Introduction
Chronic obstructive pulmonary disease (COPD) is a
chronic respiratory disease characterized by airflow limitation,
which is irreversible with a progressive decline in lung function.
The prevalence of COPD of people aged over 30 years in Asia is 6.3%
(1), and the increasing
environmental pollution and smoking lead to its increasing
incidence, which brings a heavy economic burden to patients and
their families (2). The pathogenesis
of COPD has not yet been elucidated. The present research suggests
that its pathogenesis is related to inflammation, oxidative stress
and protease. These factors act on the lungs, leading to lung
lesions (3–5).
COPD can seriously damage the structure of the
respiratory tract and cause repeated pulmonary infections. Coupled
with chronic hypoxia and irreversible airflow limitation in COPD
patients, these factors can seriously impair patient's respiratory
function and even lead to respiratory failure (6). As an important auxiliary ventilation
measure, mechanical ventilation plays an important role in the
acute exacerbation and general anesthesia of COPD patients
(7). As an invasive therapy,
mechanical ventilation provides oxygen to the body. However, at the
same time, it causes pain, induces multiple stress reactions and
aggravates patient's lung injury (8). Dexmedetomidine is a new type of highly
selective α2 adrenergic receptor agonist widely used in intensive
care and anesthesiology, and dexmedetomidine (Dex) can produce
calming, analgesic, hypnosis, anti-sympathetic effects by
inhibiting the release of catecholamines in the plasma (9). In recent years, a large number of
studies (10–12) have shown that Dex has protective
effects on various organs, and can improve lung function and reduce
the impact on lung hemodynamics. However, regarding its mechanical
ventilation to patients with COPD, the mechanism remains unclear.
Therefore, this study used passive smoking and intratracheal
instillation of lipopolysaccharide to establish a rat COPD model,
and explored the effects of different doses of Dex on lung
oxidative stress and inflammatory response in mechanical
ventilation of COPD rats.
Materials and methods
Materials and reagents
The experimental SPF grade SD rats (8 weeks old,
male, weighing 200–250 g) were purchased from the Animal
Experimental Center of Henan Province, and the experiment was
carried out after 1 week of adaptive feeding. Superoxide dismutase
2 (SOD2) (cat. no. 13194), catalase (cat. no. 14097), and
monoclonal antibody were purchased from CST. Internal reference
β-actin (cat. no. 20536-1-AP) and horseradish peroxidase (HRP)
labeled secondary antibody (cat. no. SA00001-2) were purchased from
Proteintech. Interleukin-8 (IL-8) (cat. no. HM10222), tumor
necrosis factor-alpha (TNF-α) (cat. no. HM10001-S), malondialdehyde
(MDA) (cat. no. HM10250), and enzyme-linked immunosorbent assay
(ELISA) kit were from Bio-swamp. Hematoxylin and eosin (HE)
staining kit and the RIPA lysate were purchased from Biotime
Biological Co., Ltd. Dexmedetomidine hydrochloride injection (2 ml:
200 µg) was purchased from Jiangsu Hengrui Pharmaceutical Co.,
Ltd.
All animal experiments were in accordance with the
ethical standards for laboratory animals and approved by the Ethics
Committee of the First Hospital of Qiqihar (Qiqihar, China).
Establishment of a COPD rat model
Eleven of the 38 SD rats were randomly selected as
the blank control group. The other rats were used to establish COPD
rat model according to the method in the literature (13), by passive cigarette smoking and
intratracheal instillation of lipopolysaccharide. On the first day
and the 14th day, 200 µl of 1 g/l lipopolysaccharid was injected
into the trachea. From the 2nd to the 28th day (except the 14th
day), all rats were placed in a sealed 1 m3 smoke
chamber. Pressure was controlled at a standard atmospheric pressure
(101.325 kPa), and the cigarette inhalation was continued for 30
min every day. Blank control group was not performed with any
intervention. All rats had free access to food and water, and the
animal room had natural light, a temperature of 20–25°C, and a
relative humidity of 45–50%. The general condition of the survival
of the rats was observed daily. On the 29th day, 11 rats in the
blank control group and 11 rats randomly selected from the model
group received non-invasive test for lung function. Three rats from
the blank control and 3 rats from the model were randomly selected
from the model group. After blood was taken, lung tissues were
taken for pathological examination to determine whether the
modeling was successful.
Non-invasive lung function test
Twenty-two rats were placed in the EMKA small animal
non-invasive pulmonary function monitoring system, and calm
breathing was recorded first. Then the rat tidal volume (TV), peak
expiratory flow rate (PEF), 50% lung vitality maximum expiratory
flow (PE50), 0.3 sec forced expiratory volume (FEV0.3), and 0.3 sec
forced expiratory volume to forced vital capacity ratio
(FEV0.3/FVC) were recorded. The averages of 3 measurements per
parameter were taken.
Lung histopathology test
After the lung tissue was fixed by 4%
paraformaldehyde, it was dehydrated in 50, 70, 80, 95, 100 and 100%
alcohol, for 20 min each time. Then the dehydrated tissue was
placed in: Εthanol + xylene (1:1) for 2 h, xylene I and II for 10
min each, then placed in xylene + paraffin (1:1) for 2 h, paraffin
I (1 h), paraffin II (2 h) to immerse. After embedding in an ice
box, 4 µm paraffin slices were made, which were then dewaxed with
xylene and gradient alcohol, and stained with hematoxylin and eosin
for observation under a microscope.
Experimental grouping and
administration
The remaining 24 model rats were randomly divided
into 3 groups, 8 in each group, which were the model control group,
Dex low-dose group, and Dex high-dose group. All rats fasted for 12
h before anesthesia and fasted of liquid for 4 h. After weighing,
they were anesthetized with 10% chloral hydrate at a dose of 0.3
ml/100 g. The rats were fixed on a table covered with an electric
blanket and placed in a supine position. The temperature of the rat
was monitored by a rectal temperature probe and maintained at about
37°C. The tail of the rat was disinfected with vital iodine, then a
puncture was performed. The infusion pump was used to infuse 2
ml/kg/h normal saline, and the tail vein was injected with 1 mg/kg
vecuronium to inhibit spontaneous breathing in rats. Hydroxyethyl
starch (4–8 ml/kg/h) was intermittently infused to maintain blood
pressure stability. The neck was disinfected, and the towel was
placed to perform tracheostomy. The tracheal tube was inserted and
fixed. The mechanical ventilation parameters were set as:
mechanical ventilation tidal volume: VT: 8 ml/kg, ventilation
frequency: 60–80 times/min, maintaining PErCO2 at 35–45
mmHg, suction/hit ratio: 1:3, and the inspired oxygen concentration
was set at 45%. The mechanical ventilation of all groups last 2 h.
In the Dex low-dose group, Dex 1.0 µg/kg/h continuous infusion was
given during mechanical ventilation, and Dex high-dose group was
given Dex 5.0 µg/kg/h continuous infusion. Blank control and model
control group were given the same amount of normal saline infusion
for 2 h. After mechanical ventilation, the chest was opened to
obtain the lungs. One part was placed in 4% paraformaldehyde and
fixed, the other part was stored at −80°C for later use.
Blood gas analysis
Arterial blood was taken by carotid artery puncture
before and after mechanical ventilation in rats, and some blood was
taken for blood gas analysis using ABL800 blood gas analyzer
(Dadiometer, Denmark), including partial pressure of carbon dioxide
(PaCO2), partial pressure of oxygen (PaO2)
and PH value.
ELISA test
The remaining blood in the blood gas analysis was
centrifuged (12,000 × g at 4°C for 15 min) and the serum was stored
at a low temperature. ELISA kit was equilibrated to room
temperature, and the standard solution was diluted in accordance
with the specifications. Six concentration gradients were set, and
50 μl of different concentration of standards was added to
each well. Two wells, blank wells and sample wells, were set for
each. A total of 40 μl of the sample was added to the sample
well, and then 10 μl of biotin-labeled IL-8, TNF-α, and MDA
antibodies were added, respectively, to the bottom of the well,
without touching the well wall. A total of 50 μl of the
enzyme labeling reagent was added to each well except the blank
wells, and then incubated at 37°C for 30 min after the plate was
closed. The liquid was discarded and dried. A total of 200 µl of
washing solution was added per well and discarded after standing
for 30 sec, and repeated 5 times. After patted dry, each well was
added with 50 μl of chromogenic reagents A and B,
respectively, the chromogenic reaction was performed in the dark at
37°C for 10 min, and 50 μl of stop solution was added to
terminate the reaction. The blank well was zeroed, the absorbance
of each well was measured at 450 nm wavelength. The data were
entered into the computer to make a standard curve and the
regression equation, then the content in the sample well was
determined.
Detection of SOD2 and catalase in lung
tissue
Lung tissue (30 mg) was added with 300 µl lysate and
grinded on ice, standing for 30 min to make it fully lysed, then
centrifuged at 4°C and at 12,000 × g for 15 min. Supernatant (10
µl) was obtained to quantify BCA protein. The remaining supernatant
was added with 5× sample buffer by 4:1 volume, placed in a 100°C
water bath for 10 min, and the sample was loaded according to equal
total protein amount. Electrophoresis was carried out with 5%
laminating gel and 10% separating gel at 80 V voltage until
bromophenol blue entered separating the gel, the voltage was
changed to 120 V until the target strip was separated. Then the
protein was transferred to the PVDF membrane by wet transfer method
at 275 mA for 80 min, and blocked with 5% milk at room temperature
for 2 h. Then diluted (1:1,000) SOD2, catalase and β-actin
antibodies were added and incubated overnight at 4°C. After
washing, 2% milk-configured secondary antibody (1:10,000) was added
for 1 h at room temperature, and developed. Image J was used for
analyzing the band gray value, β-actin as internal reference. The
protein relative expression amount was the ratio of the gray value
of the target protein to the gray value of β-actin.
Statistical analysis
The results are expressed as mean ± standard
deviation. The data were analyzed by SPSS 20 (IBM Corp., Armonk,
NY, USA). t-test was used for comparison between two groups.
One-way ANOVA was used for comparison of multi groups. LSD test was
used if the variance was equal. If the variance was unequal,
Dunnett's t-test was used. The statistical difference was set at
p<0.05, and each experiment was repeated at least three
times.
Results
General survival of rats
In the normal control group, all rats had good
mental state, flexible response, normal skin gloss, normal diet,
normal breathing, and normal weight gain. In the model group, rats
developed slowly and were in poor spirit. The amount of activity
was reduced, and the response to external stimuli was slow, skin
was yellow, gloss was poor, appetite was weak, body was thin, and
the weight gain was slow (Table I).
There were respiratory diseases such as cough and wheezing.
 | Table I.Weight gain of SD rats before and
after modeling. |
Table I.
Weight gain of SD rats before and
after modeling.
| Groups | Before modeling | After modeling | Weight gain |
|---|
| Blank control | 228.63±22.58 | 408.13±20.52 | 179.5±11.19 |
| COPD model | 228.16±27.33 | 383.96±29.0 |
155.8±20.16a |
Lung histopathological changes
Compared with the blank control group, the TV, PEF,
FEV0.3, and FEV0.3/FVC of the COPD model group were significantly
decreased (Table II), suggesting
airflow limitation in the COPD model group.
 | Table II.Pulmonary function indicators of SD
rats after modeling. |
Table II.
Pulmonary function indicators of SD
rats after modeling.
|
| Groups |
|---|
|
|
|
|---|
| Indicators | Blank control
(n=11) | COPD model
(n=11) |
|---|
| TV (ml) | 2.73±0.32 |
1.47±0.25a |
| PEF (ml/s) | 37.15±1.83 |
16.38±2.13a |
| EP50 (ml/s) | 1.79±0.20 |
1.35±0.22a |
| FEV0.3 (ml) | 4.42±0.33 |
2.33±0.32a |
| FEV0.3/FVC (%) | 87.61±4.41 |
64.49±4.30a |
Gross view of rat lung tissue
Lung tissue volume of normal control group was
basically normal, surface was smooth, elasticity was good and whole
lung was light red. In model group, lung tissue volume of the rat
was significantly increased, and surface was rough with a large
amount of black particulate matter; the edges became dull, the
elasticity was reduced, and the lungs were dark purple-red. HE
staining showed that in normal control group, lung lobule structure
of the rat lung tissue was normal; the cells had no obvious
congestion or edema, no degeneration or necrosis, no or only a
small amount of inflammatory cell infiltration; the alveolar of the
model group became large, the alveolar wall became thinner; some
alveolar walls were broken, and the cell lobule showed obvious cell
degeneration, necrosis and shedding; a large amount of inflammatory
cell infiltration appeared in the interstitial space, suggesting
that the COPD rat model was successfully established (Fig. 1).
Blood gas analysis
Blood gas analysis showed (Fig. 2) that compared with the blank control
group, the PaO2 of the model control group decreased
significantly, and the PaCO2 increased significantly,
the blood pH value decreased (p<0.05); while after treatment
with different dose of Dex during mechanical ventilation,
PaO2 increased and PaCO2 decreased
significantly. This effect was significantly increased with
increasing dose of Dex, and blood pH returned to normal levels
(Fig. 2B).
Effect of Dex on inflammatory factors
and oxidative stress in COPD rats
As shown in Fig. 3,
levels of inflammatory factors IL-8 and TNF-α were significantly
increased in COPD rats (p<0.05), and the contents of MDA were
also significantly increased (p<0.05). After treatment with low
dose Dex and high dose Dex, serum IL-8, TNF-α and MDA in COPD model
rats were significantly decreased (p<0.05), and decreased with
the increase of Dex dose.
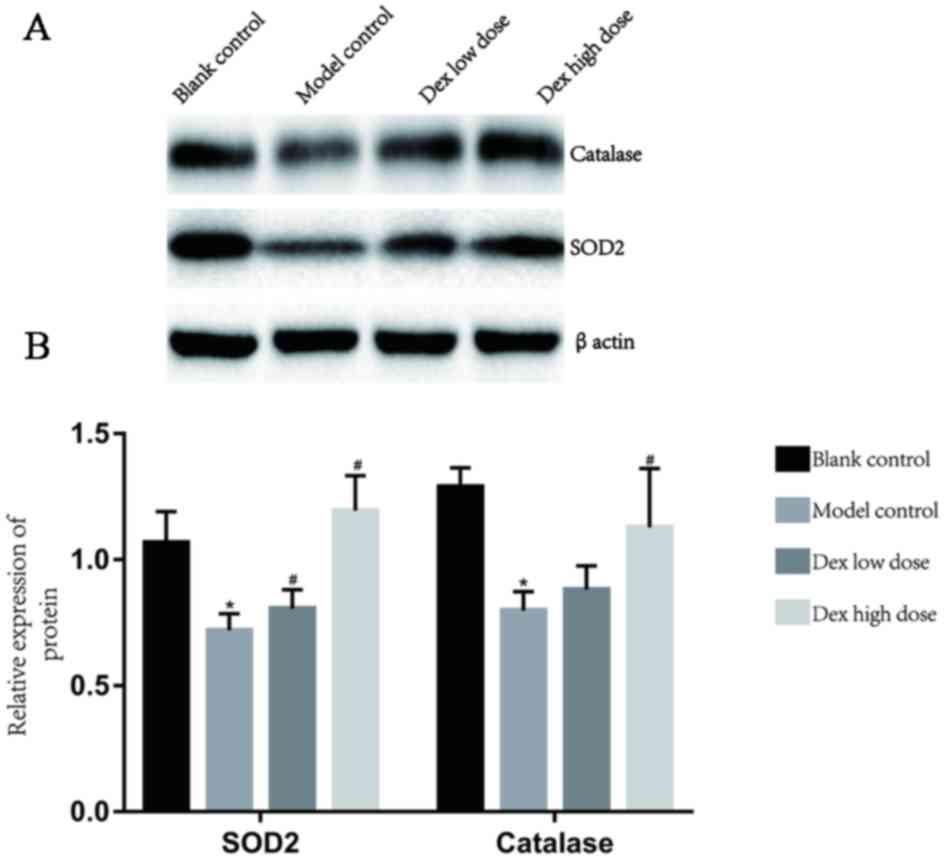
Western blot analysis of SOD2 and
catalase
Compared with the blank control group, SOD2 and
catalase were significantly decreased in the lung tissue of the
model control group (p<0.05). After low dose Dex and high dose
Dex infusion, SOD2 and catalase were significantly increased
(p<0.05) in a dose-dependent manner (Fig. 4).
Discussion
Smoking is not only the leading cause of death in
preventable diseases, but is also the most important risk factor
for COPD. A large amount of particulate matter and harmful gases
such as IL-8 and TNF-α in cigarette smoke can cause and aggravate
oxidative stress and inflammatory reactions in the lungs. These
inflammatory mediators can destroy the elastic fibers of the lungs
and airways, causing decreased compliance, increased airway
secretions, and limited irreversibility of the respiratory tract
(14). LPS is an antigen on the cell
wall surface of Gram-negative bacteria, which can promote the
activation of inflammatory cells and release a large number of
inflammatory factors (15). The
administration of a certain dose of lipopolysaccharide can induce
chronic inflammation of the airway and alter lung function
(16). Although a previous study
showed that Dex provided clinically relevant benefits for patients
with moderate COPD who underwent lung cancer surgery, there was no
direct evidence that Dex had an effect on mechanical ventilation in
patients with COPD. In this study, we established a COPD rat model
to investigate the effects of Dex on oxidative stress and
inflammatory response, providing a theoretical basis for the
application of Dex and mechanical ventilation in patients with
clinical COPD.
In this study, a cigarette smoke assay + multiple
intratracheal instillation of LPS was used to establish a COPD rat
model. After 28 days of modeling, the rats showed loss of appetite,
body weight loss, slow weight gain, and cough, wheezing and other
respiratory system diseases. After the HE staining analysis, the
alveolar wall of the model group became thinner, and some alveolar
walls were broken. There was a large amount of inflammatory cell
infiltration in the interstitial space, which was consistent with
the pathological features of COPD, indicating that the COPD rat
model was successfully established.
Chronic inflammatory response is an important
pathogenesis of COPD. A variety of inflammatory cells, cytokines
and inflammatory mediators are involved in the formation of COPD.
After inflammatory cells are activated, a large amount of
inflammatory mediators can be released, leading to the destruction
of lung structure causing emphysema, edema, and excessive secretion
of mucus, airway stenosis and increased airflow resistance
(17). Inflammatory mediators such
as IL-6, IL-8, TNF-α, and IL-1β are involved in the development of
COPD, which can induce histamine release and neutrophil
decomposition, promote the infiltration and release of inflammatory
mediators, and induce tissue fibrosis, further aggravating lung
injury (18). Dexmedetomidine is a
highly selective α2 adrenergic receptor agonist widely used for
sedation of endotracheal intubation and mechanical ventilation. Its
sedative effect is very similar to the natural sleep state, which
can minimize patients' stress response and has a protective effect
on various organs (19). This study
found that compared with the blank control group, the inflammatory
mediators IL-8 and TNF-α in the serum of the model control group
were significantly increased, suggesting that the inflammatory
response increased in the COPD rat model. After treatment with
different doses of Dex, the post-inflammatory mediators were
significantly reduced, indicating that Dex can significantly
inhibit the inflammatory response in COPD rats. This
anti-inflammatory effect of Dex may be related to its control of
inflammatory response and reduction of inflammatory mediators by
inhibiting the excessive activation of macrophages (20).
Oxidative stress is one of the characteristics of
COPD. The accumulation of oxidants in the body and the large
consumption of antioxidants lead to an oxidation-antioxidation
imbalance, which leads to oxidative stress and damage to lung
tissue (21). Oxides in the body can
cause peroxidation of lipids, resulting in a significant decrease
in the fluidity of the cell membrane, causing increased
permeability of the cell membrane, release of cell lysosomes,
dissolution of cells, and large amounts of oxygen free radicals and
aldehydes produced from peroxidation activate caspase inducing
apoptosis (22). When the body's
oxidative stress level is significantly increased, the body
releases a large number of antioxidant enzymes such as SOD2,
catalase. These antioxidant enzymes can resist oxidative stress and
prevent the lungs from being damaged by oxidative stress. In this
study, Dex significantly increased SOD2 and catalase proteins and
the ability of scavenging oxygen free radicals, and improved
ventilation and increased blood oxygen partial pressure in COPD
rats. Chen et al (23)
reported that Dex can reduce the synthesis of cyclic adenosine by
inhibiting the activity of adenylate cyclase, thereby reducing the
influx of calcium ions to the nerve endings and inhibiting the
release of transmitters, then performing the anti-oxidative stress
role. However, in this study, it is still unclear whether Dex
directly promotes the expression of antioxidant enzymes through
some regulation or Dex reduces lung oxidative stress by inhibiting
inflammatory response, improving lung ventilation and lung oxygen
supply.
Based on our study, Dex can improve oxidative stress
in mechanical ventilation in rats with COPD, and can reduce lung
tissue inflammation, thereby protecting lung tissue in COPD
mechanically ventilated rats.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL wrote the manuscript. PL and CS were responsible
for lung histopathology test and ELISA. JH and SC contributed to
the construction of the animal model. DZ performed western blot
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Hospital of Qiqihar (Qiqihar, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Regional COPD Working Group, : COPD
prevalence in 12 Asia-Pacific countries and regions: Projections
based on the COPD prevalence estimation model. Respirology.
8:192–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biancardi E, Fennell M, Rawlinson W and
Thomas PS: Viruses are frequently present as the infecting agent in
acute exacerbations of chronic obstructive pulmonary disease in
patients presenting to hospital. Intern Med J. 46:1160–1165. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foschino Barbaro MP, Carpagnano GE,
Spanevello A, Cagnazzo MG and Barnes PJ: Inflammation, oxidative
stress and systemic effects in mild chronic obstructive pulmonary
disease. Int J Immunopathol Pharmacol. 20:753–763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Min T, Bodas M, Mazur S and Vij N:
Critical role of proteostasis-imbalance in pathogenesis of COPD and
severe emphysema. J Mol Med (Berl). 89:577–593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacNee W: Pulmonary and systemic
oxidant/antioxidant imbalance in chronic obstructive pulmonary
disease. Proc Am Thorac Soc. 2:50–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amri Maleh V, Monadi M, Heidari B, Maleh
PA and Bijani A: Efficiency and outcome of non-invasive versus
invasive positive pressure ventilation therapy in respiratory
failure due to chronic obstructive pulmonary disease. Caspian J
Intern Med. 7:99–104. 2016.PubMed/NCBI
|
|
7
|
Westhoff M, Bachmann M, Braune S,
Karagiannidis C, Kluge S, Lepper PM, Müller T and Schönhofer B:
Severe hypercapnic respiratory failure in acute exacerbation of
COPD: Significance of ventilation and extracorporal CO2
removal. Dtsch Med Wochenschr. 141:1758–1762. 2016.(In German).
PubMed/NCBI
|
|
8
|
Hoegl S, Bachmann M, Scheiermann P, Goren
I, Hofstetter C, Pfeilschifter J, Zwissler B and Muhl H: Protective
properties of inhaled IL-22 in a model of ventilator-induced lung
injury. Am J Respir Cell Mol Biol. 44:369–376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu J, Chen J, Xia P, Tao G, Zhao H and Ma
D: Dexmedetomidine attenuates remote lung injury induced by renal
ischemia-reperfusion in mice. Acta Anaesthesiol Scand.
55:1272–1278. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cosar M, Eser O, Fidan H, Sahin O,
Buyukbas S, Ela Y, Yagmurca M and Ozen OA: The neuroprotective
effect of dexmedetomidine in the hippocampus of rabbits after
subarachnoid hemorrhage. Surg Neurol. 71:54–59; discussion 59.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duffy BA, Chun KP, Ma D, Lythgoe MF and
Scott RC: Dexamethasone exacerbates cerebral edema and brain injury
following lithium-pilocarpine induced status epilepticus. Neurobiol
Dis. 63:229–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsing CH, Lin CF, So E, Sun DP, Chen TC,
Li CF and Yeh CH: α2-Adrenoceptor agonist dexmedetomidine protects
septic acute kidney injury through increasing BMP-7 and inhibiting
HDAC2 and HDAC5. Am J Physiol Renal Physiol. 303:F1443–F1453. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizutani N, Fuchikami J, Takahashi M, Nabe
T, Yoshino S and Kohno S: Pulmonary emphysema induced by cigarette
smoke solution and lipopolysaccharide in guinea pigs. Biol Pharm
Bull. 32:1559–1564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhatt SP, Washko GR, Dransfield MT, Sieren
JC, Newell JD Jr and Hoffman EA: Comparison of spirometric
thresholds in diagnosing smoking-related airflow obstruction:
Authors' response. Thorax. 69:1147–1148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li G, Li J, Zhou Q, Song X, Liang H and
Huang L: Growth hormone releasing peptide-2, a ghrelin agonist,
attenuates lipopolysaccharide-induced acute lung injury in rats.
Tohoku J Exp Med. 222:7–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wright JL, Tai H and Churg A: Vasoactive
mediators and pulmonary hypertension after cigarette smoke exposure
in the guinea pig. J Appl Physiol (1985). 100:672–678. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuo L, He F, Sergakis GG, Koozehchian MS,
Stimpfl JN, Rong Y, Diaz PT and Best TM: Interrelated role of
cigarette smoking, oxidative stress, and immune response in COPD
and corresponding treatments. Am J Physiol Lung Cell Mol Physiol.
307:L205–L218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nie G, Xie CL, Cao YJ, Xu MM, Shi X, Zou
AL and Qi JH: Meta-analysis of IL-6 −174G/C polymorphism and
psoriasis risk. Genet Mol Res. 15:152016. View Article : Google Scholar
|
|
19
|
Xia R, Xu J, Yin H, Wu H, Xia Z, Zhou D,
Xia ZY, Zhang L, Li H and Xiao X: Intravenous infusion of
dexmedetomidine combined isoflurane inhalation reduces oxidative
stress and potentiates hypoxia pulmonary vasoconstriction during
one-lung ventilation in patients. Mediators Inflamm.
2015:2380412015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Callahan P, Pinto SJ, Kurland G, Cain JG,
Motoyama EK and Weiner DJ: Dexmedetomidine for infant pulmonary
function testing. Pediatr Pulmonol. 50:150–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nemmar A, Raza H, Subramaniyan D, John A,
Elwasila M, Ali BH and Adeghate E: Evaluation of the pulmonary
effects of short-term nose-only cigarette smoke exposure in mice.
Exp Biol Med (Maywood). 237:1449–1456. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirkham PA and Barnes PJ: Oxidative stress
in COPD. Chest. 144:266–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Hou C, Chen X, Wang D, Yang P, He
X, Zhou J and Li H: Protective effect of cannabidiol on hydrogen
peroxide induced apoptosis, inflammation and oxidative stress in
nucleus pulposus cells. Mol Med Rep. 14:2321–2327. 2016. View Article : Google Scholar : PubMed/NCBI
|