Introduction
Gestational diabetes mellitus (GDM) is clinically
classified as a high-risk pregnancy condition (1). In recent years, the incidence of GDM is
increasing with continuous changes in living standards and eating
habits (2). It has been estimated
that 1 out of 15 pregnant females is susceptible to GDM, and in
developed countries, including the US and the UK, the incidence of
GDM exceeds 10%. The uterus of patients with GDM has a high-glucose
environment, which may exert long-term effects on the mother and
fetus (3). GDM may greatly increase
the incidence of complications during pregnancy, including
polyhydramnios, eclampsia and premature delivery. More seriously,
it may lead to abortion (4). In
addition, the probability of fetal malformation and developmental
limitation is markedly increased in patients with GDM. If the fetus
remains in a high-glucose environment during pregnancy, it is prone
to developing respiratory distress syndrome and hypoglycemia once
delivered, which may be life-threatening to the infant (5). According to a survey, ~24.84% of
females with GDM developed GDM-associated diseases, including
respiratory distress syndrome, organ dysplasia, following
childbirth (6). Owing to the high
incidence of GDM and high risk associated with it, the present
study investigated methods which may facilitate effective clinical
diagnosis and treatment of GDM. To date, no breakthrough has been
achieved with this regard. Therefore, an increasing number of
studies performed worldwide focus on the identification of
effective indicators for pregnancy outcomes in patients with GDM,
which may allow for the prevention and treatment of GDM. Studies
have indicated that glycosylated hemoglobin (HbA1c) and urinary
microalbuminuria (24 h mAlb) are closely associated with GDM
(7–9), while serum cystatin C (Cys-C) is a
highly sensitive indicator of renal impairment (10). In the present study, it was
hypothesized that Cys-C may also be abnormally elevated in patients
with GDM, and that detection of HbA1c, 24 h mAlb and Cys-C may be
an effective predictor of pregnancy outcomes in patients with GDM.
Therefore, a retrospective analysis of patients with GDM was
conducted to provide support for future clinicians in the diagnosis
and treatment of adverse pregnancy in patients with GDM.
Materials and methods
General information
A total of 144 pregnant females with GDM admitted to
the Department of Obstetrics, Qingpu Branch, Zhongshan Hospital
Affiliated to Fudan University (Shanghai, China) between August
2016 and September 2017, were selected for retrospective analysis.
Their age ranged from 22 to 35 years, with an average of 27.12±4.93
years. A further 117 cases of normal pregnancy (age, 22–34 years;
average age, 26.83±4.62 years) were selected as the control group.
The experiment was approved by the Ethics Committee of Qingpu
Branch, Zhongshan Hospital Affiliated to Fudan University
(Shanghai, China) and all subjects provided written informed
consent.
Inclusion and exclusion criteria
Criteria for inclusion were as follows: i) Pregnant
women diagnosed with GDM via oral glucose tolerance test, in line
with the 2016 GDM diagnostic guidelines (11); ii) Blood glucose <7.0 mmol/l; iii)
delivery at the Zhongshan Hospital Affiliated to Fudan University;
and iv) complete medical data. The exclusion criteria were as
follows: i) Family history of genetic diabetes; ii) presence of a
tumor; iii) mental illness; iv) cardio-cerebral vascular disease;
v) severe liver and kidney dysfunction; vi) organ failure; vii)
fetal congenital malformation diagnosed by B-ultrasound; viii)
patients who were long-term bedridden; and ix) patients transferred
from another hospital.
Collection of biological fluids and
measurement of biomarkers
Pregnant women with GDM were classified as the GDM
group and normal pregnant women were categorized as the control
group. The 24-h urine samples were collected from 7 to 7 am the
next day, and 10 ml urine per sample was used. Following
centrifugation for 5 min at 2,432 × g (20°C), the supernatant of
the urine was obtained, and the 24-h urine 24 h mAlb levels of the
two groups were determined via the biuret method. In the morning, 5
ml fasting venous blood was drawn from patients and centrifuged for
10 min at 2,432 × g (20°C). The serum in the supernatant was
divided into two portions that were stored in a −80°C refrigerator
for testing. One portion was used to detect HbA1c levels with an
automatic biochemical analyzer (AU5800; Beckman Coulter), while the
other portion was used to detect Cys-C levels via latex-enhanced
immunoturbidimetric assay according to manufacturer's protocol
(cat. no. UFWD0121; Shanghai Junrui Biotechnology Co., Ltd.)
(12).
Observation indicators
The following information was collected: Maternal
clinical information including age, body weight and gestational
age; HbA1c level; Cys-C level; 24 h mAlb level; pregnancy outcome
and adverse pregnancy rate; preterm birth (28–37 weeks pregnancy);
premature rupture of membranes (progressive uterine cervix decline
before delivery; cervical tube disappearance and fetal malposition
decline); polyhydramnios (largest pocket depth of abdominal by
ultrasound prior to delivery ≥8 cm or amniotic fluid volume after
delivery >2,000 ml; fetal distress (average 10-min fetal heart
rate >180 or <120 beats/min); abnormal fetal development
(body weight <2,500 g for developmental obstruction, weight
>4,000 g for a huge infant). The correlations of HbA1c, Cys-C
and 24 h mAlb with adverse pregnancy outcomes, including adverse
pregnancy rates and neonatal Apgar scores [according to 2016
Newborn Apgar scoring standard (13)] were then determined.
Statistical analysis
The data were analyzed and processed using the SPSS
22.0 statistical package (IBM Corp.). Enumeration data, including
the place of residence, lifestyle habits and adverse pregnancies,
were expressed as rates. Comparisons between groups were performed
using the chi-squared test. Continuous variables, including age,
body weight and HbA1c levels were expressed as the mean
± standard deviation. The Independent Samples t-test was used for
comparison of means. Correlation between HbAlc, Cys-C, mAlb and
neonatal Apgar scores was analyzed by Pearson correlation
coefficient; Logistic regression analysis was used to correlate
HbAlc, Cys-C, mAlb and adverse pregnancy outcomes. Predictive
values were analyzed using receiver operating characteristic (ROC)
curves. P<0.05 was considered to indicate a statistically
signficant difference.
Results
Comparison of clinical data
There was no difference in age, weight, gestational
age, blood routine examination and the place of residence between
the two groups (P>0.05), suggesting that the two groups were
comparable (Table I).
 | Table I.Comparison of general data between the
two groups. |
Table I.
Comparison of general data between the
two groups.
| Parameter | GDM group
(n=144) | Normal group
(n=117) | t or χ
2 | P-value |
|---|
| Age (years) | 27.12±4.93 | 26.83±4.62 | 0.486 | 0.627 |
| Body weight (kg) | 61.24±6.24 | 60.55±6.51 | 0.871 | 0.384 |
| Gestational week | 38.62±5.21 | 39.27±5.84 | 0.949 | 0.343 |
| WBC
(×109/l) | 16.24±5.04 | 16.81±4.86 | 0.923 | 0.357 |
| RBC
(×1012/l) | 6.28±2.07 | 5.94±2.44 | 1.218 | 0.224 |
| PLT
(×109/l) | 187.24±34.51 | 194.53±29.85 | 1.802 | 0.072 |
| Place of
residence |
|
| 0.880 | 0.348 |
| Town | 81 (56.25) | 59 (50.43) |
|
|
| Rural
area | 63 (43.75) | 58 (49.57) |
|
|
| Primipara |
|
| 0.312 | 0.576 |
| Yes | 131 (90.97) | 104 (88.89) |
|
|
| No | 13 (9.03) | 13 (11.11) |
|
|
Comparison of HbA1c, Cys-C and 24 h
mAlb levels
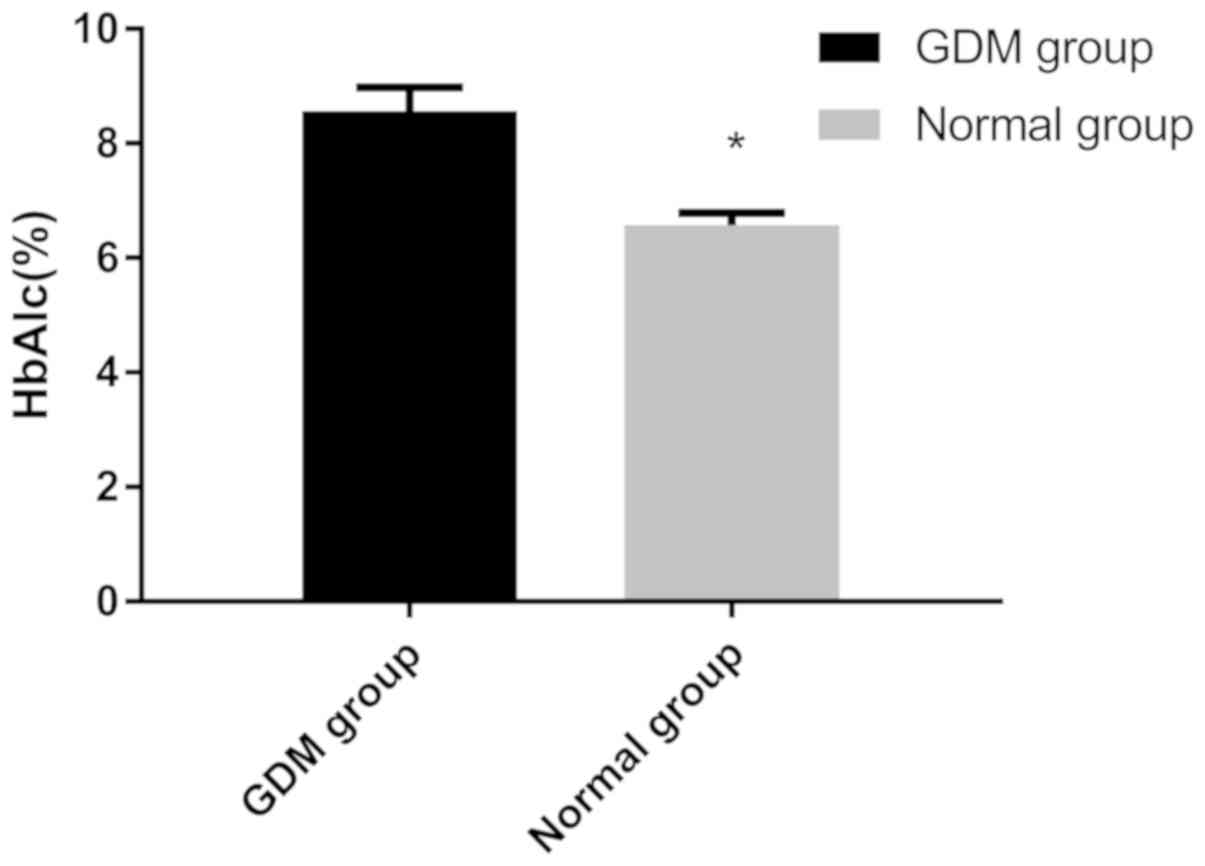
The level of HbA1c of the GDM group (8.56±0.42%) was
significantly higher than that in the control group (6.57±0.22%;
P<0.001; Fig. 1). The serum
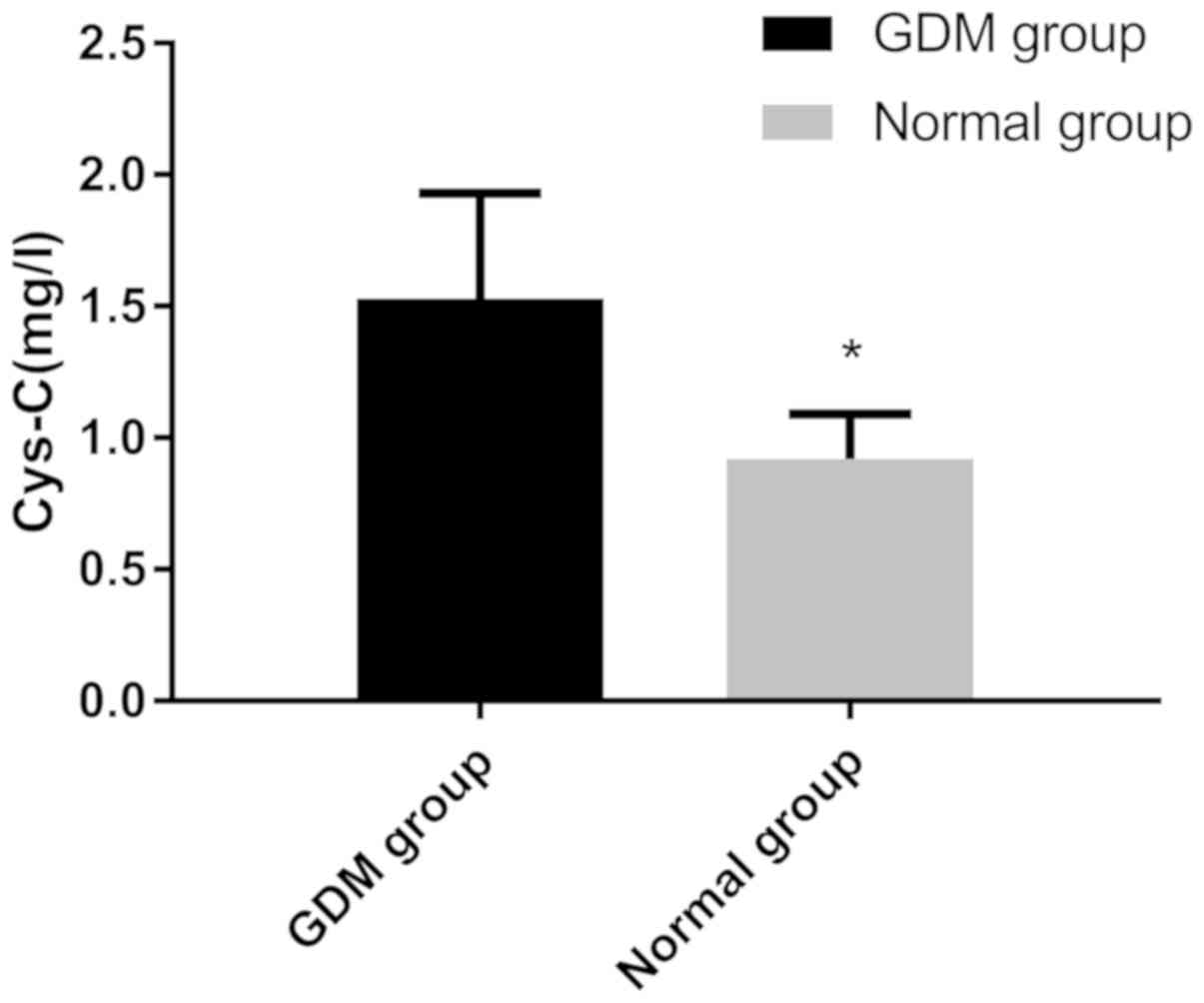
levels of Cys-C in the GDM group (1.53±0.40 mg/l) were
significantly higher than those in the control group (0.92±0.17
mg/l; P<0.001; Fig. 2).
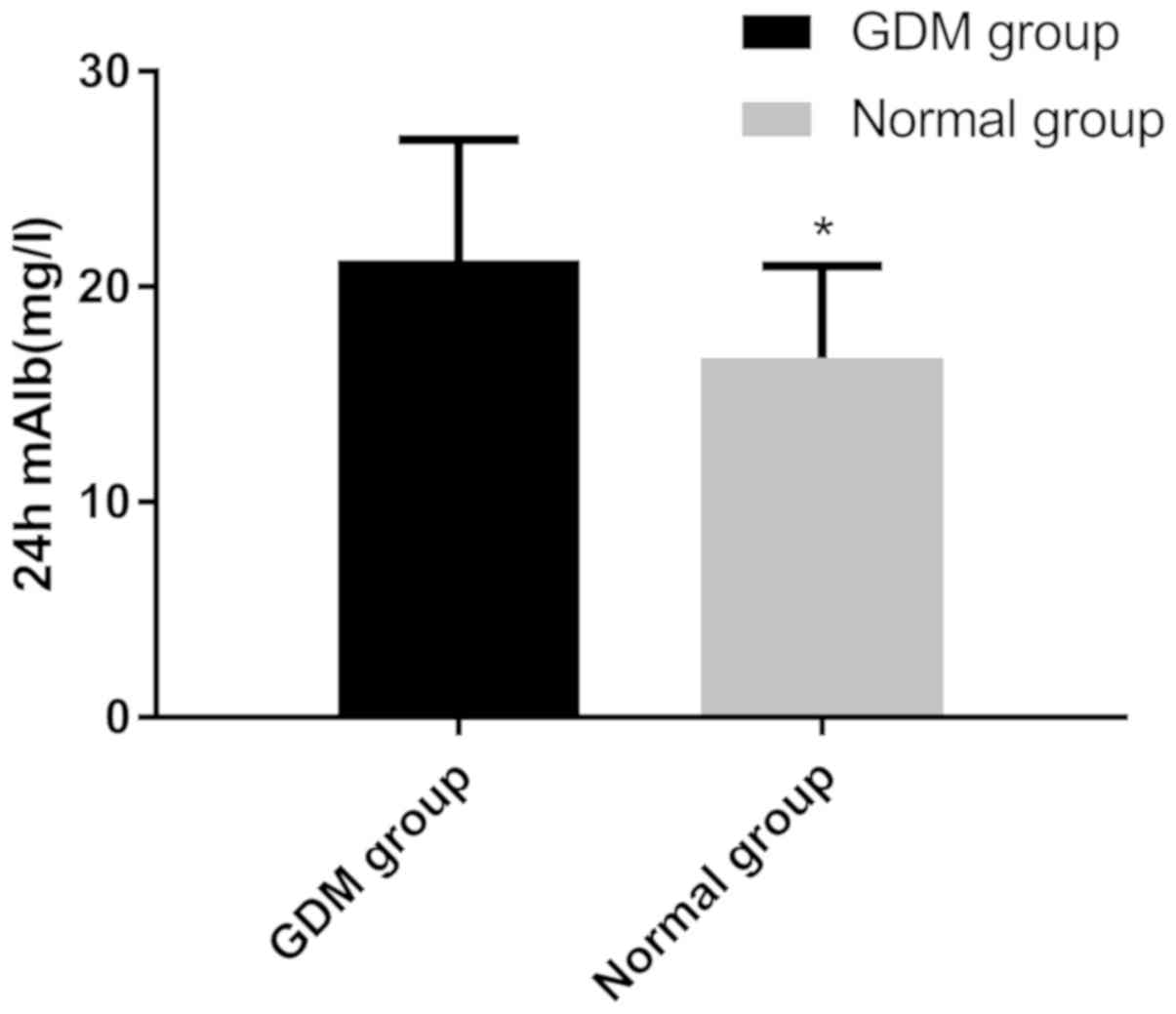
Furthermore, the levels of 24 h mAlb in the GDM group (21.24±5.59
mg/l) were significantly higher than those in the control group
(16.69±4.27 mg/l; P<0.001; Fig.
3).
Comparison of adverse pregnancy
rates
In the GDM group, the premature delivery rate was
9.03%, the premature membrane rupture rate was 10.42%, the
polyhydramnios rate was 5.56%, the fetal distress rate was 9.03%
and the abnormal fetal development rate was 6.94%. In the control
group, the premature delivery rate was 5.13%, the premature
membrane rupture rate was 4.27%, the polyhydramnios rate was 2.56%,
the fetal distress rate was 1.71% and the abnormal fetal
developmental rate was 2.56%. The rate of adverse pregnancy in the
GDM group (40.97%) was significantly higher than that in the
control group (16.24%; P<0.001; Table II).
 | Table II.Comparison of adverse pregnancy rates
between the two groups. |
Table II.
Comparison of adverse pregnancy rates
between the two groups.
| Item | GDM group
(n=144) | Normal group
(n=117) | χ 2 | P-value |
|---|
| Premature birth | 13 (9.03) | 6 (5.13) |
1.454 |
0.228 |
| Premature rupture of
membranes | 15 (10.42) | 5 (4.27) |
3.443 |
0.064 |
| Excessive amniotic
fluid | 8 (5.56) | 3 (2.56) |
1.431 |
0.232 |
| Fetal intrauterine
distress | 13 (9.03) | 2 (1.71) |
6.383 |
0.012 |
| Abnormal fetal
development | 10 (6.94) | 3 (2.56) |
2.617 |
0.106 |
| Overall adverse
pregnancy rate (%) | 40.97 | 16.24 | 18.841 | <0.001 |
Association of HbA1c, Cys-C and 24 h
mAlb with adverse pregnancy outcomes
Logistic regression analysis indicated that in
maternal patients with GDM, but not in healthy pregnant women,
HbA1c, Cys-C and 24 h mAlb were closely associated with adverse
pregnancy outcomes (P<0.050) and were risk factors leading to
adverse pregnancy outcomes in GDM (Table III).
 | Table III.Correlation analysis between HbA1c,
Cys-C and 24 h mAlb and adverse pregnancy rate in patients with
GDM. |
Table III.
Correlation analysis between HbA1c,
Cys-C and 24 h mAlb and adverse pregnancy rate in patients with
GDM.
| Factor | Reference range | Hazards ratio | SE | B | 95% CI | P-value |
|---|
| HbAlc | 4–6% | 0.247 | 0.468 | 12.314 | 1.369–3.816 | 0.002 |
| Cys-C | 0.51–1.09
mg/dl | 0.670 | 0.191 |
8.723 | 1.093–2.473 | 0.017 |
| 24 h mAlb | <150 mg/24
h | 0.229 | 0.555 |
6.942 | 1.045–2.185 | 0.028 |
Correlation between HbA1c,
Cys-C, 24 h mAlb and neonatal Apgar score
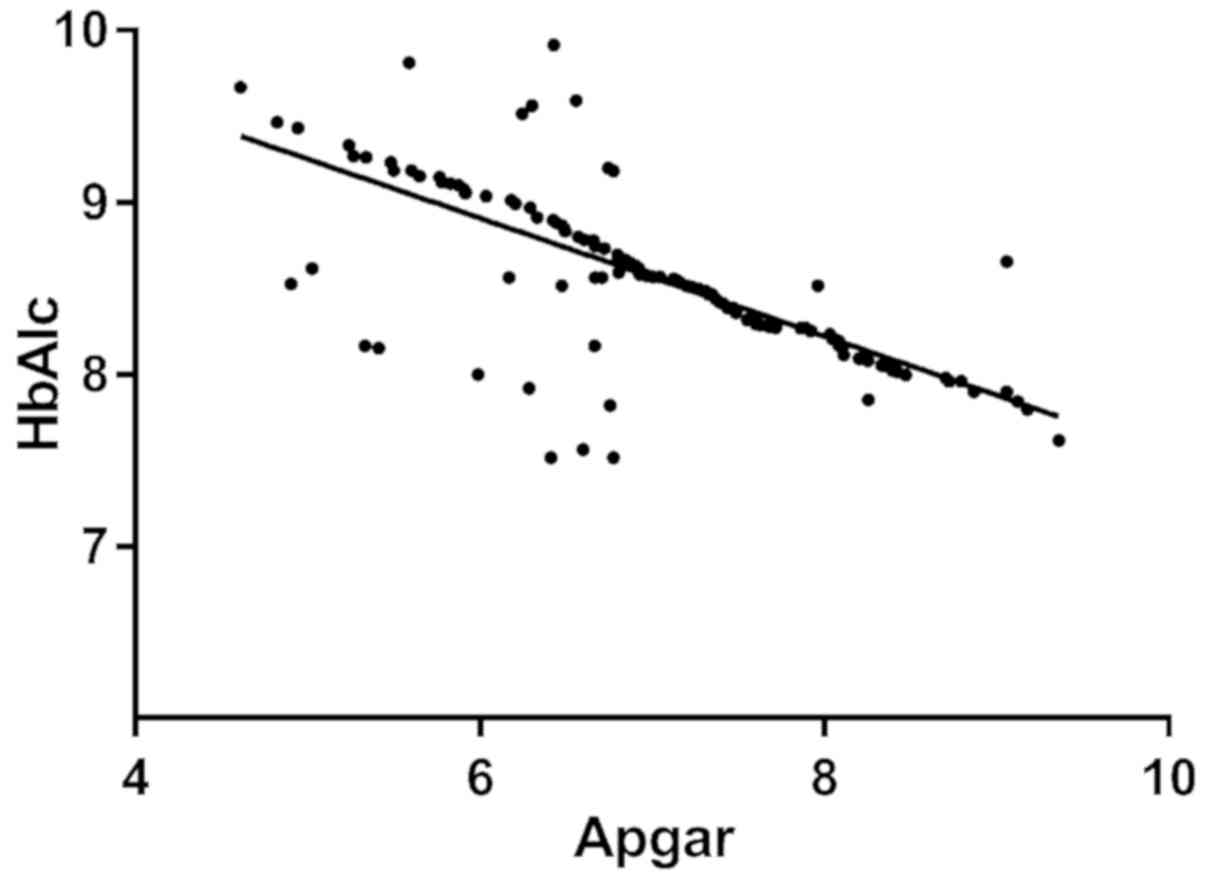
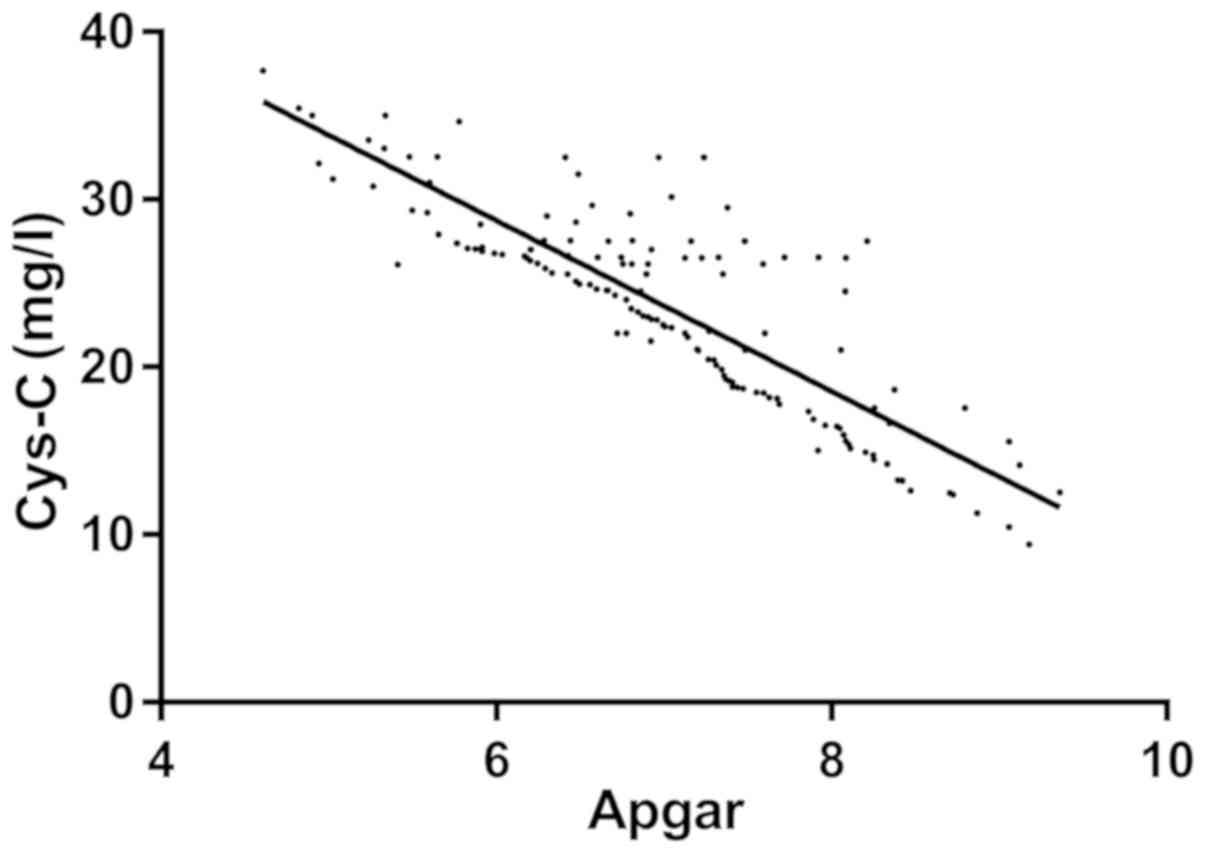
The Apgar score of the neonates in the GDM group was
7.12±1.07. Linear correlation analysis indicated that HbA1c, Cys-C
and 24 h mAlb were negatively correlated with the neonatal Apgar
score (r=−0.509, −0.678 and −0.733, respectively; P<0.001;
Figs. 4–6).
Predictive value of HbAlc, Cys-C and
24 h mAlb regarding adverse pregnancy outcomes for patients with
GDM
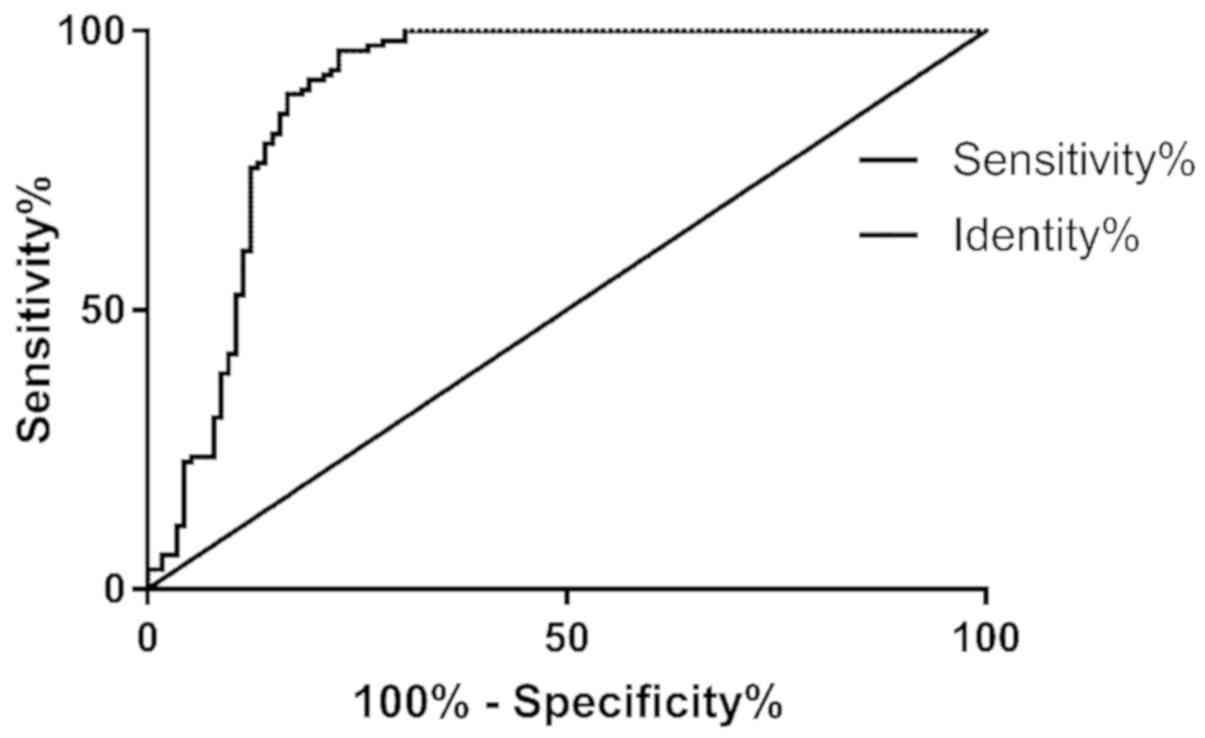
Binary logistic analysis was performed using HbAlc,
Cys-C, and 24 h mAlb as three independent variables to obtain joint
predictive factors, using the following formula: Log(P) value =
1.626 + 0.051 × HbAl + 0.426 × Cys-C + 0.623 × 24 h mAlb. According
to ROC analysis, the predictive sensitivity of HbAlc, Cys-C and 24
h mAlb for adverse pregnancy outcome was calculated to be 96.49%
and the specificity was calculated to be 77.19% (Fig. 7; Table
IV).
 | Table IV.Predictive value of maternal HbAlc,
Cys-C and 24 h mAlb combined for adverse pregnancy outcomes. |
Table IV.
Predictive value of maternal HbAlc,
Cys-C and 24 h mAlb combined for adverse pregnancy outcomes.
| Variable | Numerical
value |
|---|
| AUC | 0.891 |
| Standard
deviation | 0.024 |
| 95% CI | 0.843–0.939 |
| Cut-off
(ng/ml) | 1.249 |
| Sensitivity
(%) | 96.49 |
| Specificity
(%) | 77.19 |
| P-value | <0.001 |
Discussion
Pregnancy is a unique circumstance under which the
female body undergoes certain physiological changes. Alterations in
glucose metabolism are among the most significant physiological
changes that occur during this process (14). The fasting blood glucose levels of
the mother are generally reduced during pregnancy, and this is
associated with a high incidence of GDM (15). The primary reason for the decrease in
maternal blood glucose is that the fetus consumes a large amount of
sugar during development, and insulin accelerates the release of
glucose into the blood for metabolization, which then also
decreases the blood sugar levels (16). It has been indicated that blood
glucose metabolism in pregnant females is significantly lower
compared with that in non-pregnant females. The key factor
affecting maternal blood glucose is insulin, the sensitivity to
which is significantly reduced during pregnancy (17). Therefore, in order to maintain normal
functioning of the maternal body, accelerated secretion of insulin
in pregnant females must be regulated. In pregnant females with
impaired insulin secretion, smooth acceleration of insulin
secretion may not be possible, leading to abnormal glucose
metabolism and onset of GDM (18,19). GDM
is a high-risk disease during pregnancy. If the optimal treatment
time is missed, GDM causes functional and environmental changes
affecting fetal blood sugar metabolism, leading to a variety of
adverse pregnancy outcomes (20).
For GDM, clinical treatment is essentially according to the
principle ‘early detection, early treatment’. At present, HbA1c
remains the gold standard for monitoring blood sugar function. The
formation rate of HbA1c, which is produced by the interaction of
hemoglobin and carbohydrates, and the blood glucose concentration
are positively correlated (20,21).
Therefore, detection of HbA1c in the clinic may reflect simple
blood glucose levels during pregnancy and facilitate the
identification of the development of GDM. 24 h mAlb and Cys-C are
sensitive indicators of renal injury and serve as important
reference points for glomerular microangiopathy in females with GDM
(22). To date, only a few
associated reference studies between GDM pregnancy outcomes and
HbA1c, Cys-C and 24 h mAlb have been performed (23,24).
Therefore, the present study aimed to establish maternal HbA1c,
Cys-C and 24 h mAlb as predictors of pregnancy outcomes in subjects
with GDM.
The results of the present study demonstrated that
the levels of HbA1c, Cys-C and 24 h mAlb in the GDM group were
significantly higher than those in the control group, which is
consistent with the results of Chawla and Malik (25). The significantly increased level of
24 h mAlb in the GDM group was attributed to an increase in blood
volume, renal blood flow and the glomerular filtration rate during
pregnancy, which markedly increased urinary protein excretion. In
addition, a high-glucose environment is always present in patients
with GDM. In these subjects, endothelial cells promote the release
of vasoactive substances, thus altering plasma protein filtration
in the glomerular basement membrane. They exhibited a large amount
of protein in the blood circulation which were leaked into the
urine. Females with normal pregnancies did not exhibit a
significant increase in 24 h mAlb, whereas patients with GDM had a
significantly increased 24 h mAlb expression, suggesting that 24 h
mAlb may be used as an effective indicator of glomerular damage.
Cys-C is a low-molecular, non-glycosylated protein, consisting of
122 amino acids residues, which is cleared by the kidneys,
resulting in low levels in normal individuals (26). However, impaired kidney function
reduces the clearance rate of Cys-C, resulting in high plasma
levels. A mother and her fetus are intimately connected during
pregnancy, and any abnormal condition in the mother influences the
fetus to varying degrees. In the present study, the adverse
pregnancy rate in the GDM group was significantly higher than that
in the control group, and it was suggested that HbA1c, Cys-C and 24
h mAlb may be used as indicators for predicting pregnancy outcomes
in pregnant females. The major cause for the association of HbA1c,
Cys-C and 24 h mAlb with the pregnancy outcome is presumed to be
blood glucose. Abnormal glucose metabolism in the mother and high
insulin levels in the long-term high-glucose fetal environment
activate the amino acid transfer system, which greatly increases
the synthetic ability of proteins. However, fat disintegration is
reduced, resulting in the accumulation of a large amount of fat and
glucose in the fetal tissues, leading to various developmental
dysfunctions in the neonate. At the same time, due to the high
permeability of the uterus between the mother and fetus, their
blood sugar levels may increase with the mother (27). Stimulation by high glucose destroys
blood vessel walls, causing spasms. Capillary stenosis in the
placenta causes narrowing of the vascular lumen, thereby
considerably reducing hemodynamics and causing a shortage of blood
supply to the fetus, which may lead to several fetal issues. In the
present study, HbA1c, Cys-C and 24 h mAlb were negatively
correlated with the neonatal Apgar scores in patients with GDM,
suggesting that combined HbAlc, Cys-C and 24 h mAlb reading can be
used to predict the outcome of delivery in GMD patients. ROC
analysis indicated that HbAlc, Cys-C and 24 h mAlb levels of
pregnant females with GDM have a relatively high sensitivity and
specificity for predicting adverse pregnancy outcomes, suggesting
that future clinical trials may determine pregnancy outcomes by
detecting the levels of these indicators to provide protective
intervention as early as possible.
Of note, the present study does contain certain
limitations. Effective data analyses of other types of high-risk
pregnancies was not possible, as the experimental population was
relatively small. The age range of the study subjects was
relatively narrow, and it may not be excluded that HbAlc, Cys-C and
24 h mAlb are different in pregnant females of different ages and
geographical regions. In addition, due to the short experimental
period, it was impossible to determine the association of HbAlc,
Cys-C and 24 h mAlb with the prognosis of GDM-associated health
conditions in the mothers after pregnancy. In the future, a more
detailed follow-up of the subjects will be performed and the number
of subjects will be increased to obtain the best possible
experimental results.
In conclusion, the present study indicated that
HbA1c, Cys-C and 24 h mAlb are elevated in females with GDM, and
may be effective indicators of adverse outcomes of high-risk
pregnancies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contribution
HJ conceived and designed the study and interpreted
the results, analyzed the data, prepared the figures, drafted,
edited and revised manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Qingpu Branch, Zhongshan Hospital Affiliated to Fudan University
(Shanghai, China) and all subjects provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The author declare that she has no competing
interests.
References
|
1
|
Spaight C, Gross J, Horsch A and Puder JJ:
Gestational diabetes mellitus. Endocr Dev. 31:163–178. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Euser AG, Hammes A, Ahrendsen JT, Neshek
B, Weitzenkamp DA, Gutierrez J, Koivunen P, Julian CG and Moore LG:
Gestational diabetes prevalence at moderate and high altitude. High
Alt Med Biol. 19:367–372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Senat MV and Deruelle P: Gestational
diabetes mellitus. Gynecol Obstet Fertil. 44:244–247. 2016.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Damm P, Houshmand-Oeregaard A, Kelstrup L,
Lauenborg J, Mathiesen ER and Clausen TD: Gestational diabetes
mellitus and long-term consequences for mother and offspring: A
view from Denmark. Diabetologia. 59:1396–1399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schoenaker DA, Mishra GD, Callaway LK and
Soedamah-Muthu SS: The role of energy, nutrients, foods, and
dietary patterns in the development of gestational diabetes
mellitus: A systematic review of observational studies. Diabetes
Care. 39:16–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu M, Xu Y, Lv L and Zhang M: Association
between vitamin D status and the risk of gestational diabetes
mellitus: A meta-analysis. Arch Gynecol Obstet. 293:959–966. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rudland VL, Hinchcliffe M, Pinner J, Cole
S, Mercorella B, Molyneaux L, Constantino M, Yue DK, Ross GP and
Wong J: Identifying glucokinase monogenic diabetes in a multiethnic
gestational diabetes mellitus cohort: New pregnancy screening
criteria and utility of HbA1c. Diabetes Care. 39:50–52. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baack ML, Forred BJ, Larsen TD, Jensen DN,
Wachal AL, Khan MA and Vitiello PF: Consequences of a maternal
high-fat diet and late gestation diabetes on the developing rat
lung. PLoS One. 11:e01608182016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sherwani SI, Khan HA, Ekhzaimy A, Masood A
and Sakharkar MK: Significance of HbA1c test in diagnosis and
prognosis of diabetic patients. Biomark Insights. 11:95–104. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pottel H, Delanaye P, Schaeffner E,
Dubourg L, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST and
Glassock RJ: Estimating glomerular filtration rate for the full age
spectrum from serum creatinine and cystatin C. Nephrol Dial
Transplant. 32:497–507. 2017.PubMed/NCBI
|
|
11
|
Zhu Y and Zhang C: Prevalence of
gestational diabetes and risk of progression to type 2 diabetes: A
global perspective. Curr Diab Rep. 16:72016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dipalo M, Buonocore R, Gnocchi C, Picanza
A, Aloe R and Lippi G: Analytical evaluation of Diazyme
procalcitonin (PCT) latex-enhanced immunoturbidimetric assay on
Beckman Coulter AU5800. Clin Chem Lab Med. 53:593–597. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ray JG, Medcalf KE and Park AL:
Association of newborn Apgar score with maternal admission to the
intensive care unit. JAMA Pediatr. 170:88–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rani PR and Begum J: Screening and
diagnosis of gestational diabetes mellitus, where do we stand. J
Clin Diagn Res. 10:QE01–QE04. 2016.PubMed/NCBI
|
|
15
|
Sovio U, Murphy HR and Smith GC:
Accelerated fetal growth prior to diagnosis of gestational diabetes
mellitus: A prospective cohort study of nulliparous women. Diabetes
Care. 39:982–987. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nahum Sacks K, Friger M, Shoham-Vardi I,
Abokaf H, Spiegel E, Sergienko R, Landau D and Sheiner E: Prenatal
exposure to gestational diabetes mellitus as an independent risk
factor for long-term neuropsychiatric morbidity of the offspring.
Am J Obstet Gynecol. 215:380.e1–7. 2016. View Article : Google Scholar
|
|
17
|
Muralimanoharan S, Maloyan A and Myatt L:
Mitochondrial function and glucose metabolism in the placenta with
gestational diabetes mellitus: Role of miR-143. Clin Sci (Lond).
130:931–941. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Retnakaran R and Shah BR: Role of type 2
diabetes in determining retinal, renal, and cardiovascular outcomes
in women with previous gestational diabetes mellitus. Diabetes
Care. 40:101–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marso SP, Bain SC, Consoli A, Eliaschewitz
FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren
ML, et al: Semaglutide and cardiovascular outcomes in patients with
type 2 diabetes. N Engl J Med. 375:1834–1844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karges B, Kapellen T, Wagner VM,
Steigleder-Schweiger C, Karges W, Holl RW and Rosenbauer J; DPV
Initiative, : Glycated hemoglobin A1c as a risk factor for severe
hypoglycemia in pediatric type 1 diabetes. Pediatr Diabetes.
18:51–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Faruque LI, Wiebe N, Ehteshami-Afshar A,
Liu Y, Dianati-Maleki N, Hemmelgarn BR, Manns BJ and Tonelli M;
Alberta Kidney Disease Network, : Effect of telemedicine on
glycated hemoglobin in diabetes: A systematic review and
meta-analysis of randomized trials. CMAJ. 189:E341–E364. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lau L, Al-Ismaili Z, Harel-Sterling M,
Pizzi M, Caldwell JS, Piccioni M, Lands LC, Mottes T, Devarajan P,
Goldstein SL, et al: Serum cystatin C for acute kidney injury
evaluation in children treated with aminoglycosides. Pediatr
Nephrol. 32:163–171. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hughes RC, Moore MP, Gullam JE, Mohamed K
and Rowan J: An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is
optimal for detecting diabetes and identifies women at increased
risk of adverse pregnancy outcomes. Diabetes Care. 37:2953–2959.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu X, Qiu Y, Dai Y, et al: A research of
correlation between renal damage of patients with gestational
diabetes mellitus and level of serum cystatin C. International
Journal of Laboratory Medicine. 10:1289–1290. 2014.
|
|
25
|
Chawla R and Malik S: Microalbuminuria
detected at mid term as a marker for adverse pregnancy outcome. Int
J Health Sci Res. 8:41–52. 2018.
|
|
26
|
Bargnoux AS, Piéroni L, Cristol JP, Kuster
N, Delanaye P, Carlier MC, Fellahi S, Boutten A, Lombard C,
González-Antuña A, et al: Multicenter evaluation of cystatin C
measurement after assay standardization. Clin Chem. 63:833–841.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fitzpatrick KE, Tuffnell D, Kurinczuk JJ
and Knight M: Incidence, risk factors, management and outcomes of
amniotic-fluid embolism: A population-based cohort and nested
case-control study. BJOG. 123:100–109. 2016. View Article : Google Scholar : PubMed/NCBI
|