(i) All children have a clear history of minor trauma; (ii)
hemiplegia or facial paralysis often occurs within a few hours
after trauma; and (iii) CT or MRI could show unilateral or
bilateral infarction in some episodes. In children <2 years of
age, ultrasound could sometimes detect strong echoes of the
lenticular artery.
Treatment and prognosis
There is no consensus on the treatment of traumatic
BGIC infarction in children. However, all the reported children
were treated with conservative treatment, using aspirin at a dose
of 3–5 mg/kg body once daily (11,26,47).
Ivanov et al (33) used fresh
frozen plasma infusion, dipiridamol, pyracetam and physiotherapy to
treat the disease. In addition, post-traumatic rehabilitative
physiotherapy is a safe and effective method (5,26,48,49).
In previous studies, most children who experienced
infarction after MHI recovered completely between 1 week and 3
months except for recurrence (2,4). To the
best of the authors' knowledge, there were no cases of death
reported in the literature used in the present study. The median
duration for complete recovery was 12 weeks for MHI. Neuronal
plasticity during childhood, a theory describing the cellular
potential for damaged neurons to recover and healthy neurons to
reorganize, can account for marked recovery following BGIC
infarction after MHI (2,17).
Conclusion
MHI may cause BGIC infarction due to mechanical
vasospasm of the perforating vessels in the pediatric age range.
The anatomical characteristics of the growing brain in infancy,
mineralization of the LAs and viral infection may all play a part
in BGIC infarction after MHI, which often occurs within 24 months.
Symptoms are not as severe and tend to disappear in the early
period. CT or MRI often showed BGIC infarction. There are also
children showing scattered calcification in the basal ganglia. In
children <2 years of age, ultrasound could sometimes detect
strong echoes of the lenticular artery. Neural rehabilitation is a
commonly accepted treatment. The prognosis of patients with BGIC
infarction after MHI consistently improves.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The literature search was performed using the PubMed
database and relevant search terms.
Authors' contributions
YL and LF searched the literature and analyzed the
data. GW and JY designed the study and wrote the manuscript. All of
the authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The case report received written consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nabika S, Kiya K, Satoh H, Mizoue T,
Oshita J and Kondo H: Ischemia of the internal capsule due to mild
head injury in a child. Pediatr Neurosurg. 43:312–315. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kieslich M, Fiedler A, Heller C, Kreuz W
and Jacobi G: Minor head injury as cause and co-factor in the
aetiology of stroke in childhood: A report of eight cases. J Neurol
Neurosurg Psychiatry. 73:13–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Provenzale JM and Sorensen AG:
Diffusion-weighted MR imaging in acute stroke: Theoretic
considerations and clinical applications. AJR Am J Roentgenol.
173:1459–1467. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain P, Kishore P, Bhasin JS and Arya SC:
Mineralizing angiopathy with basal ganglia stroke in an infant. Ann
Indian Acad Neurol. 18:233–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang FH, Wang H, Zhang JM and Liang HY:
Clinical features and risk factors of cerebral infarction after
mild head trauma under 18 months of age. Pediatr Neurol.
48:220–226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mirvis SE, Wolf AL, Numaguchi Y, Corradino
G and Joslyn JN: Posttraumatic cerebral infarction diagnosed by CT:
Prevalence, origin, and outcome. AJR Am J Roentgenol.
154:1293–1298. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hutton B, Salanti G, Caldwell DM, Chaimani
A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen
JP, et al: The PRISMA extension statement for reporting of
systematic reviews incorporating network meta-analyses of health
care interventions: Checklist and explanations. Ann Intern Med.
162:777–784. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
James MA: Use of the medical research
council muscle strength grading system in the upper extremity. J
Hand Surg. 32:154–156. 2007. View Article : Google Scholar
|
|
9
|
McMillan T, Wilson L, Ponsford J, Levin H,
Teasdale G and Bond M: The glasgow outcome scale - 40 years of
application and refinement. Nat Rev Neurol. 12:477–485. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seals DR, Johnson DG and Fregosi RF:
Hypoxia potentiates exercise-induced sympathetic neural activation
in humans. J Appl Physiol (1985). 71:1032–1040. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marinkovic SV, Milisavljevic MM, Kovacevic
MS and Stevic ZD: Perforating branches of the middle cerebral
artery. Microanatomy and clinical significance of their
intracerebral segments. Stroke. 16:1022–1029. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rana KS, Behera MK and Adhikari KM:
Ischemic stroke following mild head injury is it the cause. Indian
Pediatr. 43:994–997. 2006.PubMed/NCBI
|
|
13
|
Kodama N and Suzuki J: Cerebrovascular
moyamoya disease. IIIrd report-the study on the aging of the
perforating branches and the possibility of collateral pathway.
Neurol Med Chir (Tokyo). 14:55–67. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buompadre MC, Arroyo HA and Stroke G:
Basal ganglia and internal capsule stroke in childhood-risk
factors, neuroimaging, and outcome in a series of 28 patients: A
tertiary hospital experience. J Child Neurol. 24:685–691. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donzelli R, Marinkovic S, Brigante L, de
Divitiis O, Nikodijevic I, Schonauer C and Maiuri F: Territories of
the perforating (lenticulostriate) branches of the middle cerebral
artery. Surg Radiol Anat. 20:393–398. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaffer L, Rich PM, Pohl KR and Ganesan V:
Can mild head injury cause ischaemic stroke? Arch Dis Child.
88:267–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dharker SR, Mittal RS and Bhargava N:
Ischemic lesions in basal ganglia in children after minor head
injury. Neurosurgery. 33:863–865. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mosberg WH and Lindenberg R: Traumatic
hemorrhage from the anterior choroidal artery. J Neurosurg.
16:209–221. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landi A, Marotta N, Mancarella C, Marruzzo
D, Salvati M and Delfini R: Basal ganglia stroke due to mild head
trauma in pediatric age - clinical and therapeutic management: A
case report and 10 year literature review. Ital J Pediatr.
37:22011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang ZR, Yu LP, Yang XC, Zhang F, Chen
YR, Feng F, Qian XS and Cai J: Human cytomegalovirus linked to
stroke in a chinese population. CNS Neurosci Ther. 18:457–460.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Grau AJ, Urbanek C and Palm F: Common
infections and the risk of stroke. Nat Rev Neurol. 6:681–694. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bodensteiner JB, Hille MR and Riggs JE:
Clinical features of vascular thrombosis following varicella. Am J
Dis Child. 146:100–102. 1992.PubMed/NCBI
|
|
23
|
Kors EE, Terwindt GM, Vermeulen FL,
Fitzsimons RB, Jardine PE, Heywood P, Love S, van den Maagdenberg
AM, Haan J, Frants RR and Ferrari MD: Delayed cerebral edema and
fatal coma after minor head trauma: Role of the CACNA1A calcium
channel subunit gene and relationship with familial hemiplegic
migraine. Ann Neurol. 49:753–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jauhari P, Sankhyan N, Khandelwal N and
Singhi P: Childhood basal ganglia stroke and its association with
trivial head trauma. J Child Neurol. 31:738–742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fidan E, Cummings DD and Manole MD: A case
of lenticulostriate stroke due to minor closed head injury in a
2-year-old child: Role of mineralizing angiopathy. Pediatr Emerg
Care. 34:e233–e235. 2017. View Article : Google Scholar
|
|
26
|
Lingappa L, Varma RD, Siddaiahgari S and
Konanki R: Mineralizing angiopathy with infantile basal ganglia
stroke after minor trauma. Dev Med Child Neurol. 56:78–84. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang FH, Wang H, Zhang JM and Liang HY:
Cerebral infarction after mild head trauma in children. Indian
Pediatr. 50:875–878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caplan LR, Schmahmann JD, Kase CS,
Feldmann E, Baquis G, Greenberg JP, Gorelick PB, Helgason C and
Hier DB: Caudate infarcts. Arch Neurol. 47:133–143. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deveber G: Stroke in infancy: A
convergence of causes. Dev Med Child Neurol. 56:9–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kowalik TF, Wing B, Haskill JS, Azizkhan
JC, Baldwin AS Jr and Huang ES: Multiple mechanisms are implicated
in the regulation of NF-kappa B activity during human
cytomegalovirus infection. Proc Natl Acad Sci USA. 90:1107–1111.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cantey JB and Sisman J: The etiology of
lenticulostriate vasculopathy and the role of congenital
infections. Early Hum Dev. 91:427–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ishihara C, Sawada K and Tateno A:
Bilateral basal ganglia infarction after mild head trauma. Pediatr
Int. 51:829–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ivanov I, Zlatareva D, Pacheva I and
Panova M: Does lenticulostriate vasculopathy predipose to ischemic
brain infarct? A case report. J Clin Ultrasound. 40:607–610. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Coley BD, Rusin JA and Boue DR: Importance
of hypoxic/ischemic conditions in the development of cerebral
lenticulostriate vasculopathy. Pediatr Radiol. 30:846–855. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Masuzawa H, Kubo T, Kanazawa I, Kamitani H
and Nakamura N: Shearing injuries of parasagittal white matter,
corpus callosum and basal ganglia: Possible radiological evidences
of hemiplegia in diffuse axonal injury. No Shinkei Geka.
25:689–694. 1997.(In Japanese). PubMed/NCBI
|
|
36
|
Hollin SA, Sukoff MH, Silverstein A and
Gross SW: Post-traumatic middle cerebral artery occlusion. J
Neurosurg. 25:526–535. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Seckin H, Demirci AY, Degerliyurt A, Dagli
M and Bavbek M: Posttraumatic infarction in the basal ganglia after
a minor head injury in a child: Case report. Turk Neurosurg.
18:415–419. 2008.PubMed/NCBI
|
|
38
|
Symon L: An experimental study of
traumatic cerebral vascular spasm. J Neurol Neurosurg Psychiatry.
30:497–505. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boto GR, Lobato RD, Rivas JJ, Gomez PA, de
la Lama A and Lagares A: Basal ganglia hematomas in severely head
injured patients: Clinicoradiological analysis of 37 cases. J
Neurosurg. 94:224–232. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zurynski YA and Dorsch NW: A review of
cerebral vasospasm. Part IV. Post-Traumatic vasospasm. J Clin
Neurosci. 5:146–154. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kramer DR, Winer JL, Pease BA, Amar AP and
Mack WJ: Cerebral vasospasm in traumatic brain injury. Neurol Res
Int. 2013:4158132013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahn JY, Han IB, Chung YS, Yoon PH and Kim
SH: Posttraumatic infarction in the territory supplied by the
lateral lenticulostriate artery after minor head injury. Childs
Nerv Syst. 22:1493–1496. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Erbayraktar S, Tekinsoy B, Acar F and Acar
U: Posttraumatic isolated infarction in the territory of heubner's
and lenticulostriate arteries: Case report. Kobe J Med Sci.
47:113–121. 2001.PubMed/NCBI
|
|
44
|
Teasdale G, Maas A, Lecky F, Manley G,
Stocchetti N and Murray G: The glasgow coma scale at 40 years:
Standing the test of time. Lancet Neurol. 13:844–854. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim HC, Choi DP, Ahn SV, Nam CM and Suh I:
Six-year survival and causes of death among stroke patients in
Korea. Neuroepidemiology. 32:94–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee K, Kim EH, Song D, Kim YD, Nam HS, Lee
HS and Heo JH: Lenticulostriate artery involvement is predictive of
poor outcomes in superficial middle cerebral artery territory
infarction. Yonsei Med J. 58:123–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Anderson V, Catroppa C, Morse S, Haritou F
and Rosenfeld J: Functional plasticity or vulnerability after early
brain injury? Pediatrics. 116:1374–1382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ogrenci A, Eksi MS, Gun B and Koban O:
Traumatic basal ganglia hematoma following closed head injuries in
children. Childs Nerv Syst. 32:1237–1243. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kurwale NS, Gupta DK and Mahapatra AK:
Outcome of pediatric patients with traumatic basal ganglia
hematoma: Analysis of 21 cases. Pediatr Neurosurg. 46:267–271.
2010. View Article : Google Scholar : PubMed/NCBI
|