Introduction
Glaucoma is an eye disease characterized by optic
atrophy and visual field defect. Primary glaucoma is divided into
open-angle and angle-closure glaucoma. The incidence of glaucoma
has been increasing with the development of science and technology
and the overuse of eyes in recent years (1,2). The
disease has a very covert onset, so its pathogenesis remains
unclear. Studies have shown that the onset is related to mechanical
and vascular factors (3), so early
diagnosis and timely treatment are crucial to prevent glaucoma
patients from severe visual impairment (4).
Currently, glaucoma is mainly diagnosed by optic
nerve examination and ultrasonic biomicroscopy. However, the two
methods are expensive and susceptible to the subjective judgment of
operators (5), which is not
conducive to the diagnosis and the severity evaluation of the
disease. Long non-coding RNA (lncRNA) is a long-chain non-coding
RNA and its role in various diseases has been widely valued in
recent years (6). lncRNA MALAT1, a
typical and multifunctional non-coding RNA, has been reported to
promote tumor metastasis by inducing epithelial-mesenchymal
transformation (7).
According to a previous study, lncRNA MALAT1 plays a
pivotal role in proliferative vitreoretinopathy, and affects
apoptosis of retinal ganglion cells in glaucoma rats by regulating
PI3K/Akt signaling pathway (8). As
an lncRNA that has been widely studied, lncRNA ANRIL has been found
to protect human trabecular meshwork cells of glaucoma mice by
conditioning miR-7 (9). Although
these studies have shown that lncRNA MALAT1 and lncRNA ANRIL play
an important role in the development of glaucoma, their clinical
value in glaucoma patients has been rarely analyzed.
Therefore, the expression of serum lncRNA MALAT1 and
lncRNA ANRIL in glaucoma patients was detected to analyze their
clinical significance in the patients, to provide more
possibilities for the diagnosis and treatment of the disease.
Patients and methods
General information
Altogether 86 glaucoma patients diagnosed in the
Hospital of Chengdu University of Traditional Chinese Medicine from
January 2016 to June 2018 were enrolled (the study group),
including 49 males and 37 females, with an average age of
51.19±1.33 years. A total of 86 people who underwent physical
examinations and were confirmed to be healthy in the hospital
during the same period were also enrolled (the control group).
Inclusion criteria were as follows: patients diagnosed with
glaucoma by optic nerve examination, ultrasound biomicroscopy, and
visual field examination. Exclusion criteria were as follows:
Patients complicated with other eye diseases; pregnant or lactating
patients; patients complicated with other malignant tumor diseases;
patients with severe diseases of the immune system; patients with
severe hepatic and renal dysfunction. All patients and their
families agreed to participate in the study, which was approved by
the Hospital Ethics Committee.
Detection of indices
Fasting venous blood (5 ml) was drawn from the
patients in the morning of the next day after admission,
anticoagulated with heparin, and centrifuged at 3,000 rpm for 5
min. The serum was taken out for the detection of the indices.
qRT-PCR detection of lncRNA MALAT1 and
lncRNA ANRIL
TRIzol reagent was used to extract total RNA from
the serum, and an ultraviolet spectrophotometer was used to detect
its purity and concentration. SYBR-Green Real-time PCR Master mix
was used to reverse transcribe the total RNA of lncRNA MALAT1 and
lncRNA ANRIL, with the steps carried out in strict accordance with
the manufacturer's kit. Then, PCR amplification was carried out.
The reaction conditions were: pre-degeneration at 95°C for 10 min,
degeneration at 95°C for 15 sec, annealing at 60°C for 60 sec, and
finally extension at 72°C for 30 sec, for 40 cycles. The primers
were synthesized by Sangon Biotech (Shanghai) Co., Ltd. GAPDH was
used as an internal reference and 2−ΔΔCt was used to
calculate the relative expression (Table
I).
 | Table I.Related primers. |
Table I.
Related primers.
| Factors | Upstream primers | Downstream
primers |
|---|
| MALAT1 |
5′-CAGTGGGGAACTCTGACTCG-3′ |
5′-GTGCCTGGTGCTCTCTTACC-3′ |
| ANRIL |
5′-TGCTCTATCCGCCAATCAGG-3′ |
5′-GGGCCTCAGTGGCACATACC-3′ |
| GAPDH |
5′-ACAGTCAGCCGCATCTTCTT-3′ |
5′-GACAAGCTTCCCGTTCTCAG-3′ |
Detection of other relevant
indices
The expression of serum pigment epithelium-derived
factor (PEDF), homocysteine (Hcy), and inflammatory cytokines
[interleukin-12 (IL-12), interleukin-4 (IL-4) and interferon-γ
(IFN-γ)] was detected. Enzymatic cycling assay was used to
determine the expression of serum Hcy. A fully automatic
biochemical analyzer was used to analyze the expression of serum
PEDF. Enzyme-linked immunosorbent assay (ELISA) was used to detect
the expression of serum IL-12, IL-4 and IFN-γ.
Statistical methods
In this study, SPSS 18.0 was used to statistically
analyze the experimental data. A Chi-square test was used for count
data. Measurement data were expressed as mean ± standard deviation.
A t-test was used for comparison between two groups. Pearson was
used for correlation analysis. Receiver operating characteristic
(ROC) curves were plotted to analyze the diagnostic value of lncRNA
MALAT1 alone, lncRNA ANRIL alone, and their combined detection for
glaucoma. Multivariate Logistic regression was used for the
multivariate analysis of risk factors for the disease. P<0.05
indicates a statistically significant difference.
Results
Comparison of general information
There were no significant differences in sex, age,
body mass index (BMI), and other information between the study and
control groups (P>0.05) (Table
II).
 | Table II.General information. |
Table II.
General information.
| Factors | Study group
(n=86) | Control group
(n=86) | t/χ2
value | P-value |
|---|
| Sex |
|
| 0.024 | 0.878 |
| Male | 47 (54.65) | 48 (55.81) |
|
|
|
Female | 39 (45.35) | 38 (44.19) |
|
|
| Age (years) |
|
| 0.023 | 0.879 |
| ≤51 | 41 (47.67) | 42 (48.84) |
|
|
|
>51 | 45 (52.33) | 44 (51.16) |
|
|
| BMI
(kg/m2) |
|
| 0.094 | 0.759 |
| ≤23 | 40 (45.51) | 38 (44.19) |
|
|
|
>23 | 46 (54.49) | 48 (55.81) |
|
|
| History of
smoking |
|
| 0.223 | 0.637 |
| Yes | 31 (36.05) | 34 (39.53) |
|
|
| No | 55 (63.95) | 52 (60.47) |
|
|
| Educational
background |
|
| 0.097 | 0.755 |
| Below
junior high school | 33 (38.37) | 35 (40.70) |
|
|
| Junior
high school or above | 53 (61.63) | 51 (59.30) |
|
|
| Place of
residence |
|
| 0.216 | 0.642 |
|
Countryside | 49 (56.98) | 52 (60.47) |
|
|
| City | 37 (43.02) | 34 (39.53) |
|
|
| Types |
|
| 0.024 | 0.878 |
|
Open-angle | 37 (43.02) | 38 (44.19) |
|
|
|
Angle-closure | 49 (56.98) | 48 (55.81) |
|
|
| Creatinine
(µmol/l) | 62.77±4.24 | 63.05±4.31 | 0.668 | 0.430 |
| Blood urea nitrogen
(mmol/l) |
6.08±1.08 |
6.11±1.10 |
|
|
Comparison of expression of lncRNA
MALAT1 and lncRNA ANRIL
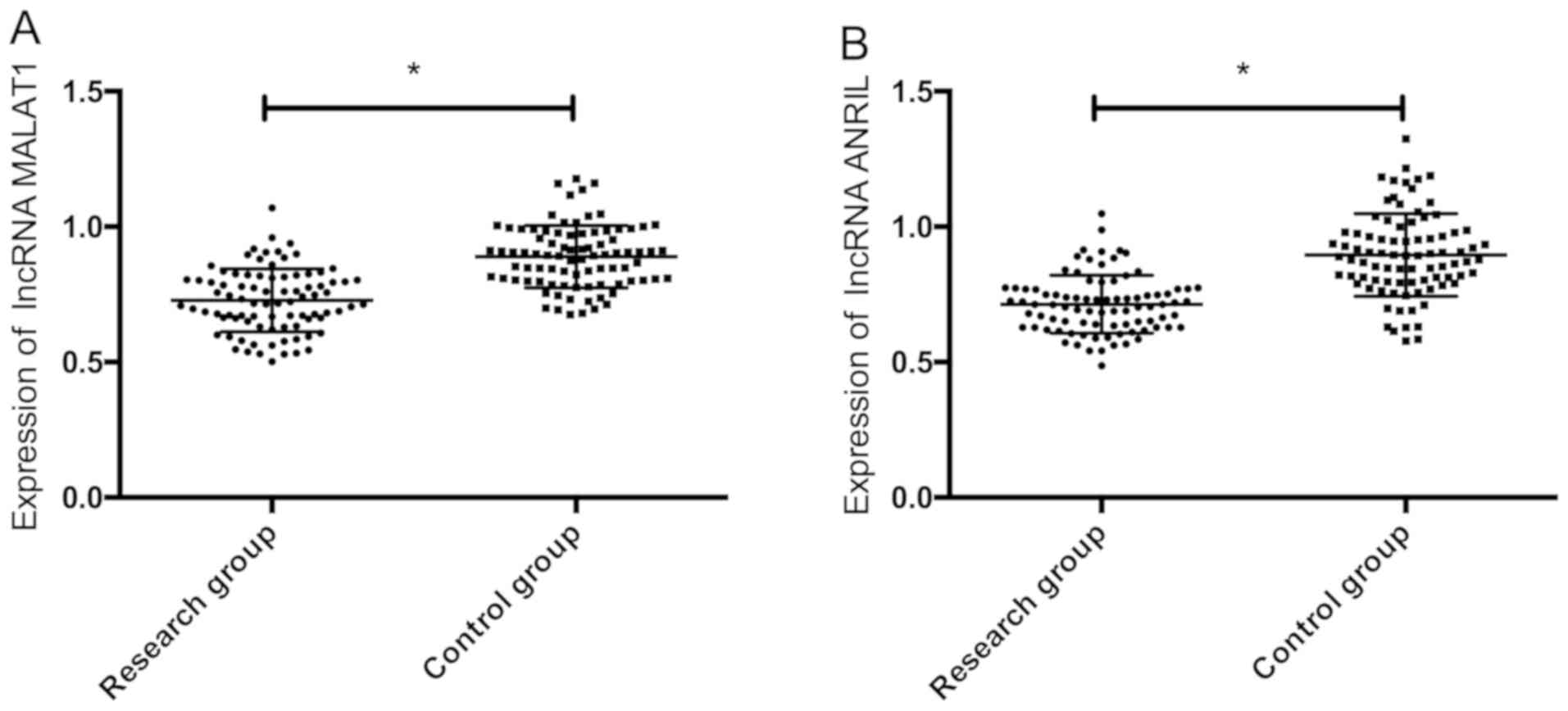
The expression of serum lncRNA MALAT1 and lncRNA
ANRIL in the study group was significantly lower than that in the
control group (P<0.05) (Fig.
1).
Comparison of other related
indices
The expression of serum PEDF and IL-12 in the study
group was significantly lower than that in the control group, while
the expression of serum Hcy and IL-4 was significantly higher than
that in the control group (P<0.05), without significant
difference in the expression of serum IFN-γ between the two groups
(P>0.05) (Table III).
 | Table III.Detection of other related
indices. |
Table III.
Detection of other related
indices.
| Indices | Study group
(n=86) | Control group
(n=86) | t value | P-value |
|---|
| PEDF (pg/ml) | 9.57±1.24 | 16.39±2.41 | 23.34 | <0.001 |
| Hcy (mmol/l) | 18.52±2.96 | 5.79±1.36 | 36.24 | <0.001 |
| IL-12 (pg/ml) | 93.24±12.83 | 138.55±18.28 | 18.81 | <0.001 |
| IL-4 (pg/ml) | 258.41±31.58 | 182.49±24.96 | 17.49 | <0.001 |
| IFN-γ (pg/ml) | 110.75±26.93 | 104.54±35.93 | 1.283 | 0.201 |
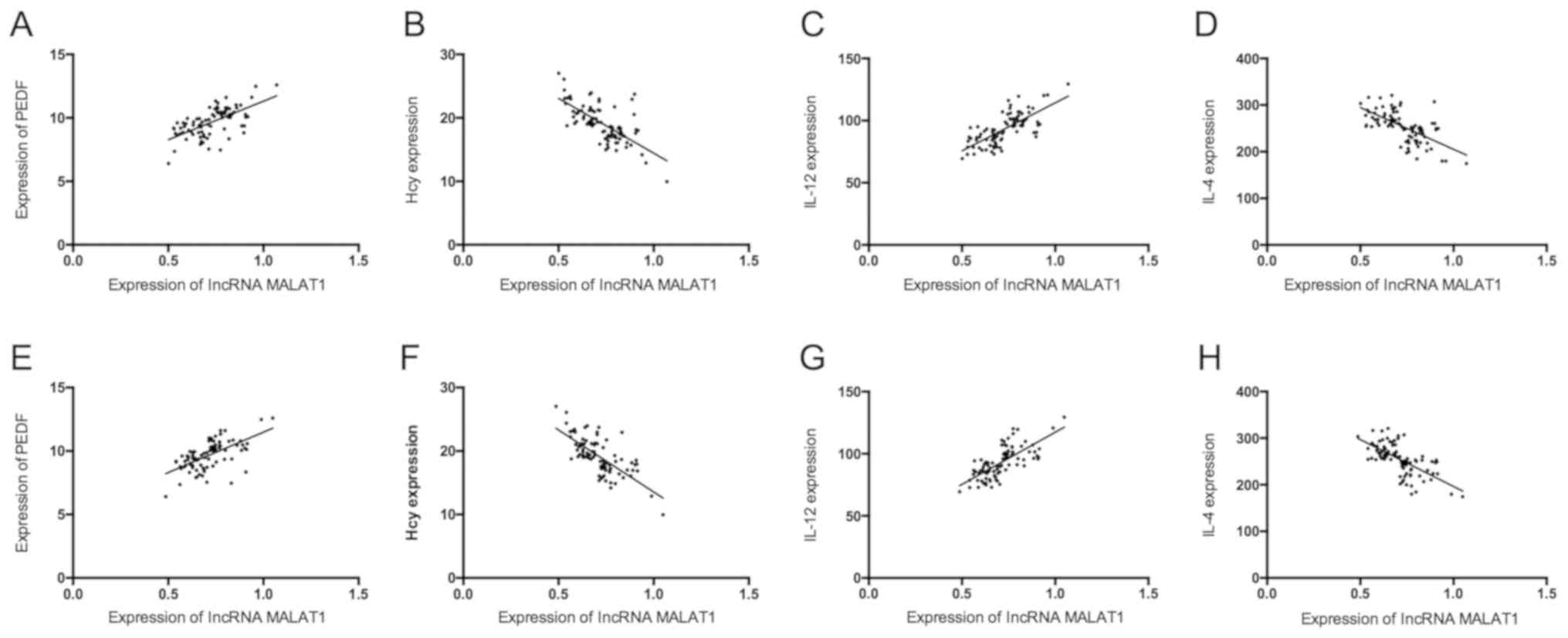
Correlation of serum lncRNA MALAT1 and
lncRNA ANRIL with PEDF, Hcy, IL-12 and IL-4
Serum lncRNA MALAT1 and lncRNA ANRIL were positively
correlated with PEDF and IL-12 (P<0.05), but negatively
correlated with Hcy and IL-4 (P<0.05) (Table IV and Fig. 2).
 | Table IV.Correlation analysis. |
Table IV.
Correlation analysis.
|
| lncRNA MALAT1 | lncRNA ANRIL |
|---|
|
|
|
|
|---|
| Factors | r value | P-value | r value | P-value |
|---|
| PEDF | 0.632 | P<0.001 | 0.606 | P<0.001 |
| Hcy | −0.686 | P<0.001 | −0.911 | P<0.001 |
| IL-12 | 0.720 | P<0.001 | 0.717 | P<0.001 |
| IL-4 | −0.633 | P<0.001 | −0.650 | P<0.001 |
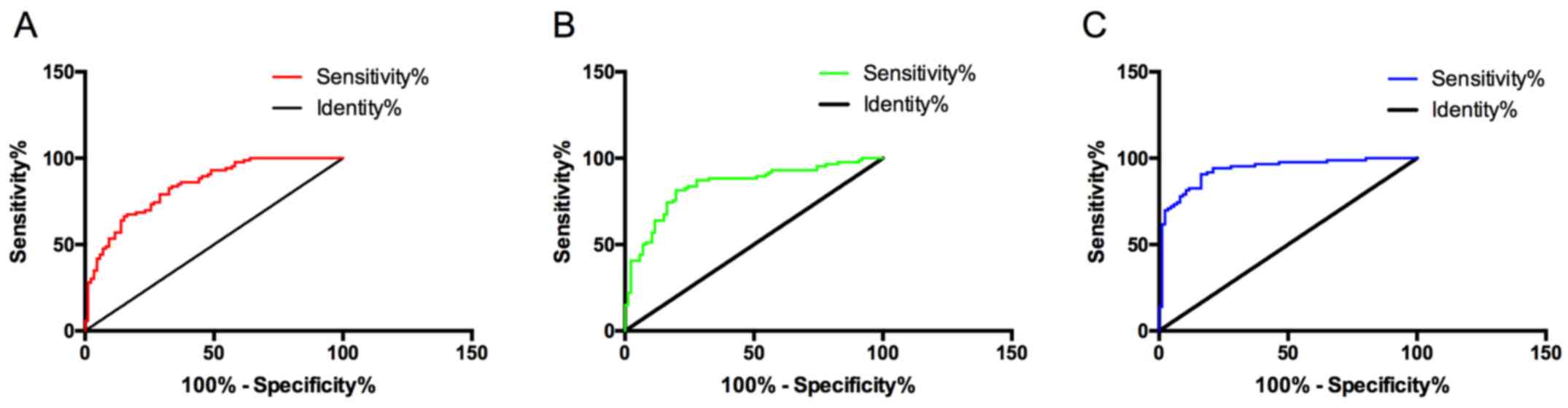
Diagnostic value of lncRNA MALAT1
alone, lncRNA ANRIL alone, and their combined detection
The sensitivity, specificity, and area under the
curve (AUC) of lncRNA MALAT1 for diagnosing glaucoma were 83.72,
66.28 and 0.836%, respectively. Those of lncRNA ANRIL were 81.40,
76.74 and 0.842%, respectively. With lncRNA MALAT1 and lncRNA ANRIL
considered as independent variables, binary Logistic regression
analysis was conducted, and a Logistic regression model: Logit
(P)=0.884+1.673 lncRNA MALAT1+-2.960 lncRNA ANRIL was obtained. The
sensitivity, specificity, and AUC of the model were 93.02, 79.07
and 0.932, respectively. The diagnostic value of the combined
detection was higher than that of lncRNA MALAT1 alone and lncRNA
ANRIL alone (Fig. 3).
Correlation of lncRNA MALAT1 and
lncRNA ANRIL with clinicopathological features
The expression of lncRNA MALAT1 and lncRNA ANRIL was
significantly related to the pathological staging of the patients
(P<0.05), but not to sex, age, BMI, types, and presence or
absence of myopia (P>0.05) (Table
V).
 | Table V.Correlation of lncRNA MALAT1 and
lncRNA ANRIL with clinicopathological features. |
Table V.
Correlation of lncRNA MALAT1 and
lncRNA ANRIL with clinicopathological features.
| Factors | Relative expression
of lncRNA MALAT1 | t value | P-value | Relative expression
of lncRNA ANRIL | t value | P-value |
|---|
| Sex |
|
| 0.674 |
| 0.464 | 0.644 |
| Male
(n=47) | 0.62±0.10 | 0.422 |
| 0.63±0.09 |
|
|
| Female
(n=39) | 0.61±0.12 |
|
| 0.64±0.11 |
|
|
| Age |
| 0.886 |
|
| 0.879 | 0.382 |
| <57
years (n=41) | 0.65±0.09 |
| 0.388 | 0.67±0.12 |
|
|
| ≥57
years (n=45) | 0.63±0.12 |
|
| 0.65±0.09 |
|
|
| BMI |
| 1.316 | 0.192 |
| 0.422 | 0.675 |
| ≤23
(n=40) | 0.67±0.10 |
|
| 0.65±0.12 |
|
|
| >23
(n=46) | 0.64±0.11 |
|
| 0.66±0.10 |
|
|
| Types |
|
| 0.130 |
|
| 0.124 |
|
Angle-closure (n=37) | 0.63±0.09 | 1.530 |
| 0.61±0.10 | 1.555 |
|
|
Open-angle (n=49) | 0.66±0.09 |
|
| 0.65±0.13 |
|
|
| Myopia |
| 1.463 | 0.147 |
| 1.414 | 0.161 |
| Yes
(n=52) | 0.65±0.08 |
|
| 0.64±0.10 |
|
|
| No
(n=34) | 0.62±0.11 |
|
| 0.67±0.09 |
|
|
| Stages |
|
|
|
|
| <0.001 |
| I and
II (n=47) | 0.75±0.07 | 18.28 | <0.001 | 0.74±0.08 | 17.63 |
|
| III and
IV (n=39) | 0.49±0.06 |
|
| 0.48±0.05 |
|
|
Discussion
Glaucoma is an eye disease that can lead to
blindness. Intraocular hypertension causes damage to retinal
ganglion cells, and then to vision and visual field, eventually
resulting in glaucoma (10,11). There are many theories about the
pathogenesis of the disease, such as mechanical theory, vascular
theory, and nervous activity theory. These theories believe that
intraocular hypertension leads to apoptosis of retinal ganglion
cells through different pathways (12,13).
LncRNA is a widely studied biomolecule. Its expression changes are
closely related to the development and progression of many
diseases, so it can be used as a molecular target for diagnosis or
treatment (14).
lncRNA ANRIL regulates adjacent tumor suppressor
gene CDKN2A/CDKN2B through an epigenetic mechanism and then
regulates the proliferation and apoptosis of cells (15). According to a previous study
exploring the mechanism of action of lncRNA ANRIL in glaucoma,
lncRNA ANRIL reduces oxidative stress responses of human trabecular
meshwork cells, thus inhibiting the pathogenesis of the disease
(15). Previous studies have found
that lncRNA MALAT1 plays a regulatory role in angiogenesis of
endothelial cells (16), and
inhibits apoptosis of neuronal cells by upregulating the expression
of Bcl-2 (17). The clinical
significance of lncRNA ANRIL and lncRNA MALAT1 in glaucoma was
evaluated in our study. The two genes were poorly expressed in the
serum of glaucoma patients. A previous study has shown that the
downregulation of lncRNA MALAT1 expression leads to the decline of
visual function and apoptosis of retinal cells, which also confirms
the downregulation of lncRNA MALAT1 expression in patients with eye
diseases (18), consistent with our
findings.
Subsequently, the expression of serum Hcy, PEDF,
IL-12, IL-4 and IFN-γ in the patients was detected to analyze the
correlation of lncRNA ANRIL and lncRNA MALAT1 expression with
glaucoma. The results showed that the expression of serum PEDF and
IL-12 in the study group was significantly lower than that in the
control group, while the expression of serum Hcy and IL-4 was
significantly higher than that in the control group, without
significant difference in the expression of serum IFN-γ between the
two groups. Hcy, a sulfur-containing amino acid that is highly
expressed in glaucoma patients, can damage vascular endothelial
cells of eyes through multiple pathways, and induce optic atrophy
(19). PEDF can be produced in many
parts of the eyes inhibiting angiogenesis, so the reduction in its
expression significantly increases the number of new vessels in the
eyes, thus promoting the progression of glaucoma (20). IL-12 inhibits apoptosis of ganglion
cells and then protects the optic nerve. IL-4 plays an important
role during the process of optic nerve injury, and its high
expression represents serious optic nerve injury (21,22).
These studies are consistent with our conclusions. The correlation
of lncRNA ANRIL and lncRNA MALAT1 with Hcy, PEDF, IL-12 and IL-4
were analyzed. The results showed that serum lncRNA MALAT1 and
lncRNA ANRIL were positively correlated with PEDF and IL-12, but
negatively correlated with Hcy and IL-4, which further indicates
that lncRNA MALAT1 and lncRNA ANRIL may be closely related to the
progression of glaucoma.
The diagnostic value of lncRNA MALAT1 alone, lncRNA
ANRIL alone, and their combined detection for glaucoma, and the
correlation of the two genes with the clinicopathological features
were analyzed. The results showed that the AUCs of lncRNA MALAT1
alone and lncRNA ANRIL alone were >0.8, but the AUC of the
combined detection was 0.93, which suggests that the latter has
higher diagnostic value for glaucoma. According to the analysis of
the clinicopathological features, the expression of lncRNA MALAT1
and lncRNA ANRIL was correlated with the pathological staging of
glaucoma, which indicates that the low expression of the two genes
represents serious glaucoma. This demonstrates that lncRNA MALAT1
and lncRNA ANRIL may be used as important markers for the severity
evaluation of the disease. There are currently only few studies on
this aspect, so more research is needed to confirm our
conclusions.
In conclusion, lncRNA MALAT1 and lncRNA ANRIL are
poorly expressed in the serum of glaucoma patients, significantly
correlated with serum Hcy, PEDF, IL-12 and IL-4, and related to the
pathological staging of the disease. Their combined detection has
high diagnostic value for the disease. Therefore, lncRNA MALAT1 and
lncRNA ANRIL may be used as new molecular targets for the diagnosis
and severity evaluation of glaucoma patients. However, whether the
two genes have a common mechanism of action in glaucoma is still
unclear. Our conclusions should be further verified due to the few
relevant studies and the small sample size in this study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ conceived the study and drafted the manuscript.
YZ, MG and HM detected indices. XZ and YL collected subjects and
compared their information. FW and HH performed qRT-PCR. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
the Hospital of Chengdu University of Traditional Chinese Medicine.
Patients who participated in this research, signed an informed
consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bashir H, Sridhar U, Mazumdar S and
Tripathy K: Panscleritis masquerading as an attack of primary acute
angle closure glaucoma. GMS Ophthalmol Cases.
9:Doc312019.PubMed/NCBI
|
|
2
|
Youngblood H, Hauser MA and Liu Y: Update
on the genetics of primary open-angle glaucoma. Exp Eye Res.
188:1077952019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinreb RN and Khaw PT: Primary open-angle
glaucoma. Lancet. 363:1711–1720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribeiro A, Veiga F, Santos D,
Torres-Labandeira JJ, Concheiro A and Alvarez-Lorenzo C:
Hydrophilic acrylic hydrogels with built-in or pendant
cyclodextrins for delivery of anti-glaucoma drugs. Carbohydr Polym.
88:977–985. 2012. View Article : Google Scholar
|
|
5
|
Garcia-Medina JJ, Garcia-Medina M,
Garrido-Fernandez P, Galvan-Espinosa J, Garcia-Maturana C,
Zanon-Moreno V and Pinazo-Duran MD: A two-year follow-up of oral
antioxidant supplementation in primary open-angle glaucoma: An
open-label, randomized, controlled trial. Acta Ophthalmol.
93:546–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Islam MN, Moriam S, Umer M, Phan HP,
Salomon C, Kline R, Nguyen NT and Shiddiky MJ: Naked-eye and
electrochemical detection of isothermally amplified HOTAIR long
non-coding RNA. Analyst (Lond). 143:3021–3028. 2018. View Article : Google Scholar
|
|
7
|
Ying L, Chen Q, Wang Y, Zhou Z, Huang Y
and Qiu F: Upregulated MALAT-1 contributes to bladder cancer cell
migration by inducing epithelial-to-mesenchymal transition. Mol
Biosyst. 8:2289–2294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li HB, You QS, Xu LX, Sun LX, Abdul Majid
AS, Xia XB and Ji D: Long non-coding RNA-MALAT1 mediates retinal
ganglion cell apoptosis through the PI3K/Akt signaling pathway in
rats with glaucoma. Cell Physiol Biochem. 43:2117–2132. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao J, Sun H, Zhang JM, Wang M, Du XJ and
Zhang JL: Long non-coding RNA ANRIL downregulates microRNA-7 to
protect human trabecular meshwork cells in an experimental model
for glaucoma. Eur Rev Med Pharmacol Sci. 23:3173–3182.
2019.PubMed/NCBI
|
|
10
|
Francis BA, Varma R, Vigen C, Lai MY,
Winarko J, Nguyen B and Azen S; Los Angeles Latino Eye Study Group,
: Population and high-risk group screening for glaucoma: The Los
Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 52:6257–6264.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson CA: The Glenn A. Fry Award
Lecture. Early losses of visual function in glaucoma. Optom Vis
Sci. 72:359–370. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ashok A, Kang MH, Wise AS, Pattabiraman P,
Johnson WM, Lonigro M, Ravikumar R, Rhee DJ and Singh N: Prion
protein modulates endothelial to mesenchyme-like transition in
trabecular meshwork cells: Implications for primary open angle
glaucoma. Sci Rep. 9:130902019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swamy R, Francis BA, Akil H, Yelenskiy A,
Francis BA, Chopra V and Huang A: Clinical results of ab interno
trabeculotomy using the trabectome in patients with uveitic
glaucoma. Clin Exp Ophthalmol. Sep 10–2019.(Epub ahead of print).
doi: 10.1111/ceo.13639. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Zhang F, Pan Z, Luo H, Liu K and
Duan X: LncRNA NR_003923 promotes cell proliferation, migration,
fibrosis, and autophagy via the miR-760/miR-215-3p/IL22RA1 axis in
human Tenon's capsule fibroblasts. Cell Death Dis. 10:5942019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Congrains A, Kamide K, Ohishi M and Rakugi
H: ANRIL: Molecular mechanisms and implications in human health.
Int J Mol Sci. 14:1278–1292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou RM, Wang XQ, Yao J, Shen Y, Chen SN,
Yang H, Jiang Q and Yan B: Identification and characterization of
proliferative retinopathy-related long noncoding RNAs. Biochem
Biophys Res Commun. 465:324–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao J, Wang XQ, Li YJ, Shan K, Yang H,
Wang YN, Yao MD, Liu C, Li XM, Shen Y, et al: Long non-coding RNA
MALAT1 regulates retinal neurodegeneration through CREB signaling.
EMBO Mol Med. 8:346–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakazawa T, Shimura M, Tomita H, Akiyama
H, Yoshioka Y, Kudou H and Tamai M: Intrinsic activation of
PI3K/Akt signaling pathway and its neuroprotective effect against
retinal injury. Curr Eye Res. 26:55–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee YJ, Ke CY, Tien N and Lin PK:
Hyperhomocysteinemia causes chorioretinal angiogenesis with
placental growth factor upregulation. Sci Rep. 8:157552018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Midena E, Bini S, Frizziero L, Pilotto E,
Esposito G and Micera A: Aqueous humour concentrations of PEDF and
Erythropoietin are not influenced by subthreshold micropulse laser
treatment of diabetic macular edema. Biosci Rep. 39:392019.
View Article : Google Scholar
|
|
21
|
Benitez-Del-Castillo J, Cantu-Dibildox J,
Sanz-González SM, Zanón-Moreno V and Pinazo-Duran MD: Cytokine
expression in tears of patients with glaucoma or dry eye disease: A
prospective, observational cohort study. Eur J Ophthalmol.
29:437–443. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aketa N, Yamaguchi T, Suzuki T, Higa K,
Yagi-Yaguchi Y, Satake Y, Tsubota K and Shimazaki J: Iris damage is
associated with elevated cytokine levels in aqueous humor. Invest
Ophthalmol Vis Sci. 58:BIO42–BIO51. 2017. View Article : Google Scholar : PubMed/NCBI
|