Introduction
With the improvement of living standards, coronary
atherosclerotic disease remains one of the leading causes of
morbidity and mortality worldwide (1). Coronary atherosclerosis is the
pathological basis of coronary atherosclerotic heart disease
(2,3). Therefore, it is of great significance
to explore the pathogenesis of atherosclerosis (AS) and search
effective therapeutic methods. At present, the specific mechanism
of AS remains unclear, and most scholars regard it as a chronic
inflammatory response (4–6). Mononuclear macrophages have the most
significant role in formation of atherosclerotic plaques, and the
germinal cells of the innate immune system, which exist in each
stage of atherosclerotic lesions (7,8). The
pro-inflammatory factors released by macrophages play key roles, in
which interleukin-1β (IL-1β) and IL-18 are the most important ones
accelerating the development of AS (9,10).
Studies have demonstrated that metabolites formed by the body can
be sensed by Nod-like receptor (NLR) in the cytoplasm of
macrophages, and then NLR forms a complex with apoptosis-associated
speck like protein containing CARD (ASC) and caspase-1 (Casp-1).
The complex, which is called inflammatory corpuscle, can promote
the maturation of inflammatory cytokines (11,12).
Nod-like receptor protein 3 (NLRP3), an inflammatory corpuscle most
closely related to chronic inflammatory response, is a kind of
pattern recognition receptor in innate immune cells that has been
studied widely, which plays a decisive role in innate immunity
(13). After the ligand binds to
NLRP3, the formation of inflammatory corpuscles is promoted, and
Casp-1 is activated, ultimately promoting the maturation and
secretion of pro-IL-1β and pro-IL-18, so that the pro-inflammatory
factors IL-1β and IL-18 are produced (14).
Tacrolimus, also known as FK506, is a potent
immunosuppressor, which, as a first-line drug in liver, kidney and
heart transplantation, has come into the market in Japan and the
United States in recent years (15).
At the same time, it also plays a positive role in the treatment of
such autoimmune diseases as atopic dermatitis, systemic lupus
erythematosus and autoimmune eye diseases (16–18). A
large number of clinical studies have proved that tacrolimus can
significantly reduce the incidence of early initial poor function
(IPF), primary nonfunction (PNF) and delayed nonfunction (DNF)
caused by ischemia-reperfusion injury after transplantation
(19–21), and ischemia-reperfusion injury is
essentially a non-specific inflammatory response, indicating that
tacrolimus has an anti-inflammatory property. Moreover, many
studies have shown that tacrolimus is topical calcineurin
inhibitors (22–24). However, whether tacrolimus can affect
the occurrence and development of AS through the anti-inflammatory
effect has not been reported yet. In this investigation, the animal
model of AS was established to observe the effect of tacrolimus on
atherosclerotic plaques and its influence on the NLRP3 inflammatory
pathway.
Materials and methods
Laboratory animals and models
A total of 20 male apolipoprotein E (ApoE, a
polymorphic protein involved in the transformation and metabolism
of lipoproteins)−/− mice aged 6 weeks, weighing 16–18 g
and 10 male C57BL/6 mice (as a wild-type control group) aged 6
weeks old weighing 16–18 g were purchased from Qingdao University
Animal Center. After adaptation for 1 week, ApoE−/− mice
were fed with high-fat diet (The formula: 79.85% general fodder +
15% fat + 5% yolk powder + 0.15% cholesterol), while C57BL/6J mice
were fed with general fodder. This study was approved by the Animal
Ethics Committee of the Third People's Hospital of Qingdao Animal
Center (Qingdao, China).
Experimental grouping and
treatment
The mice were divided into 3 groups: C57BL/6 mice
group (WT group), ApoE−/− mouse group
(ApoE−/− group), and ApoE−/− mouse +
tacrolimus intervention group (ApoE−/− + Tac group). In
ApoE−/− + Tac group, after high-fat diet for 6 weeks,
tacrolimus at 3 mg/kg/day, according to pre-experimental results
was intraperitoneally injected for 12 weeks.
Extraction of aorta
After tacrolimus intervention for 12 weeks, the
blood was taken from the orbit, the mice were anesthetized and
fixed on an anatomy plate, and the heart was exposed. A fine needle
was inserted into the left ventricle to the ascending aorta, and 50
ml phosphate buffered saline (PBS) was slowly perfused at room
temperature. The aorta was isolated under a surgical microscope,
the excess adipose tissues around the aorta were removed, and the
aorta was extracted from the mouse. The aortic root was embedded
into OCT embedding agent, sliced into frozen sections
(approximately 5-µm thick) and stored at −20°C. In addition, a
small part of the upper aortic segment was preserved in 4%
paraformaldehyde, embedded into paraffin, sliced into paraffin
sections (approximately 5-µm thick) and stored at room temperature.
The adipose tissues around the upper segments of thoracic aorta and
abdominal aorta were removed, followed by oil red O staining.
Oil red O staining
Oil red O staining for aorta: The upper segments of
thoracic aorta and abdominal aorta were differentiated in 60%
isopropanol for a few minutes, laid on a glass slide, and added
dropwise with oil red O working solution. After soaking for 2 h,
the oil red O dye was discarded, followed by differentiation with
60% isopropanol. Then the differentiation solution was replaced
until the whitening of aorta, and reddening of plaque. After the
aorta was washed with tap water, it was observed and photographed
under an optical microscope.
Oil red O staining for frozen sections: The frozen
sections of aortic root were taken from the −20°C refrigerator,
re-warmed at room temperature for 30 min, washed with 60%
isopropanol for 10 min, and stained with heated oil red O working
solution for 10 min, followed by differentiation with 60%
isopropanol for 3–10 sec until the mesenchyme became clear,
washing, hematoxylin counterstaining for 1 min, and washing with
tap water. The water was dried carefully with filter paper, and the
sections were sealed with gelatin glycerin and observed and
photographed under an optical microscope.
Hematoxylin and eosin (H&E)
staining
The paraffin sections prepared were transparentized
with xylene, deparaffinized with ethanol, soaked in tap water for 5
min, stained with hematoxylin for 5 min, differentiated with 1%
hydrochloric acid alcohol for 30 sec and observed under the
microscope, followed by eosin staining completely soaking tissues.
Finally, the sections were sealed with neutral balsam, and observed
and photographed under the optical microscope.
Immunofluorescence staining
The frozen sections were taken from the −20°C
refrigerator, re-warmed at room temperature for 30 min and fixed in
ice acetone for 10 min. After antigen retrieval for 30 min, the
sections were soaked in 0.3% triton X-100 at room temperature for
30 min to rupture the cell membrane, and sealed with 5% bovine
serum albumin (BSA) at room temperature for 60 min. The serum
around the tissues was wiped off, and the primary antibodies (α-SMA
diluted at 1:400, and MOMA-2 diluted at 1:50) were added dropwise
onto the sections for incubation in a wet box at 4°C overnight. The
next day, the sections were taken from the refrigerator, re-warmed
at room temperature for 30 min and added with secondary antibodies
(diluted at 1:400 and 1:200) for incubation in the dark at room
temperature for 3 h, followed by 4′,6-diamidino-2-phenylindole
(DAPI) counterstaining in the dark for 5 min. Finally, the sections
were sealed with anti-fluorescence quenching sealing solution,
covered with the cover glass, and observed and photographed under a
fluorescence confocal microscope.
Enzyme-linked immunosorbent assay
(ELISA)
The blood was collected and centrifuged at 2,750 × g
and 4°C for 15 min, and the supernatant was taken. The
concentrations of serum IL-1β, IL-18 and NLRP3 in mice were
measured according to the instructions of the ELISA kit.
Aortic reactive oxygen species
(ROS)
The frozen sections were taken, re-warmed for 10 min
and added with dihydroethidium (DHE) prepared in the dark, followed
by incubation in the dark at 37°C for 30 min. Finally, the sections
were observed and photographed under the fluorescence confocal
microscope.
Western blotting
The arterial tissues isolated were cut off, added
with 1 ml protein lysis buffer, homogenized for approximately 2 min
and lysed for 30 min, followed by centrifugation at 10,500 × g and
4°C for 15 min. The supernatant was taken, and the protein
concentration in the aorta was detected according to the
instructions of the bicinchoninic acid (BCA) protein assay kit
(Pierce; Thermo Fisher Scientific, Inc.). After denaturation, 50 µg
protein was slowly loaded for electrophoresis. After that, the
protein was transferred onto a polyvinylidene fluoride (PVDF)
membrane (EMD Millipore), washed with tris buffered saline-tween
(TBST) and sealed with 5% skim milk powder for 2 h, followed by
incubation with primary antibodies (diluted pro rata) on a shaking
table at 4°C overnight. The next day, the membrane was washed, and
the protein was incubated with secondary antibodies for 2 h. After
the membrane was washed again, the image was developed in an imager
using the electrochemiluminescence (ECL) solution, followed by
scanning and quantitative calculation.
Statistical analysis
Image-Pro Plus 6.0 (Silver Springs) was used for
image analysis and Statistical Product and Service Solutions (SPSS)
20.0 software (IBM Corp.) was used for data analysis. All data were
expressed as (mean ± SD). Comparison between groups was done using
One-way ANOVA test followed by post hoc test (least significant
difference). P<0.05 was considered to indicate a statistically
significant difference.
Results
Tacrolimus reduces the area of
atherosclerotic plaques in mice
The oil red O staining of aorta in each group
revealed that the area of atherosclerotic plaques in
ApoE−/− mice was increased significantly compared with
that in WT mice, and the difference was statistically significant,
indicating that the AS model was successfully established. After
tacrolimus intervention, the area of atherosclerotic plaques was
significantly reduced in ApoE−/− mice fed with high-fat
diet, and the difference was statistically significant (Fig. 1A and B). The oil red O staining of
aortic root further showed that tacrolimus could reduce the area of
atherosclerotic plaques in ApoE−/− mice fed with
high-fat diet, and there was a statistically significant difference
(Fig. 1C and D).
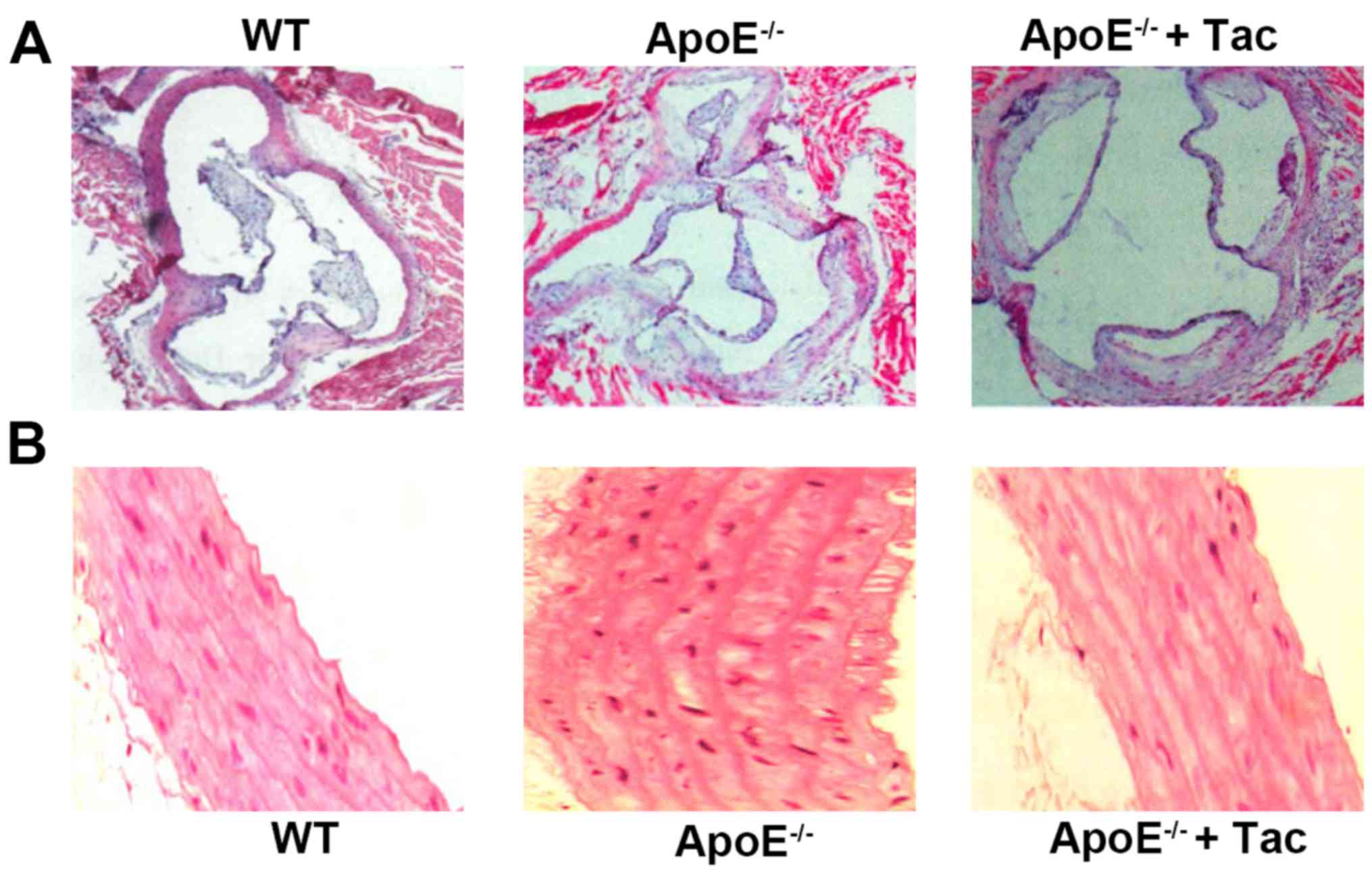
Tacrolimus alleviates the pathological
lesions of atherosclerotic plaques in mice
The H&E staining of aortic root in each group
revealed that the thickness of the aortic wall was uniform and
there was very little AS in WT mice. In the other two groups, there
was intima thickening of the arterial wall in different degrees in
ApoE−/− mice, the fibrous caps were formed, the
thickness of the aortic wall was non-uniform and the
atherosclerotic plaques were obvious. There were obvious arterial
intima thickening and formation of cholesterol crystals in
ApoE−/− mice fed with high-fat diet, and after
tacrolimus intervention, the arterial intima became obviously
thinner and no obvious cholesterol crystals were observed (Fig. 2).
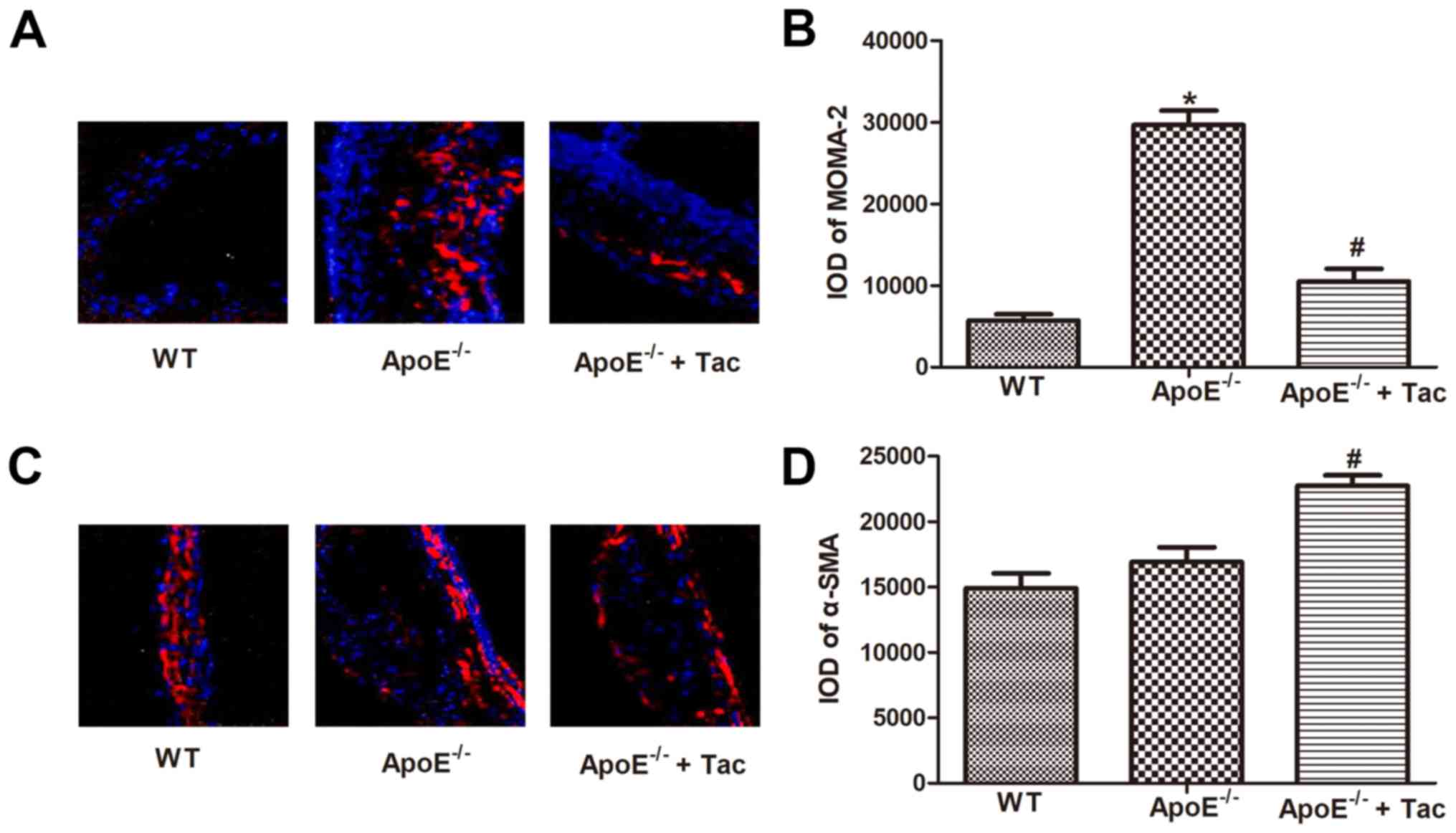
Tacrolimus reduced the macrophage
infiltration in atherosclerotic plaques in mice
To evaluate the effect of tacrolimus on inflammation
in atherosclerotic plaques, the macrophage infiltration and changes
in smooth muscle cells in plaques were detected using MOMA-2 and
α-SMA as specific markers. In atherosclerotic lesions, the
macrophage infiltration was obviously increased in
ApoE−/− mice compared with that in WT mice, showing a
statistically significant difference, and the content of smooth
muscle cells was also increased, but there was no statistically
significant difference. After tacrolimus intervention, the
macrophage infiltration was obviously reduced, and the content of
smooth muscle cells was obviously increased in atherosclerotic
lesions in ApoE−/− mice fed with high-fat diet,
displaying statistically significant differences (Fig. 3).
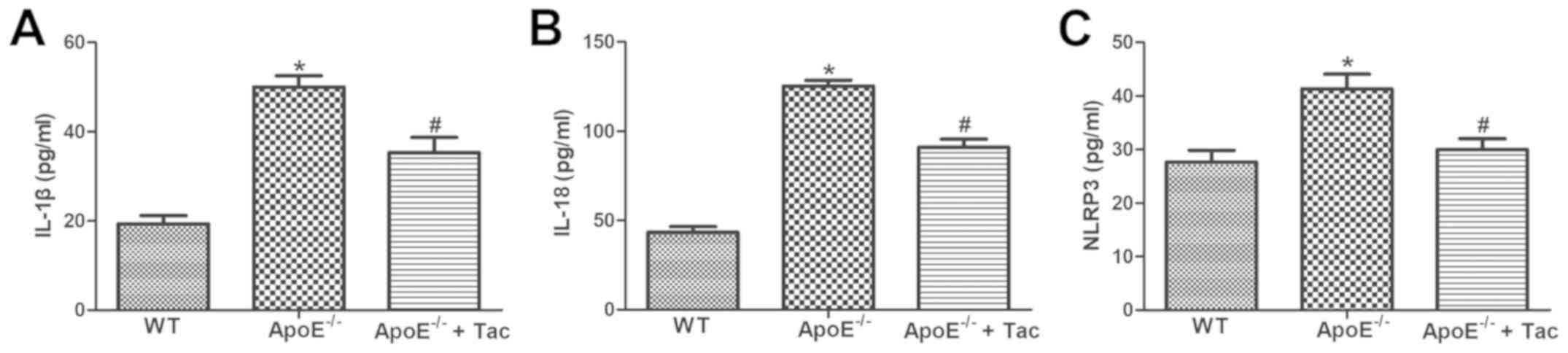
Tacrolimus reduces the concentrations
of serum inflammatory factors in AS mice
According to the ELISA results, the levels of serum
IL-1β, IL-18 and NLRP3 in ApoE−/− mice fed with high-fat
diet were significantly increased compared with those in WT mice,
and the differences were statistically significant. After
tacrolimus intervention, the levels remarkably declined in
ApoE−/− mice fed with high-fat diet, showing
statistically significant differences (Fig. 4).
Tacrolimus inhibits ROS production and
activation of NLRP3 inflammatory corpuscles in atherosclerotic
plaques in mice
The ROS content in atherosclerotic plaques was
detected using DHE. The results manifested that the ROS production
in atherosclerotic plaques was remarkably increased in
ApoE−/− mice compared with that in WT mice, showing a
statistically significant difference, and it was remarkably
decreased after tacrolimus intervention, displaying a statistically
significant difference (Fig. 5A and
B). The results of western blotting showed that the protein
content of NLRP3, ASC, Casp-1, IL-1β and IL-18 in the aorta in
ApoE−/− mice fed with high-fat diet was obviously
increased compared with that in WT mice, and the differences were
statistically significant. After tacrolimus intervention, the
expression of these five kinds of proteins was inhibited to some
extent, indicating that tacrolimus can inhibit ROS production and
activation of NLRP3 inflammatory corpuscles in the aortic root of
AS mice (Fig. 5C and D).
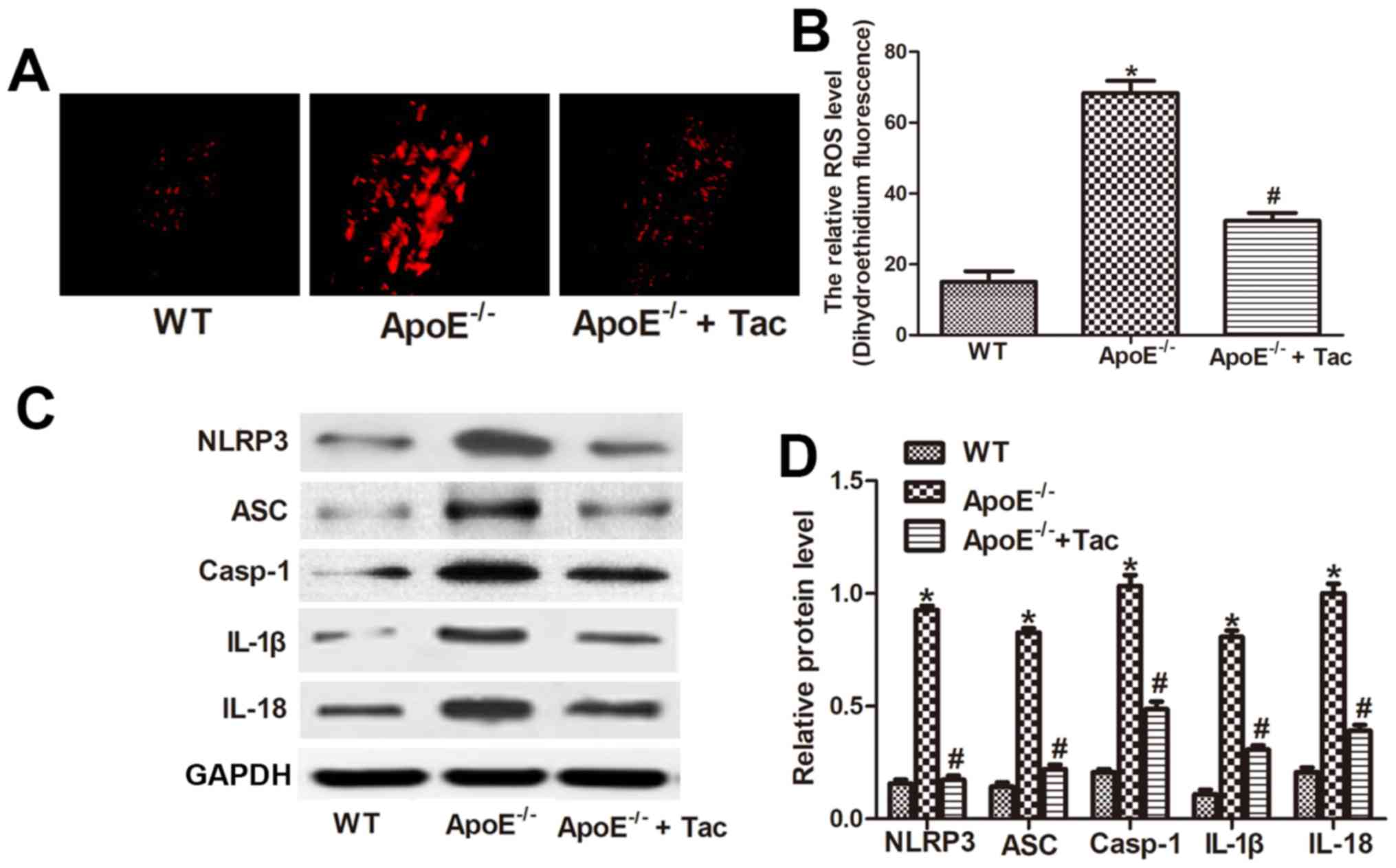 | Figure 5.Tacrolimus inhibits ROS production
and activation of NLRP3 inflammatory corpuscles in atherosclerotic
plaques in mice. (A) The ROS content in atherosclerotic plaques
detected by DHE (magnification of ×400). (B) Analysis of the ROS
level in different groups. (C) Western blotting showed protein
level of NLRP3, ASC, Casp-1, IL-1β and IL-18 in different groups.
(D) Analysis of protein level of NLRP3, ASC, Casp-1, IL-1β and
IL-18. *P<0.05 vs. WT group, #P<0.05 vs.
ApoE−/− group. WT, C57BL/6 mouse group;
ApoE−/−, ApoE−/− mouse group;
ApoE−/− + Tac, ApoE−/− mouse + tacrolimus
intervention group; IL, interleukin; NLRP3, Nod-like receptor
protein 3; ROS, reactive oxygen species; DHE, dihydroethidium; ASC,
apoptosis-associated speck-like protein containing CARD; Casp-1,
caspase-1. |
Discussion
AS is a kind of chronic inflammatory disease caused
by the imbalance of lipid metabolism and deposition of lipid-rich
foam cells under the arterial wall, and inflammation is one of the
important pathophysiological mechanisms of its occurrence and
development (25). AS-induced
cardiovascular and cerebrovascular diseases have become the main
cause of human disability and death in the world, and the coronary
heart disease caused by coronary AS is the main cause of death of
cardiovascular diseases (1).
Tacrolimus, an immunosuppressive drug widely applied in the
transplantation currently, belongs to the macrolide in molecular
structure, which has anti-inflammatory property (26), but its role in AS has not been
reported yet. In this investigation, the effect of tacrolimus on
atherosclerotic plaques was studied via oil red O staining and
H&E staining, and the results revealed that the area of
atherosclerotic plaques in ApoE−/− mice fed with
high-fat diet was obviously increased compared with that in WT
group, suggesting that tacrolimus can inhibit the formation and
development of atherosclerotic plaques in ApoE−/− mice
fed with high-fat diet.
Macrophages are considered as major inflammatory
cells during the formation of AS (27,28).
Macrophages can develop into foam cells, and the lipid nucleus is
formed after necrosis of foam cells, which is a major component of
atherosclerotic plaques (29). In
addition, macrophages are also the major inflammatory cellular
components in atherosclerotic plaques, and a variety of
pro-inflammatory factors secreted by them alter the local
environment of plaques and affect the plaque stability and disease
development (27,29). The collagen fibers produced by smooth
muscle cells migrating to plaques are the main source of fibrous
caps in plaques, and smooth muscle cells in the fibrous cap are
decreased (30,31). If inflammatory cells lead to death of
smooth muscle cells, macrophages are increased, and thin fibrous
caps are prone to rupture, so the plaque stability declines and
they rupture easily (32,33). Therefore, inhibiting the macrophage
infiltration and preventing the decrease of smooth muscle cells in
plaques are the therapeutic goals for stabilizing plaques. In this
study, the infiltration of macrophages and changes in smooth muscle
cells in plaques were detected using MOMA-2 and α-SMA as markers
for macrophages and smooth muscle cells, and it was found that
tacrolimus inhibited the macrophage infiltration and the decrease
of smooth muscle cells in plaques in mice, thereby stabilizing
plaques and inhibiting the occurrence and development of AS.
Macrophages are the main source of IL-1β and IL-18
produced in the body, and their accumulation in vascular lesions is
the main cause of local inflammatory response and plaque formation
(9,10). According to previous studies, ligands
bind to NLRP3 to promote the formation of inflammatory corpuscles
and activate Casp-1, ultimately resulting in maturation and
secretion of pro-IL-1β and pro-IL-18 (14). In this investigation, the levels of
serum NLRP3, IL-1β and IL-18 in mice were detected. The results
showed that the levels of serum IL-1β, IL-18 and NLRP3 in
ApoE−/− mice were significantly increased compared with
those in WT group, and they declined in ApoE−/− mice fed
with high-fat diet after tacrolimus intervention, indicating that
tacrolimus possesses potential anti-inflammatory effect.
At present, there are three hypotheses on the
mechanism of metabolites in the body in activating NLRP3
inflammatory corpuscles: ion channel mode, lysosome mode and ROS
mode (34–36). The ROS model has been well studied at
present, mainly because ROS production promotes the dissociation
between thioredoxin in cells and its ligand in Txnip, the latter of
which may bind to NLRP3 and lead to its activation (37). Oxidative stress is caused by the
imbalance between antioxidants and ROS, and its effect of promoting
AS has been widely recognized (38).
Studies have demonstrated that ROS significantly promotes the
occurrence and development of AS (39,40).
Therefore, the expression levels of ROS and NLRP3 inflammatory
corpuscles in atherosclerotic plaques were further detected, and it
was found that the rapid formation of AS might be related to ROS
production and activation of NLRP3 inflammatory corpuscles that
promote the production and secretion of IL-1β and IL-18. After
tacrolimus intervention, ROS production was inhibited and IL-1β and
IL-18 were decreased, inhibiting the formation of atherosclerotic
plaques.
In conclusion, it is speculated that tacrolimus may
reduce the formation of AS through inhibiting ROS in macrophages
and activation of NLRP3 inflammatory corpuscles and reducing the
release of IL-1β and IL-18. However, its specific mechanism remains
to be further studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XL designed the study and performed the experiments,
XL and XS collected the data, XL and LS analyzed the data, XL
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Ethics
Committee of the Third People's Hospital of Qingdao Animal Center
(Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wong MC, Zhang DX and Wang HH: Rapid
emergence of atherosclerosis in Asia: A systematic review of
coronary atherosclerotic heart disease epidemiology and
implications for prevention and control strategies. Curr Opin
Lipidol. 26:257–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lyu Y, Jiang X and Dai W: The roles of a
novel inflammatory neopterin in subjects with coronary
atherosclerotic heart disease. Int Immunopharmacol. 24:169–172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu F, Chen YG, Geng YJ, Zhang H, Jiang CX,
Sun Y, Li RJ, Sagar MB, Xue L and Zhang Y: The polymorphism in
acetaldehyde dehydrogenase 2 gene, causing a substitution of Glu
> Lys(504), is not associated with coronary atherosclerosis
severity in Han Chinese. Tohoku J Exp Med. 213:215–220. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li J, Lei HT, Cao L, Mi YN, Li S and Cao
YX: Crocin alleviates coronary atherosclerosis via inhibiting lipid
synthesis and inducing M2 macrophage polarization. Int
Immunopharmacol. 55:120–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ravi S, Schuck RN, Hilliard E, Lee CR, Dai
X, Lenhart K, Willis MS, Jensen BC, Stouffer GA, Patterson C, et
al: Clinical evidence supports a protective role for CXCL5 in
coronary artery disease. Am J Pathol. 187:2895–2911. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keles N, Aksu F, Aciksari G, Yilmaz Y,
Demircioglu K, Kostek O, Cekin ME, Kalcik M and Caliskan M: Is
triglyceride/HDL ratio a reliable screening test for assessment of
atherosclerotic risk in patients with chronic inflammatory disease?
North Clin Istanb. 3:39–45. 2016.PubMed/NCBI
|
|
7
|
Samsonova NG, Zvenigorodskaia LA,
Cherkashova EA and Lazebnik LB: Intestinal dysbiosis and
atherogenic dyslipidemia. Eksp Klin Gastroenterol. 3:88–94.
2010.(In Russian).
|
|
8
|
Zeng Z, Cao B, Guo X, Li W, Li S, Chen J,
Zhou W, Zheng C and Wei Y: Apolipoprotein B-100 peptide 210
antibody inhibits atherosclerosis by regulation of macrophages that
phagocytize oxidized lipid. Am J Transl Res. 10:1817–1828.
2018.PubMed/NCBI
|
|
9
|
Ma J, Liu C, Yang Y, Yu J, Yang J, Yu S,
Zhang J and Huang L: C/EBPβ acts upstream of NF-κB P65 subunit in
Ox-LDL-induced IL-1β production by macrophages. Cell Physiol
Biochem. 48:1605–1615. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Formanowicz D, Gutowska K and Formanowicz
P: Theoretical studies on the engagement of interleukin 18 in the
immuno-inflammatory processes underlying atherosclerosis. Int J Mol
Sci. 19:192018. View Article : Google Scholar
|
|
11
|
Cheng Y, Li S, Wang M, Cheng C and Liu R:
Peroxisome proliferator activated receptor gamma (PPARγ) agonist
rosiglitazone ameliorate airway inflammation by inhibiting
Toll-like receptor 2 (TLR2)/Nod-like receptor with pyrin domain
containing 3 (NLRP3) inflammatory corpuscle activation in asthmatic
mice. Med Sci Monit. 24:9045–9053. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu FQ, Gao Q, Wang DD and Zhang ZX:
Effects of GBE50 on LPS/ATP induced NLRP3 inflammasome activation
in primary rat microglia. Zhongguo Zhong Yao Za Zhi. 43:3346–3352.
2018.(In Chinese). PubMed/NCBI
|
|
13
|
Ratajczak MZ, Adamiak M, Thapa A, Bujko K,
Brzezniakiewicz-Janus K and Lenkiewicz AM: NLRP3 inflammasome
couples purinergic signaling with activation of the complement
cascade for the optimal release of cells from bone marrow.
Leukemia. 33:815–825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baljon JJ, Dandy A, Wang-Bishop L, Wehbe
M, Jacobson ME and Wilson JT: The efficiency of cytosolic drug
delivery using pH-responsive endosomolytic polymers does not
correlate with activation of the NLRP3 inflammasome. Biomater Sci.
7:1888–1897. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Xi J, Zhang S, Wu H, Zhou L, Lu J,
Zhang T and Zhao C: Effectiveness and safety of tacrolimus therapy
for myasthenia gravis: A single arm meta-analysis. J Clin Neurosci.
63:160–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Darlenski R: Probable contact urticaria
caused by tacrolimus-containing ointment in the treatment of atopic
dermatitis. J Allergy Clin Immunol Pract. 7:1665–1667. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tani C, Elefante E, Martin-Cascón M,
Belhocine M, Lavilla Olleros C, Vagelli R, Stagnaro C,
Costedoat-Chalumeau N, Ruiz-Irastorza G and Mosca M: Tacrolimus in
non-Asian patients with SLE: A real-life experience from three
European centres. Lupus Sci Med. 5:e0002742018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Majumdar S, Subinya M, Korward J,
Pettigrew A, Scherer D and Xu H: A Low concentration of
tacrolimus/semifluorinated alkane (SFA) eyedrop suppresses
intraocular inflammation in experimental models of uveitis. Curr
Mol Med. 17:211–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pratschke S, Arnold H, Zollner A, Heise M,
Pascher A, Schemmer P, Scherer MN, Bauer A, Jauch KW, Werner J, et
al: Results of the TOP Study: Prospectively randomized multicenter
trial of an ex vivo tacrolimus rinse before transplantation in EDC
livers. Transplant Direct. 2:e762016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kannegieter NM, Hesselink DA, Dieterich M,
de Graav GN, Kraaijeveld R, Rowshani AT, Leenen PJM and Baan CC:
Pharmacodynamic monitoring of tacrolimus-based immunosuppression in
CD14+ monocytes after kidney transplantation. Ther Drug
Monit. 39:463–471. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kaabak M, Babenko N, Shapiro R, Zokoyev A,
Dymova O and Kim E: A prospective randomized, controlled trial of
eculizumab to prevent ischemia-reperfusion injury in pediatric
kidney transplantation. Pediatr Transplant. 22:222018. View Article : Google Scholar
|
|
22
|
Lei WT, Lin HH, Tsai MC, Hung HH, Cheng
YJ, Liu SJ, Lin CY and Yeh TL: The effects of macrolides in
children with reactive airway disease: A systematic review and
meta-analysis of randomized controlled trials. Drug Des Devel Ther.
12:3825–3845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rizvi S, Tariq S, Mehdi M and Hassan AJ:
Synthesis of 99mTc-roxithromycin: A novel diagnostic
agent to discriminate between septic and aseptic inflammation. Chem
Biol Drug Des. 93:1166–1174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ersoy B, Aktan B, Kilic K, Sakat MS and
Sipal S: The anti-inflammatory effects of erythromycin,
clarithromycin, azithromycin and roxithromycin on histamine-induced
otitis media with effusion in guinea pigs. J Laryngol Otol.
132:579–583. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Issa Bhaloo S, Chen T, Zhou B and
Xu Q: Role of resident stem cells in vessel formation and
arteriosclerosis. Circ Res. 122:1608–1624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vigil SV, de Liz R, Medeiros YS and Fröde
TS: Efficacy of tacrolimus in inhibiting inflammation caused by
carrageenan in a murine model of air pouch. Transpl Immunol.
19:25–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Howard AN and Thurnham DI: Lutein and
atherosclerosis: Belfast versus Toulouse revisited. Med Hypotheses.
98:63–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohara K, Wakabayashi H, Taniguchi Y,
Shindo K, Yajima H and Yoshida A: Quercetin-3-O-glucuronide induces
ABCA1 expression by LXRα activation in murine macrophages. Biochem
Biophys Res Commun. 441:929–934. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang D, Wang W, Lin W, Yang W, Zhang P,
Chen M, Ding D, Liu C, Zheng J and Ling W: Apoptotic cell induction
of miR-10b in macrophages contributes to advanced atherosclerosis
progression in ApoE−/− mice. Cardiovasc Res.
114:1794–1805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watanabe R, Watanabe H, Takahashi Y,
Kojima M, Konii H, Watanabe K, Shirai R, Sato K, Matsuyama TA,
Ishibashi-Ueda H, et al: Atheroprotective effects of tumor necrosis
factor-stimulated Gene-6. JACC Basic Transl Sci. 1:494–509. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shindyapina AV, Mkrtchyan GV, Gneteeva T,
Buiucli S, Tancowny B, Kulka M, Aliper A and Zhavoronkov A:
Mineralization of the connective tissue: A complex molecular
process leading to age-related loss of function. Rejuvenation Res.
17:116–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li C, Chen JW, Liu ZH, Shen Y, Ding FH, Gu
G, Liu J, Qiu JP, Gao J, Zhang RY, et al: CTRP5 promotes
transcytosis and oxidative modification of low-density lipoprotein
and the development of atherosclerosis. Atherosclerosis.
278:197–209. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q, Zhang J, Li Y, Shi H, Wang H, Chen
B, Wang F, Wang Z, Yang Z and Wang L: Green tea polyphenol
epigallocatechin-3-gallate increases atherosclerotic plaque
stability in apolipoprotein E-deficient mice fed a high-fat diet.
Kardiol Pol. 76:1263–1270. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silverman M: Structure and function of
hexose transporters. Annu Rev Biochem. 60:757–794. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hornung V, Bauernfeind F, Halle A, Samstad
EO, Kono H, Rock KL, Fitzgerald KA and Latz E: Silica crystals and
aluminum salts activate the NALP3 inflammasome through phagosomal
destabilization. Nat Immunol. 9:847–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pelegrin P, Barroso-Gutierrez C and
Surprenant A: P2X7 receptor differentially couples to distinct
release pathways for IL-1beta in mouse macrophage. J Immunol.
180:7147–7157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sodhi K, Srikanthan K, Goguet-Rubio P,
Nichols A, Mallick A, Nawab A, Martin R, Shah PT, Chaudhry M,
Sigdel S, et al: pNaKtide attenuates steatohepatitis and
atherosclerosis by blocking Na/K-ATPase/ROS amplification in C57Bl6
and ApoE knockout mice fed a western diet. Sci Rep. 7:1932017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rajkumari J, Dyavaiah M, Sudharshan SJ and
Busi S: Evaluation of in vivo antioxidant potential of Syzygium
jambos (L.) Alston and Terminalia citrina Roxb. towards
oxidative stress response in Saccharomyces cerevisiae. J Food Sci
Technol. 55:4432–4439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu J, Xu C, Ning R, Chai D and Lin J:
Effects and related mechanism of retinoid X receptor agonist
bexarotene on atherosclerosis progression in diabetic apoE(−/-)
mice. Zhonghua Xin Xue Guan Bing Za Zhi. 42:492–497. 2014.(In
Chinese). PubMed/NCBI
|
|
40
|
Huang H, Koelle P, Fendler M, Schröttle A,
Czihal M, Hoffmann U, Conrad M and Kuhlencordt PJ: Induction of
inducible nitric oxide synthase (iNOS) expression by oxLDL inhibits
macrophage derived foam cell migration. Atherosclerosis.
235:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|