Introduction
During normal pregnancy (NP), the circulation of
placenta is high-flow and low-resistance to meet the growth needs
of the fetus. Inadequate or excessive cardiovascular system
adaptation in pregnant women before 20 weeks of gestation is
associated with pregnancy complications such as pregnancy-induced
hypertension (PIH) and pre-eclampsia (PE) (1–3).
The main cause of death in pregnant women and
fetuses is hypertensive syndrome in pregnancy, a common disease in
obstetrics with an incidence of 3–10% (4). The disease is divided into gestational
hypertension, mild pre-eclampsia, severe pre-eclampsia and
eclampsia, with gestational hypertension being the least severe
form and eclampsia being the most severe form. There have been a
number of studies on hypertensive syndromes in pregnancy, but its
etiology is not fully understood. So far, the potential
pathological mechanisms of recognized PIH include: i) Abnormal
invasion of maternal uterine blood vessels by the placental
trophoblast; ii) intolerant maternal and fetal tissue immune
molecules; and iii) genetic factors (5,6). A
combination of these factors results in decreased placental blood
flow and oxygen supply and represses infiltrating cells after
trophoblastic involvement (7,8).
Furthermore, these pathological mechanisms induce inflammatory
reactions and local oxidative stress, leading to the release of
inflammatory mediators and free radicals (9–11). These
factors can activate a large number of neutrophils, directly or
indirectly causing vascular endothelial damage, which eventually
leads to the development of PIH. Thus, determining the major
contributing factors of PIH, particularly placentation and systemic
inflammatory states, would benefit in early screenings of PIH and
PE.
In the current study, Doppler ultrasound was used to
detect parameters of blood flow in the uterine arteries of pregnant
women. The effects of cardiovascular function and structural
adaptation on placentation were investigated. In addition,
non-invasive photoplethysmography (PPG) was used to measure the
effects of systemic inflammation (12–15). The
results of Doppler ultrasonography and PPG measurements can be used
for assessment of maternal factors that contribute to high-risk
pregnancy.
Subjects and methods
Study subjects
The current study was performed at the Obstetrics
Department of Shenzhen People's Hospital. Subjects were recruited
from nulliparous women (n=228) who attended their routine first and
second trimester screening in the Ultrasound Laboratory. The
inclusion criteria were: i) A singleton pregnancy; and ii) a
gestational age of 22 to 23 weeks. All pregnancies were dated by
crown-rump length measurement and last menstrual period. All
subjects refrained from caffeine and drugs that could alter the
cardiovascular system function on the day before the tests. The
exclusion criteria were: i) Current or prior history of
hypertension; ii) the use of regular medication; and iii) the
development of complications during pregnancy. The diagnostic
criteria of PIH and PE were in accordance with the Obstetrics and
Gynecology of the People's Medical Publishing House (16). The study was approved by the
Institutional Review Board of Shenzhen People's Hospital and all
subjects had signed their written informed consent.
Measurements of the PPG reflection
index (PPG RI)
In the current study, PPG signals were measured
using a HC2180-D research platform, an enhanced analytical system
for data processing of physiological waveforms (Comperson
Biotechnology Co., Ltd.), at a sampling rate of 500 Hz (8). PPG RI is derived from PPG amplitude
changes of systolic and diastolic peak/inflection points in the PPG
waveforms. PPG RI is defined as the ratio of the reflection peak
amplitude to the pulse maximum amplitude (12). The equation for PPG RI was expressed
as PPG RI=systolic peak amplitude/diastolic peak amplitude ×100
(Fig. 1A), or in the absence of a
diastolic peak, PPG RI=systolic peak amplitude/inflection point
amplitude ×100 (18–20) (Fig.
1B).
PPG signals were recorded from the right index
finger of all subjects during resting state at a sitting position
with the right hand being held at heart level. After the subjects
rested for 5 min to ensure cardiovascular stability, PPG recordings
were performed at a duration of 90 sec, with an averaging period
covering at least ≥60 pulse intervals. After a 5-min rest, PPG
signals were recorded again in a relaxed state, during which all
the subjects were asked to calm down and breathe normally.
Measurements of uterine artery (UtA)
pulsatile index (PI) and reflection index (RI)
Blood flow velocity waveforms from both sides of the
UtA in all subjects were obtained using a Philips iU22 Ultrasound
system (Philips Medical Systems B.V.) with a 3.5- or 5-MHz probe.
After PPG detection, Doppler ultrasound examinations of the
transabdominal UtA were performed. UtA PI and RI were obtained
immediately after nuchal translucency or anomaly fetal scan.
Upon the ultrasound examination, the UtA were
identified in the oblique plane of pelvis, at the apparent
crossover with the external iliac artery of its respective side. A
previous study reported that examination of the UtA close to
placental insertion revealed more diastolic flow and lower vascular
resistance even under pathological conditions compared with healthy
individuals (12). Hence, to
standardize the sample site in the current study, the proximal part
of the UtA was examined for UtA PI and UtA RI measurements.
Pulsed-wave Doppler was applied to capture UtA Doppler flow
velocity waveforms from which UtA PI and UtA RI were calculated
automatically by the system. The following equations were used: UtA
PI=(peak systolic velocity-end diastolic velocity)/mean velocity
and UtA RI=(systolic maximal velocity-diastolic maximal
velocity)/systolic maximal velocity (12).
Measurement of angiogenic proteins,
including placental growth factor (PlGF) and soluble endoglin
(sEng)
The serum concentrations of PlGF and sEng of all
participants were obtained from their medical records. PlGF (1:2;
cat. no. DPG00) and sEng (1:5; cat. no. DNDG00) data recorded at
the gestation week 22 (measured using R&D Systems Inc. kits)
were used in the analysis.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (IBM Corp.). Results are presented as the mean ± SD.
Comparisons between groups were performed using a one-way ANOVA
followed by Student-Newman-Keuls test. In addition, the effects of
the diastolic and systolic state on the three subject groups were
evaluated using the Pearson's product moment correlation
coefficient for the vascular tone measured using PPG RI and the
serum concentrations of PIGF and sEng. Strong correlation was
defined as r>|0.8|, moderate correlation as |0.8|>r>|0.3|,
and weak correlation as r<|0.3|.
The effect of cardiovascular adaptation on
placentation was evaluated through the correlation between the
Doppler ultrasound results of UtA PI and UtA RI, and the serum
concentrations of PIGF and sEng.
Results
Clinicopathological
characteristics
Among the participants studied, 14 developed PIH and
16 developed PE in the third trimester. These 30 patients were
divided into two groups 6 weeks after delivery as follows: PIH
participants (n=14) and PE participants (n=16). A total of 24
normotensive pregnant women were selected as a control group. The
general characteristics of participants, including age, smoking
status, ethnicity, height, body weight, pre-pregnancy body mass
index (BMI), are shown in Table
I.
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Characteristic | NP (n=24) | PIH (n=14) | PE (n=16) |
|---|
| Ethnicity | Chinese | Chinese | Chinese |
| Smoking status | no | no | no |
| Maternal age
(years) | 26.46±4.29 | 25.14±2.74 | 27.06±3.64 |
| Height (cm) | 160.08±4.92 | 160.07±3.25 | 157.50±3.98 |
| Weight (kg) | 53.13±6.82 | 51.71±7.55 | 51.69±10.22 |
| BMI
(kg/m2) | 20.69±2.03 | 20.15±2.66 | 20.79±3.73 |
PIH and PE patients exhibits higher
PPG RI values
Participants in PIH and PE groups exhibited
significantly higher UtA RI, UtA PI and PPG RI values compared with
the NP group (all P<0.05; Table
II). Analysis between PIH and PE groups revealed a
statistically significant difference in the UtA PI and RI values
(P<0.05), however, PPG RI values between the two groups did show
any significant changes (Table II).
The results indicated that UtA PI and RI and PPG data were distinct
among NP, PIH and PE groups.
 | Table II.PPG, Doppler ultrasonography, PlGF and
sENG data obtained at week 22 of gestation. |
Table II.
PPG, Doppler ultrasonography, PlGF and
sENG data obtained at week 22 of gestation.
| Index | NP (n=24) | PIH (n=14) | PE (n=16) |
|---|
| UtA RI |
0.49±0.07a,b |
0.62±0.06b,c |
0.69±0.09a'c |
| UtA PI |
0.78±0.19a,b |
1.19±0.26b,c |
1.50±0.44a,c |
| PPG RI |
0.44±0.09a,b |
0.53±0.01c |
0.58±0.08c |
| PlGF |
320.81±68.38b |
312.21±90.01b |
254.25±53.32a |
| sEng |
5.78±1.16a,b |
6.63±1.00b,c |
7.48±1.05a,c |
PIH and PE patients exhibits different
PlGF and sEng levels
Additionally, the serum levels of PlGF and sEng
between PIH and PE patients at week 22 showed a significant
difference (P<0.05; Table II).
This result indicated that these two components might be involved
in vascular regulation of blood circulation.
Correlation between PlGF, sEng, UtA
PI, UtA RI, PPG RI values
An significant inverse across-subject correlation
was found between PlGF and sEng serum levels (r=−0.702, P<0.001;
Fig. 2). An inverse correlation was
found between both PlGF and UtA PI values and PlGF and UtA RI
values, while an inverse correlation was found between PlGF and PPG
RI values (r=−0.396, P=0.003; r=−0.378, P=0.005; and r=−0.606,
P<0.001; respectively; Fig.
3A-C). A positive correlation was found between sEng and UtA RI
values and sEng and UtA PI values (r=0.606, P<0.001; and
r=0.571, P<0.001; Fig. 4A and B).
Additionally, a positive correlation was found between sEng and PPG
RI values (r=0.961, P<0.001; Fig.
4C).
 | Figure 3.Correlation of PlGF and index values
in all subjects using Pearson's product moment correlation
coefficient. (A) Correlation of PlGF and UtA RI, r=−0.378, P=0.005.
(B) Correlation of PlGF and UtA PI, r=−0.396, P=0.003. (C)
Correlation of PlGF and PPG RI, r=0.606, P<0.001. RI, reflection
index; PI, pulsatile index; PPG, photoplethysmography; PlGF,
placental growth factor; sENG, soluble endoglin; UtA, uterine
artery. |
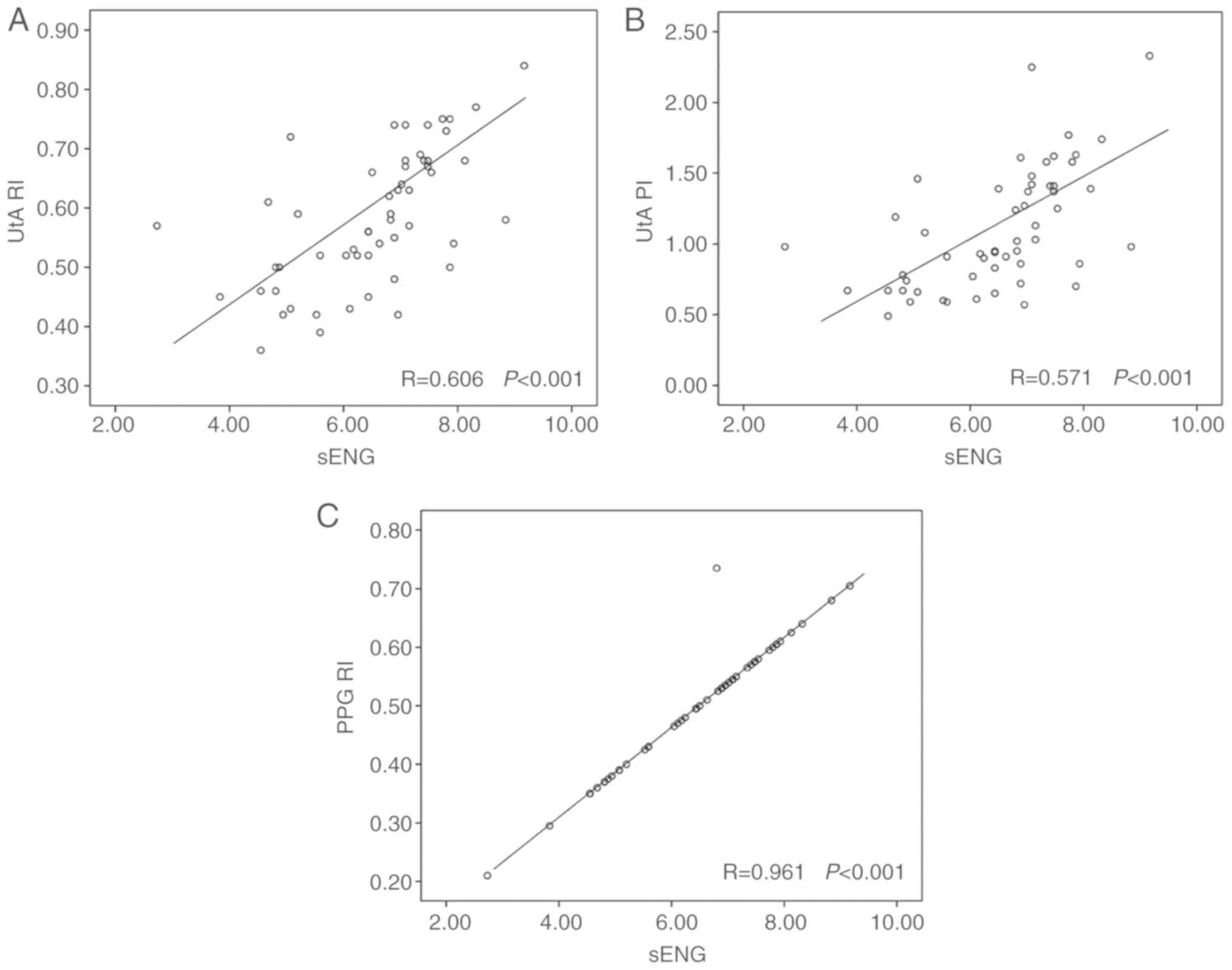 | Figure 4.Correlation of sEng and index values
in all subjects. (A) Correlation of sEng and UtA RI, r=0.606,
P<0.001. (B) Correlation of sEng and UtA PI, r=0.571,
P<0.001. (C) Correlation of sEng and PPG RI, r=0.961,
P<0.001. RI, reflection index; PI, pulsatile index; PPG,
photoplethysmography; PlGF, placental growth factor; sENG, soluble
endoglin; UtA, uterine artery. |
Discussion
The occurrence of PE is commonly associated with
large placentae or oxidatively-stressed placentae with multiple
contributing factors (17). While
poor placentation is considered a major predisposing factor for the
occurrence of PE, systemic inflammatory response is also considered
to be a contributing factor of PE development, and may be caused by
physiological shedding of apoptotic debris into the maternal
circulation as part of normal renewal of the syncytiotrophoblast
(18). In fact, PE might be the
extreme end of a universal maternal response to pregnancy (9). If PE occurs, indicative measures to
reflect the severity and the effects of systemic inflammation are
needed throughout pregnancy to improve the assessment of the
physiological condition of pregnancy, which is currently a
limitation at prenatal clinics.
In the current study, UtA PI, UtA RI and PPG RI
values exhibited inverse correlation with PlGF serum levels and a
positive correlation with sEng serum levels. Both Doppler
assessment of UtA PI and UtA RI, and PPG RI assessment were
correlated with the maternal serum concentrations of PIGF and sEng.
The correlation between PPG RI and PIGF and sEng serum
concentrations suggested that the effect of vascular regulation may
be used to estimate the circulatory state when the inflammatory
response is activated. The relatively weaker correlation between
UtA PI and UtA RI and the serum concentrations of PIGF and sEng,
compared with other correlation comparisons included in the current
study, suggested that the effects associated with poor placentation
might be, to a certain degree, reflected and used as an estimation
of defective placentation.
It remains to be confirmed whether the decreased
PIGF levels observed in the current study directly represented
increased inflammation. PlGF is an angiogenic molecule of the
vascular endothelial growth factor family. In humans, serum levels
of PlGF are reduced in women with PE (19,20).
Decreased PlGF levels are associated with increased levels of
pro-inflammatory circulating interleukin (IL)-33 (21). Thus, pro-inflammatory molecules and
cytokines may play a role in the pathogenesis of PE. IL-33 exerts
its inflammatory action through its receptor interleukin-1
receptor-like 1 (22), which is
expressed in the nuclei of endothelial cells of both large and
small vessels, as well as in the placental endothelium and smooth
muscle cells (23). In a previous
study, an inverse correlation between IL-33 and PlGF was found both
in PE and control groups, suggesting that cytokines were released
when PlGF levels decreased (24).
Numerous cytokines are released from the inter-villous space into
the maternal circulation, causing systemic maternal disease
(25).
As part of the adaptation process, normal pregnancy
is characterized by systemic inflammation, oxidative stress,
alterations in levels of angiogenic factors and vascular reactivity
(18). Inflammation induced
endothelial dysfunction and enhanced NO production and
vasodilatation. The endothelium is a crucial regulator of the
vascular tone. Impaired endothelial function is characterized by
reduced vasodilation and increased vascular tone measured using PPG
RI, represented as the amplitude of the reflected wave, which has
been shown to correlate with the severity of proinflammatory and
prothrombotic states (26). It is
currently accepted that maternal endothelial dysfunction preceeds
the development of PE (27). In the
current study, increased sEng, which is associated with endothelial
dysfunction, was found in PIH and PE groups compared with the NP
group and was the highest in the PE group. An r>0.9 also
suggested a very strong positive correlation between sEng and PPG
RI.
The vascular tone measured using PPG RI increased in
subjects with severe symptoms (28).
Inflammation in vascular tissue is an important contributor to the
pathophysiology of hypertension, the initiation and progression of
atherosclerosis, as well as the development of cardiovascular
diseases (29–31). During pregnancy, PPG RI provides a
simplified indication of the inflammatory response through which
the inflammation severity is expressed as the magnitude within the
range of universal maternal intravascular inflammatory response to
pregnancy (32). It has also been
verified that the alteration in PPG RI is a useful assessment to
reflect the status of endothelial function (33,34).
In summary, using noninvasive measures to reflect
circulatory status, PPG RI provided a scale covering a wide range
of conditions, from normal to abnormal pregnancy. The UtA PI and RI
determined using Doppler ultrasonography demonstrated the effects
associated with poor placentation. These measures are useful in
assessing high-risk pregnancy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL was responsible for research conception and
design. FS, XC and XH are responsible for data acquisition. Data
analysis and interpretation was performed by QP and XS. The article
was written by XS and critically revised by XS and XH. All authors
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Shenzhen People's Hospital and all subjects had
signed written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kintiraki E, Papakatsika S, Kotronis G,
Goulis DG and Kotsis V: Pregnancy-induced hypertension. Hormones
(Athens). 14:211–223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu FM, Zhao M, Wang M, Yang HL and Li L:
Effect of regular oral intake of aspirin during pregnancy on
pregnancy outcome of high-risk pregnancy-induced hypertension
syndrome patients. Eur Rev Med Pharmacol Sci. 20:5013–5016.
2016.PubMed/NCBI
|
|
3
|
Draganovic D, Lucic N and Jojic D:
Oxidative stress marker and pregnancy induced hypertension. Med
Arch. 70:437–440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Damodaran D: Effect of progressive muscle
relaxation technique in terms oi anxiety and physiological
parameters of antenatal mothers with pregnancy-induced
hypertension. Nurs J India. 106:254–257. 2015.PubMed/NCBI
|
|
5
|
Leffert LR, Clancy CR, Bateman BT, Bryant
AS and Kuklina EV: Hypertensive disorders and pregnancy-related
stroke: Frequency, trends, risk factors, and outcomes. Obstet
Gynecol. 125:124–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naderi S, Tsai SA and Khandelwal A:
Hypertensive disorders of pregnancy. Curr Atheroscler Rep.
19:152017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davenport MH, Ruchat SM, Poitras VJ,
Jaramillo Garcia A, Gray CE, Barrowman N, Skow RJ, Meah VL, Riske
L, Sobierajski F, et al: Prenatal exercise for the prevention of
gestational diabetes mellitus and hypertensive disorders of
pregnancy: A systematic review and meta-analysis. Br J Sports Med.
52:1367–1375. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sultana Z, Maiti K, Dedman L and Smith R:
Is there a role for placental senescence in the genesis of
obstetric complications and fetal growth restriction? Am J Obstet
Gynecol. 218:S762–S773. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phipps E, Prasanna D, Brima W and Jim B:
Preeclampsia: Updates in pathogenesis, definitions, and guidelines.
Clin J Am Soc Nephrol. 11:1102–1113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jim B and Karumanchi SA: Preeclampsia:
Pathogenesis, prevention, and long-term complications. Semin
Nephrol. 37:386–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guedes-Martins L: Superimposed
preeclampsia. Adv Exp Med Biol. 956:409–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Zhou Y, Yi R, He J, Yang Y, Luo L,
Dai Y and Luo X: Quantitative research into the deconditioning of
hemodynamic to disorder of consciousness carried out using
transcranial Doppler ultrasonography and photoplethysmography
obtained via finger-transmissive absorption. Neurol Sci.
37:547–555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han N, Luo X and Su F: A quantitative
investigation of hemodynamic adaptation to pregnancy using uterine
artery Doppler ultrasonography and finger photoplethysmography.
Hypertens Pregnancy. 33:498–507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Santo P, Harnett DT, Simard T, Ramirez
FD, Pourdjabbar A, Yousef A, Moreland R, Bernick J, Wells G, Dick
A, et al: Photoplethysmography using a smartphone application for
assessment of ulnar artery patency: A randomized clinical trial.
CMAJ. 190:E380–E388. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ling P, Quan G, Siyuan Y, Bo G and Wei W:
Can the descending aortic stroke volume be estimated by
transesophageal descending aortic photoplethysmography? J Anesth.
31:337–344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Veerbeek JH, Hermes W, Breimer AY, van
Rijn BB, Koenen SV, Mol BW, Franx A, de Groot CJ and Koster MP:
Cardiovascular disease risk factors after early-onset preeclampsia,
late-onset preeclampsia, and pregnancy-induced hypertension.
Hypertension. 65:600–606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang P, Dai A, Alexenko AP, Liu Y,
Stephens AJ, Schulz LC, Schust DJ, Roberts RM and Ezashi T:
Abnormal oxidative stress responses in fibroblasts from
preeclampsia infants. PLoS One. 9:e1031102014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tannetta D, Masliukaite I, Vatish M,
Redman C and Sargent I: Update of syncytiotrophoblast derived
extracellular vesicles in normal pregnancy and preeclampsia. J
Reprod Immunol. 119:98–106. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vieillefosse S, Guibourdenche J, Atallah
A, Haddad B, Fournier T, Tsatsaris V and Lecarpentier E: Predictive
and prognostic factors of preeclampsia: Interest of PlGF and
sFLT-1. J Gynecol Obstet Biol Reprod (Paris). 45:999–1008. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Erez O, Romero R, Maymon E, Chaemsaithong
P, Done B, Pacora P, Panaitescu B, Chaiworapongsa T, Hassan SS and
Tarca AL: The prediction of late-onset preeclampsia: Results from a
longitudinal proteomics study. PLoS One. 12:e01814682017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Enninga EA, Nevala WK, Creedon DJ,
Markovic SN and Holtan SG: Fetal sex-based differences in maternal
hormones, angiogenic factors, and immune mediators during pregnancy
and the postpartum period. Am J Reprod Immunol. 73:251–262. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Zhou X, Han TL, Baker PN, Qi H and
Zhang H: Decreased IL-33 production contributes to trophoblast cell
dysfunction in pregnancies with preeclampsia. Mediators Inflamm.
2018:97872392018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Romero R, Chaemsaithong P, Tarca AL,
Korzeniewski SJ, Maymon E, Pacora P, Panaitescu B, Chaiyasit N,
Dong Z, Erez O, et al: Maternal plasma-soluble ST2 concentrations
are elevated prior to the development of early and late onset
preeclampsia-a longitudinal study. J Matern Fetal Neonatal Med.
31:418–432. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stampalija T, Chaiworapongsa T, Romero R,
Chaemsaithong P, Korzeniewski SJ, Schwartz AG, Ferrazzi EM, Dong Z
and Hassan SS: Maternal plasma concentrations of sST2 and
angiogenic/anti-angiogenic factors in preeclampsia. J Matern Fetal
Neonatal Med. 26:1359–1370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Granne I, Southcombe JH, Snider JV,
Tannetta DS, Child T, Redman CW and Sargent IL: ST2 and IL-33 in
pregnancy and pre-eclampsia. PLoS One. 6:e244632011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mishra RC, Rahman MM, Davis MJ, Wulff H,
Hill MA and Braun AP: Alpha1-adrenergic stimulation
selectively enhances endothelium-mediated vasodilation in rat
cremaster arteries. Physiol Rep. 6:e137032018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Possomato-Vieira JS and Khalil RA:
Mechanisms of endothelial dysfunction in hypertensive pregnancy and
preeclampsia. Adv Pharmacol. 77:361–431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Couceiro R, Carvalho P, Paiva RP,
Henriques J, Quintal I, Antunes M, Muehlsteff J, Eickholt C,
Brinkmeyer C, Kelm M and Meyer C: Assessment of cardiovascular
function from multi-Gaussian fitting of a finger
photoplethysmogram. Physiol Meas. 36:1801–1825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khaddaj Mallat R, Mathew John C, Kendrick
DJ and Braun AP: The vascular endothelium: A regulator of arterial
tone and interface for the immune system. Crit Rev Clin Lab Sci.
54:458–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gray SP and Jandeleit-Dahm KA: The role of
NADPH oxidase in vascular disease - hypertension, atherosclerosis
& stroke. Curr Pharm Des. 21:5933–5944. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Madonna R and De Caterina R: Aquaporin-1
and sodium-hydrogen exchangers as pharmacological targets in
diabetic atherosclerosis. Curr Drug Targets. 16:361–365. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Redman CW, Sacks GP and Sargent IL:
Preeclampsia: An excessive maternal inflammatory response to
pregnancy. Am J Obstet Gynecol. 180:499–506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kudaravalli J: Improvement in endothelial
dysfunction in patients with systemic lupus erythematosus with
N-acetylcysteine and atorvastatin. Indian J Pharmacol. 43:311–315.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rosato E, Barbano B, Gigante A, Aversa A,
Cianci R, Molinaro I, Quarta S, Pisarri S, Afeltra A and Salsano F:
Erectile dysfunction, endothelium dysfunction, and microvascular
damage in patients with systemic sclerosis. J Sex Med.
10:1380–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|


















