Introduction
Lung function is commonly declined in airway
diseases and chronic airway inflammation is a characteristic of
asthma (1). Airway inflammation
leads to shortness of breath, hyper-responsiveness, coughing and
wheezing (2). Most cases of asthma
begin at the infant stage (3).
Evidence suggests that infants who develop asthma have normal lung
function at birth (4). Inhaled
corticosteroids are generally used to control infantile asthma
(5), but the use of corticosteroids
is not completely safe (6). Due to
the various characteristics of infantile asthma, it is difficult to
assess the benefits of inhaled corticosteroids in infantile asthma
(7).
Budesonide depresses the biological activities of
activator protein-1 and NF-κβ (8).
In addition, it is the only inhaled corticosteroid that has been
approved by the United States Food and Drug Administration for
infantile asthma, with approval granted in 2000 (5), but nebulization with budesonide of
infants with asthma is associated with a risk of relapse (9). Furthermore, fluticasone inhibits
histamine release (immunoglobulin E-dependent), was reported to
achieve increased clinical outcomes and pulmonary functions in
infantile asthma (10) and has low
systemic effects (9). The effects
reported for budesonide are similar to those of all inhaled
corticosteroids, while the inhibition of histamine release from
mast cells by fluticasone is not proven. In general, inhaled
corticosteroids are not able to prevent immediate airway responses
to allergens (only after long-term treatment), the major cause of
which is mast cell degranulation (11).
The primary aim of the present non-inferiority study
was to compare the capacity of fluticasone step-down treatment with
that of budesonide step-down treatment to achieve specific airway
resistance reduction at a level of evidence of 3. The secondary
endpoint of the analysis was to test the hypothesis that step-down
treatment with budesonide is associated with a longer
post-treatment symptom-free time compared with that of fluticasone
step-down treatment in Chinese infants with confirmed asthma.
Materials and methods
Drugs
Fluticasone and budesonide inhalation suspension
were purchased from AstraZeneca Pharma. Albuterol (Ventolin) was
purchased from GlaxoSmithKline Pharmaceuticals Ltd. Cosyntropin
(synthetic corticotropin) was purchased from Sandoz. Normal saline
was purchased from Baxter.
Inclusion criteria
Infants who were treatment naive and required
emergency treatment were included in the analysis. Patients aged
from 1 day to 2 years, who presented at the outpatient setting or
were admitted to the Department of Pediatrics of the Shanghai
University of Medicine and Health Science (Shanghai, China) and the
referring hospitals from January 2013 to the end of December 2013
of either sex with at least two episodes of asthma within two days
(the criteria were according to the institutional pediatric and
asthmatic guidelines) confirmed by pediatricians (minimum 3 years
of experience) of the institute(s) were included in the study.
Exclusion criteria
Infants with chronic asthma, chronic lung disease,
had inhaled corticosteroid(s) within 4 months previously or were
unable to be nebulized by the nursing staff (minimum 3 years of
experience) of the institute(s) were excluded from the study.
Infants who had not completed the interventions and/or were not
available for follow-up at the parent and/or the referring
hospitals were excluded from the study.
Cohort
Treatments were administered as part of routine
clinical care. Infants who had been nebulized with 500 µg
budesonide for 6 weeks followed by 250 µg budesonide for 6 weeks
(9) were included in the BS group
(n=389) and infants who had been nebulized with 250 µg fluticasone
propionate for 6 weeks followed by 125 µg fluticasone propionate
for 6 weeks (3) were included in the
FC group (n=389). Nebulization was performed with a Jet nebulizer
(Famidoc Technology Co., Ltd.) for intervention purposes twice
daily in the morning and in the evening. During the follow-up
period, 125 µg albuterol was given to infants with a metered
inhaler as and when required (dose and dosage of budesonide,
fluticasone and albuterol were decided by the institutional review
board itself) (10).
Data collection
Information regarding demographic characteristics,
clinical conditions, physical examinations, airway reactivity,
symptoms, safety study, the treatment-emergent adverse effects
(events were considered as adverse effects as per the criteria set
by the institutional review board) were collected from patients'
Digital Imaging and Communications in Medicine (DICOM) files of the
parent hospital and the referring hospitals by the nursing staff
(minimum 3 years of experience) of the institute(s).
Physical examination
During and after treatment, the patients were
followed up for two years or up to the age of 4±0.5 years using a
questionnaire every 3 months (12)
by the nursing staff (minimum 3 years of experience) of the
institute(s). Physical examinations were defined as per the
clinicians' opinions (as per Chinese guidelines, minimum 3 years of
experience) of the institute(s).
Lung function tests
The specific airway resistance (sRaw) was evaluated
by whole-body plethysmography according to Eq. 1 by pulmonologists
(minimum 3 years of experience) of the institute(s) at the time of
enrollment (BL) and after 3 months of treatment (EL) (13). Forced expiratory volume in 1 sec
(FEV1) (14) and eucapnic voluntary
hyperventilation (15) were also
recorded at BL and EL. The raised-volume rapid thoracoabdominal
compression technique as per the American Thoracic Society/European
Respiratory Society clinical practice guidelines was used to
evaluate the parameters as follows:
sRaw=plethysmograph cabin volume×Change
in air pressure in plethysmography cabinMouth air flow
An inflatable jacket, which extends from the
infant's axillae to the iliac crest, was loosely wrapped around the
infant's torso and the FEV1 was measured from a raised lung volume
(16).
Safety study
A bolus injection of 10 µg cosyntropin was given
intravenously and 1 ml blood was collected by pathologists (minimum
3 years of experience) of the institute(s) at 0, 30 and 60 min and
stored in sodium citrate cuvettes for serum cortisol concentration
measurements. Furthermore, fasting blood samples (1 ml) had been
collected from infants in the early morning and stored in sodium
citrate cuvettes for serum adrenocorticotropic hormone (ACTH) level
measurements. Patients with a plasma cortisol concentration of at
least 100 nM and an ACTH-stimulated plasma cortisol concentration
of at least 500 nM/l at the time of enrollment or plasma cortisol
that increased by at least 200 nM/l above that concentration after
stimulation were considered as having normal adrenal function
(17). The bioassay was performed as
per the chemiluminescent immunometric assay kit (Calbiotech).
Pathology was performed at BL, EL, 6 and 9 months after treatment.
Physical examinations were performed and adverse events were
evaluated during treatment and the follow-up period, while lung
function tests were performed at 3 months after treatment only.
Follow-up evaluations
During the follow-up period, since the day of
completion of treatment, the absence of at least two episodes of
asthma within two days were considered as a post-treatment effect.
Frequencies of 125 µg albuterol from a metered inhaler required
during the follow-up period were recorded. All patients were
observed for worsening of the airway condition, changes in voice,
sneezing, runny nose, stuffy nose and watering of eyes during the
follow-up period. No specific guidelines, scales or tests were
followed for these evaluations and evaluation was performed on the
basis of the opinions of the clinicians (minimum 3 years of
experience) of the institute(s) and criteria set by the
institutional review board.
Statistical analysis
InStat (for Windows 3.0; GraphPad Software, Inc.)
was used for statistical analysis. For continuous parameters, the
Wilcoxon rank-sum test was applied for comparisons between two
groups. For lung function tests, continuous parameters were
analyzed by one-way analysis of variance for multiple comparisons.
For constant parameters, the Chi-square independence test was used
for comparisons between groups (18). The Tukey-Kramer multiple-comparisons
test [considering critical value (q)>3.314] was used for
post-hoc analysis. The results were considered significant at a 95%
confidence level and P<0.05.
Results
Participants
Referring to the records of the institutes, a total
of 1,000 infants were admitted with complaints of asthma. Among
them, 28 infants had not completed the interventions and were
shifted to the other hospitals with critical facilities available
for emergency purposes, and were then excluded from the study. A
total of 18 infants had critical illness [pediatricians' opinion;
minimum 3 years of experience of the institute(s)], 13 infants had
chronic lung disease from birth, 12 infants had previous exposure
to inhaled corticosteroid(s), 8 infants were not able to be
nebulized by the nursing staff and 143 infants had missing data
from the follow-up evaluation tests from their DICOM files.
Therefore, the data of those infants were excluded from the study.
Finally, a total of 778 infants were included in the present
retrospective cohort study. The flow diagram of the study is
provided in Fig. 1.
Characteristics of infant
subjects
All patients enrolled were of <2 years of age at
the time of admission and directly residing in Shanghai city or in
the countryside. The proportion of male infants (64%) was higher
than that of female infants, 26% of infants had a history of
maternal asthma and the majority of infants (60%) had presented
with nighttime asthma. The other demographic data, characteristics
and clinical conditions of the infants enrolled are presented in
Table I.
 | Table I.Demographics, characteristics and
clinical conditions of the infants included. |
Table I.
Demographics, characteristics and
clinical conditions of the infants included.
|
| Group |
|
|---|
|
|
|
|
|---|
| Parameters | BS (n=389) | FC (n=389) | P-value |
|---|
| Age |
|
| 0.227 |
| Minimum
(days) | 1 | 1 |
|
| Maximum
(months) | 24 | 24 |
|
| Mean ± SD
(months) | 12.15±2.45 | 12.26±2.47 |
|
| Sex |
|
| 0.709 |
| Male | 245 (63) | 251 (65) |
|
|
Female | 144 (37) | 138 (35) |
|
| Body weight (kg) | 7.51±2.51 | 7.36±2.48 | 0.627 |
| Body height (cm) | 60.12±5.56 | 59.45±4.89 | 0.131 |
| History of maternal
asthma (first-level) |
|
| 0.935 |
|
Yes | 101 (26) | 99 (25) |
|
| No | 288 (74) | 290 (75) |
|
| No. of episodes of
asthma within two daysa |
|
| 0.477 |
|
2–5 | 281 (72) | 271 (70) |
|
|
>5 | 108 (28) | 118 (30) |
|
| Allergic
rhinitisa | 45 (12) | 51 (13) | 0.586 |
| Allergic
conjunctivitisb | 31 (8) | 35 (9) | 0.700 |
| Treatment
history |
|
| 0.895 |
|
Bronchodilators | 45 (12) | 41 (11) |
|
|
Antibiotics | 12 (3) | 15 (4) |
|
|
Antihistamine | 54 (14) | 57 (15) |
|
|
None | 278 (71) | 276 (70) |
|
| Time of episodes of
asthma |
|
| 0.770 |
|
Day | 156 (40) | 161 (41) |
|
|
Night | 233 (60) | 228 (59) |
|
| Total
Immunoglobulin E (IU/ml) | 19.18±3.18 | 19.76±5.01 | 0.056 |
| HbA1C (%) | 5.99±0.71 | 6.01±0.75 | 0.906 |
| Ethnicity |
|
| 0.856 |
| Han
Chinese | 349 (90) | 355 (91) |
|
|
Mongolian | 28 (7) | 22 (6) |
|
|
Tibetan | 8 (2) | 8 (2) |
|
|
Hui | 4 (1) | 4 (1) |
|
| sRaw (kPa/sec) | 1.28±0.11 | 1.27±0.1 | 0.321 |
| FEV1 (l/sec) | 0.977±0.085 | 0.971±0.069 | 0.289 |
| Serum cortisol
level (nM) | 257.51±85.51 | 261.62±87.61 | 0.699 |
| Serum ACTH level
(nM) | 20.11±8.41 | 19.89±7.89 | 0.912 |
Lung function tests
At EL, the budesonide treatment group had a reduced
sRaw as compared with that at BL (1.28±0.11 vs. 1.21±0.1 kPa/sec;
P<0.0001, q=13.45). The fluticasone treatment group also had a
reduced sRaw as compared with that at BL (1.27±0.1 vs. 1.23±0.11
kPa/sec; P<0.0001, q=7.39). Of note, budesonide treatment had a
greater effect to reduce sRaw than fluticasone treatment at EL
(P=0.008, q=3.69; Fig. 2).
At EL, the budesonide treatment group had an
improved FEV1 as compared with that at BL (0.977±0.068 vs.
0.997±0.085 l/sec; P<0.0001, q=5.54). Fluticasone treatment also
improved FEV1 as compared with that at BL (0.971±0.069 vs.
0.992±0.085 l/sec; P=0.0003, q=5.46). Of note, budesonide treatment
had a greater capacity to improve FEV1 as compared to fluticasone
treatment at EL (P<0.0001, q=6.93; Fig. 3).
Safety study
The infants enrolled had not been prescribed any
type of antibiotic by the pediatrician(s) and physician(s) at the
time of enrollment and during the study. Therefore, it was assumed
that none of the infants had any respiratory infections during the
study period. Budesonide and fluticasone nebulization had no
adverse effects on adrenal functions of the treated infants at EL
(Table II). In addition, at 6 and 9
months after treatment, adrenal functions were normal (data not
presented).
 | Table II.Adrenal function evaluation at 3
months after treatment. |
Table II.
Adrenal function evaluation at 3
months after treatment.
|
| Group |
|
|---|
|
|
|
|
|---|
| Hormone | BS (n=389) | FC (n=389) | P-value |
|---|
| Serum cortisol
(nM/l) |
|
|
|
| 0
min | 335.85±95.98 | 349.18±101.21 | 0.090 |
| 1/2
h | 751.52±112.12 | 743.49±102.21 | 0.494 |
| 1
h | 801.12±135.42 | 799.21±129.51 | 0.974 |
| Serum ACTH
(nM/l) | 26.12±9.13 | 27.15±9.88 | 0.210 |
Follow-up evaluations
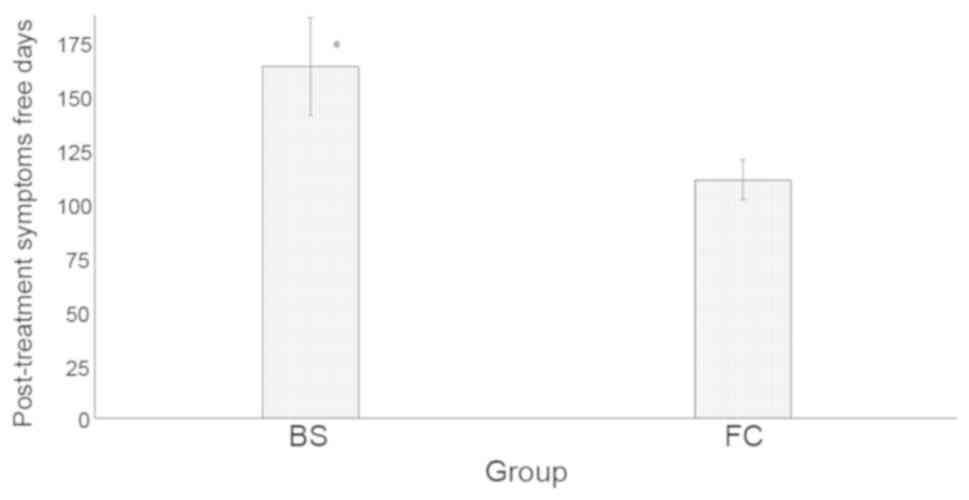
Budesonide treatment had a larger number of
post-treatment symptom-free days than fluticasone treatment
(165.56±23.15 vs. 112.21±9.45 days; P<0.0001; Fig. 4).
During the follow-up period, albuterol had been
given fewer times in the BS group than in the FC group (14.15±3.12
vs. 21.16±5.45 times; P<0.0001; Fig.
5).
In the fluticasone nebulization group, the major
adverse effects were hoarseness (135 vs. 3 cases in the budesonide
group; P<0.0001), exacerbations (105 vs. 35 cases; P<0.0001)
and oral candidiasis (11 vs. 1 case; P=0.009), while in the
budesonide group, sneezing (33 vs. 12 cases in the fluticasone
group; P=0.002), runny nose (45 vs. 13 cases; P<0.0001) and
watering of eyes (11 vs. 1 case; P=0.009) were the most frequent
adverse effects in infants during the follow-up period (Table III).
 | Table III.Treatment-emergent adverse effects
reported during follow-up. |
Table III.
Treatment-emergent adverse effects
reported during follow-up.
|
| Group |
|
|---|
|
|
|
|
|---|
| Adverse effect | BS (n=389) | FC (n=389) | P-value |
|---|
| Exacerbations | 35 (9) | 105
(27)a | <0.0001 |
| Hoarseness | 3 (1) | 135 (35) | <0.0001 |
| Oral
candidiasis | 1 (1) | 11 (3)a | 0.009 |
| Sneezing | 33 (8)b | 12 (3) | 0.002 |
| Runny or stuffy
nose | 45
(12)b | 13 (3) | <0.0001 |
| Watering of
eyesc | 11 (3)b | 1 (1) | 0.009 |
| Vomiting | 9 (2) | 2 (1) | 0.069 |
| Total | 137 (35) | 279 (72) | <0.0001 |
Discussion
After 3 months of treatment, budesonide and
fluticasone nebulization were proven effective in improvement of
sRaw and FEV1 values and no adverse effects on adrenal function
were observed. The study results regarding budesonide were similar
to those of previous studies (5,10,11,17)
but results regarding fluticasone were not in accordance with a
previous study (3). Budesonide
(19) and fluticasone (10) have potent airway anti-inflammatory
action. Unlike oral therapies, nebulized therapies have a rapid
onset of action (5), are successful
in the stabilization of clinical symptoms (19), have the least adverse effects
(5) and do not require active
inspiration (20). With respect to
the benefits offered by budesonide and fluticasone, the present
study supported the suitability of nebulized budesonide or
fluticasone in infantile asthma.
In the present study, a longer post-treatment effect
was reported under budesonide intervention as compared to
fluticasone treatment (P<0.0001). This result was not in line
with that of a previous study (11).
A possible explanation for this discrepancy is that in the present
study, a step-down approach was adopted in the intervention, which
improved the effectiveness of budesonide (21). The study recommended a step-down
budesonide approach in infantile asthma for long-term
post-treatment benefits (21).
In the BS group, fewer instances of exacerbation of
asthma and hoarseness were observed compared with the FC group and
previous studies on FC (3,10). Budesonide reduces the risk of
exacerbations of asthma and hoarseness (22,23), as
the half-life of budesonide in infants is lower than that of
fluticasone (24) and the serum
elimination rate of budesonide is higher than that of fluticasone
(25). Asthma exacerbations lead to
morbidity, increase the cost of treatment and decrease lung
function (26). With regard to the
adverse events encountered during the follow-up period
(requirements of emergency bronchodilators), BS is more potent and
suitable than FC in the infantile asthma conditions.
Several limitations of the present study should be
pointed out. For instance, the study provided a retrospective
analysis of observational cohorts only and lacked a control group.
History of maternal asthma (18),
sex, age, infections (26), and
other demographic characteristics have effects on the adverse
events or relapse occurring in the follow-up period, but no
multivariate analysis of such parameters was performed in the
present study (no adjustment for confounding factors). In a future
study, a control cohort of infants (e.g. healthy and/or not treated
with BS or FC) should be used to compare the frequencies of adverse
effects. The addition of long-acting β-agonist with nebulized
corticosteroids provides better control of asthma (27), but the present study was performed
using a step-down approach with nebulized corticosteroid alone.
High-dose budesonide (1,000 µg) twice daily may overcome recurrence
of infantile asthma (19), but
interventions were performed with 500 µg budesonide twice daily
followed by 250 µg budesonide twice daily. There was a potential
high inter-subject variability, leading to difficulty in the
interpretation of significant data. However, most of the infants
were only several days and months old and only a small number of
patients had an age of nearly 2 years.
In conclusion, the present retrospective
observational cohort study indicated that a step-down approach of
budesonide and fluticasone nebulization is effective in infantile
asthma. Nebulization of infants with asthma under budesonide
provided a longer post-treatment symptom-free duration and a lower
risk of exacerbations than fluticasone. The study recommended that
if the step-down approach for nebulization with 500 µg budesonide
for 4 weeks followed by 300 µg for 4 weeks followed by 100 µg for 4
weeks with administration twice a day is successful, it should be
pursued in clinical practice.
Acknowledgements
Not appicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors have reviewed and approved the
manuscript submitted for publication. ZW was the project
administrator and contributed to the design, data curation and
literature review of the study. XB contributed to the
conceptualization, literature review and data curation of the
study. LH contributed to the conceptualization, software
management/processing and literature review for the study. JZ
contributed to the data curation, formal analysis and literature
review for the study, and drafted, reviewed and edited the
manuscript for intellectual content. The author agrees to be
accountable for all aspects of work ensuring integrity and
accuracy.
Ethics approval and consent to
participate
The original study protocol (SSP/CL/15/13 dated 1
January 2013) was approved by the Shanghai University of Medicine
and Health Science review board (Shanghai, China). The study
reporting adhered to the law of China, the 2008 Helsinki
Declaration and the Strengthening. The Reporting of Observational
studies in Epidemiology statement. Parents/legal guardians provided
informed consent for the participation of the subjects in the study
at the time of hospitalization.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DICOM
|
Digital Imaging and Communications in
Medicine
|
|
sRaw
|
specific airway resistance
|
|
BL
|
at the time of enrollment
|
|
EL
|
at 3 months after treatment
|
|
FEV1
|
forced expiratory volume in 1 sec
|
|
ACTH
|
adrenocorticotropic hormone
|
References
|
1
|
Sears MR: Lung function decline in asthma.
Eur Respir J. 30:411–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshihara S: Early intervention for
infantile and childhood asthma. Expert Rev Clin Immunol. 6:247–255.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murray CS, Woodcock A, Langley SJ, Morris
J and Custovic A; IFWIN study team, : Secondary prevention of
asthma by the use of Inhaled Fluticasone propionate in Wheezy
INfants (IFWIN): Double-blind, randomised, controlled study.
Lancet. 368:754–762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinez FD: Inhaled corticosteroids and
asthma prevention. Lancet. 368:708–710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou X, Hong J, Cheng H, Xie J, Yang J,
Chen Q, He S, Li Y, Zhou X and Li C: Budesonide suspension
nebulization treatment in Chinese pediatric patients with cough
variant asthma: A multi-center observational study. J Asthma.
53:532–537. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boulet LP, FitzGerald JM and Reddel HK:
The revised 2014 GINA strategy report: Opportunities for change.
Curr Opin Pulm Med. 21:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Merkus PJ and de Jongste JC: Inhaled
corticosteroids in wheezy infants. Am J Respir Crit Care Med.
172:1058–1059. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pelaia G, Vatrella A, Busceti MT, Fabiano
F, Terracciano R, Matera MG and Maselli R: Molecular and cellular
mechanisms underlying the therapeutic effects of budesonide in
asthma. Pulm Pharmacol Ther. 40:15–21. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Melani AS: Nebulized corticosteroids in
asthma and COPD. An Italian appraisal. Respir Care. 57:1161–1174.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Teper AM, Kofman CD, Szulman GA,
Vidaurreta SM and Maffey AF: Fluticasone improves pulmonary
function in children under 2 years old with risk factors for
asthma. Am J Respir Crit Care Med. 171:587–590. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Benedictis FM, Del Giudice MM, Vetrella
M, Tressanti F, Tronci A, Testi R and Dasic G; Flic12 Study Group,
: Nebulized fluticasone propionate vs. budesonide as adjunctive
treatment in children with asthma exacerbation. J Asthma.
42:331–336. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saddi V, Beggs S, Bennetts B, Harrison J,
Hime N, Kapur N, Lipsett J, Nogee LM, Phu A, Suresh S, et al:
Childhood interstitial lung diseases in immunocompetent children in
Australia and New Zealand: A decade's experience. Orphanet J Rare
Dis. 12:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Urbankowski T and Przybyłowski T: Methods
of airway resistance assessment. Pneumonol Alergol Pol. 84:134–141.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai SH, Liao SL, Yao TC, Tsai MH, Hua MC,
Chiu CY, Yeh KW and Huang JL: Raised-volume forced expiratory
flow-volume curve in healthy Taiwanese infants. Sci Rep.
7:63142017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burman J, Lukkarinen H, Elenius V, Remes
S, Kuusela T and Jartti T: Eucapnic voluntary hyperventilation test
in children. Clin Physiol Funct Imaging. 38:718–720. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
American Thoracic Society; European
Respiratory Society, . ATS/ERS statement: Raised volume forced
expirations in infants: Guidelines for current practice. Am J
Respir Crit Care Med. 172:1463–1471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cetinkaya F, Kayiran P, Memioglu N, Tarim
OF, Eren N and Erdem E: Effects of nebulized corticosteroids
therapy on hypothalamic-pituitary-adrenal axis in young children
with recurrent or persistent wheeze. Pediatr Allergy Immunol.
19:773–776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou YX, Zhang J, Ma C, Li J, Zai J and Guo
YS: Clinical efficacy of montelukast sodium in treating infantile
wheezing. Eur Rev Med Pharmacol Sci. 18:775–780. 2014.PubMed/NCBI
|
|
19
|
Saito M, Kikuchi Y, Kawarai Lefor A and
Hoshina M: High-dose nebulized budesonide is effective for mild
asthma exacerbations in children under 3 years of age. Eur Ann
Allergy Clin Immunol. 49:22–27. 2017.PubMed/NCBI
|
|
20
|
Rachelefsky G: Inhaled corticosteroids and
asthma control in children: Assessing impairment and risk.
Pediatrics. 123:353–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rank MA, Johnson R, Branda M, Herrin J,
van Houten H, Gionfriddo MR and Shah ND: Long-term outcomes after
stepping down asthma controller medications: A claims-based,
time-to-event analysis. Chest. 148:630–639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bafadhel M, Peterson S, De Blas MA,
Calverley PM, Rennard SI, Richter K and Fagerås M: Predictors of
exacerbation risk and response to budesonide in patients with
chronic obstructive pulmonary disease: A post-hoc analysis of three
randomised trials. Lancet Respir Med. 6:117–126. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Castillo JR, Peters SP and Busse WW:
Asthma exacerbations: Pathogenesis, prevention, and treatment. J
Allergy Clin Immunol Pract. 5:918–927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeo SH, Aggarwal B, Shantakumar S,
Mulgirigama A and Daley-Yates P: Efficacy and safety of inhaled
corticosteroids relative to fluticasone propionate: A systematic
review of randomized controlled trials in asthma. Expert Rev Respir
Med. 11:763–778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Daley-Yates PT: Inhaled corticosteroids:
Potency, dose equivalence and therapeutic index. Br J Clin
Pharmacol. 80:372–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai TR, Vonk JM, Postma DS and Boezen HM:
Severe exacerbations predict excess lung function decline in
asthma. Eur Respir J. 30:452–456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cortese S, Gatta A, Della Valle L,
Mangifesta R, Di Giampaolo L, Cavallucci E, Petrarca C, Paganelli R
and Di Gioacchino M: Fluticasone/formoterol association favors
long-lasting decrease in bronchial reactivity to methacholine and
weekly PEF variability. Int J Immunopathol Pharmacol. 29:769–774.
2016. View Article : Google Scholar : PubMed/NCBI
|