Introduction
Osteosarcoma is the most common primary malignancy
of bone tissue, typically presenting in teenagers and young adults
(1). This tumor has a highly
invasive and distant metastatic potential (2,3), and the
5-year survival rate in patients with metastatic osteosarcoma is
5–20% (4,5). Therefore, it is important to clarify
the molecular mechanisms of action behind osteosarcoma metastasis
and to explore effective therapeutic approaches to treat patients
with osteosarcoma.
Aberrant expression and activation of transcription
factors occurs frequently in various cancer types. Differentiated
embryonic chondrocyte-expressed gene 1 (DEC1) is a basic
helix-loop-helix transcription factor that has been detected in a
variety of developing and adult tissues (6–8). DEC1
plays an important role in numerous biological events, including
cell survival, differentiation, circadian rhythms and hypoxia
response (9–12). Additionally, DEC1 has been reported
to promote tumor progression, invasion and metastasis (13–16).
Previous studies have shown that DEC1 is associated with various
types of human cancers, such as lung, gastric, liver and breast
cancer (17–20). Recently, Zhou et al (21) found that knockdown of cryptochrome
circadian regulator 1 enhanced the proliferation and migration of
osteosarcoma cells presenting downregulation of DEC1. However,
there is limited data regarding the function of DEC1 in
osteosarcoma.
The present study aimed to examine the expression
level of DEC1 in human osteosarcoma tissues and cell lines.
Furthermore, the effects of DEC1 on the proliferation, adhesion,
invasion and epithelial-mesenchymal transition (EMT) of
osteosarcoma cells were investigated.
Materials and methods
Tissue collection
A total of 21 osteosarcoma patients were recruited
for this study from January 2014 to May 2018 (12–25 years old; 11
males and 10 females). All patients provided written informed
consent in compliance with the code of ethics of the World Medical
Association (Declaration of Helsinki). The present study was
approved by the Ethics Committee of The Second Xiangya Hospital of
Central South University. The human osteosarcoma samples and
adjacent normal tissues (located >3 cm away from the tumor) were
collected from the patients who underwent surgery at The Second
Xiangya Hospital of Central South University. The tissue samples
were frozen in liquid nitrogen prior to experimentation.
Cell culture and transfection
hFOB, MG63, Saos-2 and U2OS cells were purchased
from the American Type Culture Collection and maintained in DMEM
(Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.). All cells were incubated
at 37°C in a humidified atmosphere of 5% CO2. Prior to
transfection, the cells were washed with PBS, and the medium was
replaced with serum-free DMEM. A total of 200 ng small interfering
(si)RNA control (siControl; forwards, 5′-UUCUCCGAACGUGUCACGUTT-3′
and reverse, 5′-ACGUGACACGUUCGGAGAATT-3′; Shanghai GenePharma Co.,
Ltd.), DEC1 siRNA (forwards, 5′-GAACAUCUCAAACUUACAACUTT-3′ and
reverse, 5′-AGUUGUAAGUUUGAGAUGUUCTT-3′; Shanghai GenePharma Co.,
Ltd.), empty plasmid (pcDNA3.1; Shanghai GenePharma Co., Ltd.) or
DEC1 overexpression plasmid (Shanghai GenePharma Co., Ltd.) was
transfected into the cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. After 6 h, the medium was replaced
with fresh medium and the cells were maintained in the culture
flasks for at least 24 h prior to subsequent analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the tissues and cells
using Trizol® (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's instructions. cDNA was reverse
transcribed using a First Strand cDNA Synthesis kit (Fermentas;
Thermo Fisher Scientific, Inc.) at 42°C. qPCR was performed on an
ABI 7300 real-time PCR system using a SYBR Green PCR kit (Applied
Biosystems). The primers used for amplification were as follows:
DEC1 forward, 5′-GAAAGGATCGGCGCAATTAA-3′, and reverse,
5′-CATCATCCGAAAGCTGCATC-3′; GAPDH forward,
5′-CGACCACTTTGTCAAGCTCA-3′, and reverse,
5′-AGGGGTCTACATGGCAACTG-3′. GAPDH was used as a control. Primers
were used at a final concentration of 250 nM. The reaction was
followed by 40 cycles at 95°C for 30 sec, 58°C for 30 sec and 72°C
for 30 sec. mRNA levels were quantified using the 2−ΔΔCq
relative quantification method (22).
Western blot analysis
Total protein was extracted from the tissues and
cells using RIPA buffer (Elabscience Biotechnology Co., Ltd.).
Protein concentration was determined using a Bicinchoninic Acid
protein assay. Equal amounts of total protein (50 µg) were
separated on a 12% sodium dodecyl sulfate polyacrylamide gel using
electrophoresis, and then transferred onto a nitrocellulose
membrane (EMD Millipore). The membranes were blocked with 3% bovine
serum albumin (Sigma-Aldrich; Merck KGaA) at 4°C overnight and were
subsequently subjected to 3 washes with Tris-buffered saline and
Tween-20 (0.05%) (TBST). The membranes were incubated with the
primary antibodies, including DEC1 mouse monoclonal antibody
(sc-101023; 1:500; Santa Cruz Biotechnology, Inc.), E-cadherin
mouse monoclonal antibody (sc-71007; 1:400; Santa Cruz
Biotechnology, Inc.), N-cadherin mouse monoclonal antibody
(sc-8424; 1:400; Santa Cruz Biotechnology, Inc.), vimentin mouse
monoclonal antibody (sc-66002; 1:800; Santa Cruz Biotechnology,
Inc.) and GAPDH mouse monoclonal antibody (sc-47724; 1:1,000; Santa
Cruz Biotechnology, Inc.) at 37°C for 1 h. The membranes were
washed with TBST and incubated with horseradish peroxidase-labeled
goat anti-mouse secondary antibody (sc-2005; dilution, 1:2,000;
Santa Cruz Biotechnology, Inc.) at 37°C for 1 h. The
chemiluminescent signal was detected using an enhanced
chemiluminescence detection kit (Pierce; Thermo Fisher Scientific,
Inc.). Image-Pro Plus software (version 6.0; Media Cybernetics,
Inc.) was used for densitometry analysis.
MTT assay
Cell proliferation was determined using MTT assays.
The cells were seeded into 96-well plates, allowed to grow for 24,
48, 72 and 96 h, and then incubated with 10 µl MTT (Sigma-Aldrich;
Merck KGaA) at 37°C for 4 h. A total of 200 µl DMSO (Sigma-Aldrich;
Merck KGaA) was added to solubilize the formazan crystals. The
absorbance at 570 nm was then measured using a microplate reader
(SpectraMax 190; Molecular Devices, LLC).
Cell adhesion assay
For the cell adhesion assays, 96-well plates were
pre-coated with fibronectin (Sigma-Aldrich; Merck KGaA) and blocked
with 1% BSA at 37°C for 2 h. Cells in serum-free medium were seeded
into the 96-well plates at the density of 2×105 cells/ml
(0.2 ml) and incubated at 37°C for 2 h. The adhesive cells were
then fixed in 4% paraformaldehyde at room temperature for 30 min
and stained with 0.5% crystal violet (Sangon Biotech, Co. Ltd.) at
room temperature for 2 h. SDS (Amresco LLC) was used to dissolve
the crystals. The absorbance at 570 nm was measured using a
microplate reader.
Cell invasion assay
Transwell inserts (Corning, Inc.) were coated with
Matrigel matrix (BD Biosciences) at 37°C for 30 min. Cell
suspensions were prepared in serum-free medium at a final
concentration of 5×104 cells/ml and added to the upper
chambers. Subsequently, 1 ml of 10% FBS-containing medium was added
to the lower chambers. Following incubation at 37°C overnight, a
cotton swab was used to remove non-invasive cells. The cells on the
lower surface of the membrane were fixed using 95% ethanol for 20
min and stained with hematoxylin for 10 min at room temperature.
The number of invaded cells was counted using an inverted light
microscope (TS100; Nikon Corporation).
Statistical analysis
All data are expressed as the means ± SD from at
least three independent experiments. Statistical analysis was
performed using GraphPad Prism Software (version 5; GraphPad
Software, Inc.). Differences between two groups were assessed by
the paired Student's t-test, and ANOVAs followed by
student-Keuls-Newman tests were used to compare the differences
between three or more groups. P<0.05 was considered to indicate
a statistically significant difference.
Results
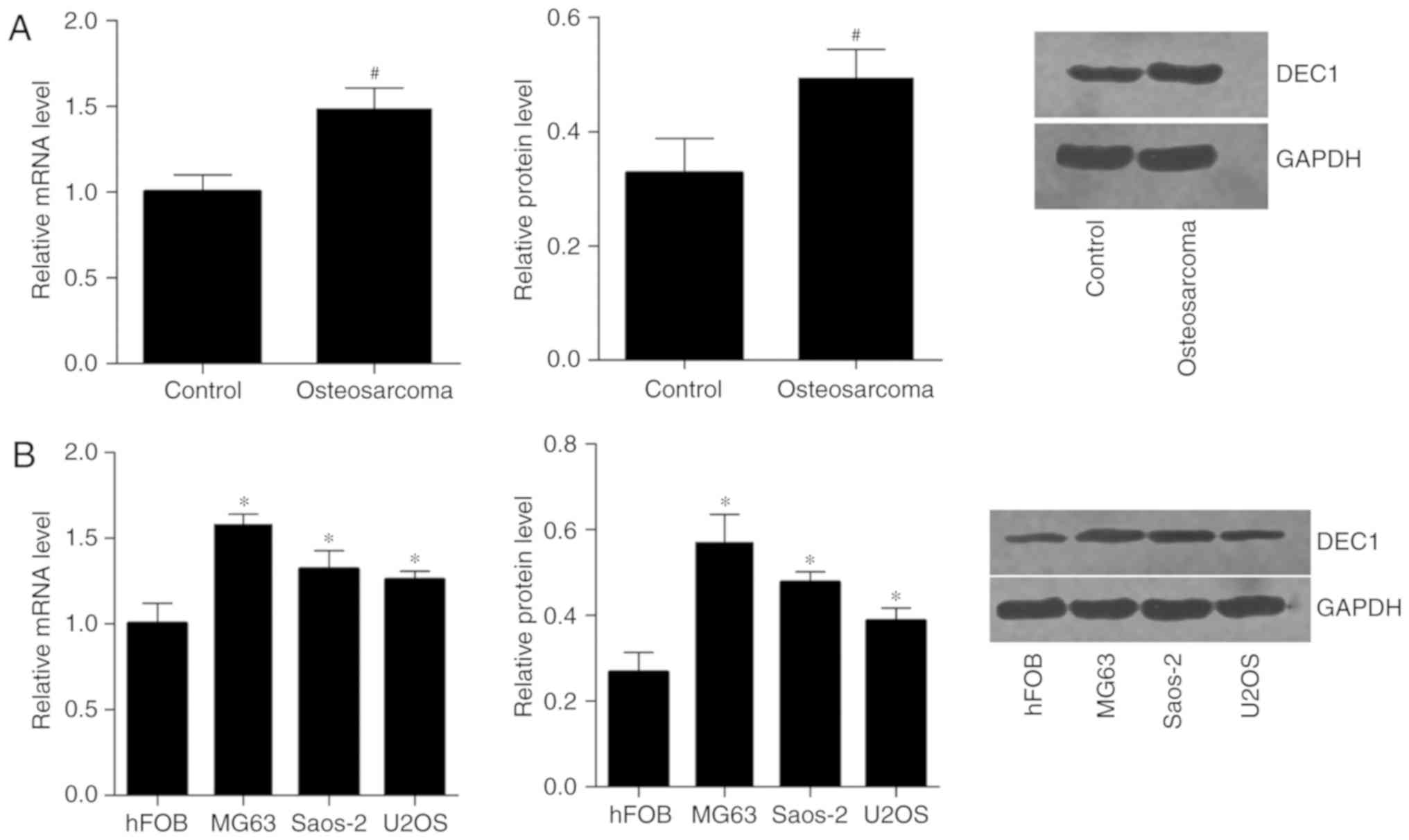
Expression of DEC1 in human
osteosarcoma tissues and cell lines
A total of 21 human osteosarcoma specimens and
adjacent non-tumor tissues were collected to investigate DEC1
expression using RT-qPCR and western blot analysis. It was found
that the expression levels of DEC1 mRNA and DEC1 protein were
higher in human osteosarcoma tissues compared with adjacent
non-tumor tissues (Fig. 1A). DEC1
expression was also analyzed in a normal bone cell line (hFOB) and
in osteosarcoma cell lines (MG63, U2OS and Saos-2). As shown in
Fig. 1B, the expression levels of
DEC1 mRNA and DEC1 protein were higher in osteosarcoma cells than
in normal bone cells.
Effect of DEC1 on the proliferation of
osteosarcoma cells
To investigate the biological functions of DEC1 in
osteosarcoma cells, both gain- and loss-of-function experiments
were performed in vitro. MG63 cells with endogenously high
DEC1 expression were selected to be transfected with DEC1 siRNA, in
order to knockdown DEC1. In contrast, U2OS cells with endogenously
low DEC1 expression were selected to be transfected with the DEC1
plasmid, in order to overexpress DEC1. Subsequently, the effect of
DEC1 on osteosarcoma cells proliferation was examined using MTT
assays. The present results showed that DEC1 overexpression
significantly promoted the proliferation of U2OS cells, while DEC1
knockdown significantly inhibited the proliferation of MG63 cells
(Fig. 2).
Effect of DEC1 on the adhesion and
invasion of osteosarcoma cells
To investigate the effects of DEC1 on the adhesive
and invasive capabilities of osteosarcoma cells, cell adhesion and
invasion experiments were performed in vitro. The present
results showed that MG63 cells transfected with DEC1 siRNA had a
reduced adhesive and invasive ability compared with those cells
transfected with siControl. Overexpression of DEC1 increased the
adhesive and invasive abilities of U2OS cells (Fig. 3).
Effect of DEC1 on the EMT in
osteosarcoma cells
To evaluate whether DEC1 regulates EMT in
osteosarcoma cells, the expression levels of EMT-related proteins,
including E-cadherin, N-cadherin and vimentin were examined. As
demonstrated by western blot analysis, DEC1 overexpression
significantly upregulated N-cadherin and vimentin but downregulated
E-cadherin in U2OS cells, whereas knockdown of DEC1 produced the
opposite results in MG63 cells (Fig.
4).
Discussion
Dysregulated expression of DEC1 has been observed in
a number of types of human cancer. Upregulated DEC1 has been found
in gastric, liver, pancreatic and breast cancer (6,13,15,23–27).
Downregulated DEC1 has been found in non-small cell lung cancer
(17,28). However, the expression of DEC1 in
osteosarcoma has not been previously reported. In the present
study, DEC1 expression levels in human osteosarcoma tissues and
cell lines were examined, and it was found that osteosarcoma
tissues and osteosarcoma cells had higher expression levels of DEC1
compared with the controls. The present findings suggested DEC1 is
aberrantly expressed in osteosarcoma, and the present results
indicated that DEC1 may be involved in the progression of
osteosarcoma.
DEC1 has multifaceted roles in cancer progression
(8). A previous study has shown that
downregulation of DEC1 inhibits gastric cancer cell proliferation
in vitro and tumorigenicity in vivo (18). DEC1 is upregulated by TGF-β in PANC-1
cells, and regulates the expression of EMT-related factors. DEC1
knockdown inhibits morphological changes during EMT processes
(13). Li et al reported that
DEC1 induces the activation of apoptosis-related factor, survivin,
under serum-starvation, which has an anti-apoptotic effect in
HEK293 cells (29). In contrast,
Thin et al found that DEC1 is induced in response to several
DNA-damaging agents and has a pro-apoptotic effect (30). Additionally, it has been reported
that high levels of DEC1 inhibit cell proliferation and colony
formation of esophageal squamous cell carcinoma cells, promoting
cellular senescence (16). The
complex mechanisms underlying the role of DEC1 in carcinogenesis
are controversial. However, all these findings suggest that
aberrant expression levels of DEC1 play important roles in tumor
progression. Currently, there are no reports into the role of DEC1
in osteosarcoma. Proliferation and metastasis are critical events
in the pathogenesis of cancer (31).
Using the osteosarcoma cell lines MG63 and U2OS, this study found
that DEC1 knockdown inhibits the proliferation of osteosarcoma cell
lines and DEC1 overexpression promotes cell proliferation in
vitro. Furthermore, the effect of DEC1 on the adhesive and
invasive abilities of MG63 and U2OS cells was evaluated. Both the
gain-of- and loss-of-function experiments demonstrated that DEC1
promotes the invasiveness of osteosarcoma cell lines. Overall,
these data supported the hypothesis that DEC1 functions as an
oncogene in osteosarcoma.
EMT is a dynamic process by which epithelial cells
lose their polarity and are converted to present a mesenchymal
phenotype, thereby gaining increased motility and invasiveness
(32). EMT is considered as a common
molecular mechanism promoting tumor metastasis (33). E-cadherin, N-cadherin and vimentin
are typical regulators of EMT. In the present study, DEC1 was found
to upregulate the mesenchymal markers N-cadherin and vimentin, and
downregulate the epithelial marker E-cadherin (34). These results suggest that DEC1 has an
inducible effect on EMT in osteosarcoma cell lines, thus
contributing to the aggressiveness of osteosarcoma.
DEC1 is frequently dysregulated in human cancer, and
the molecular mechanism of action which regulates its expression is
complex. Previous studies have reported that DEC1 can be induced in
a cell type-specific manner by various extracellular stimuli, such
as hypoxia, growth factors, hormones and cytokines (35–38).
DEC1 can also act as a transcriptional factor through interactions
with the PI3K/Akt/glycogen synthase kinase 3β signaling pathway to
promote osteogenic activity and to relieve
1-methyl-4-phenylpyridinium-induced cytotoxicity (39,40). A
recent study showed that activation of the Akt/P53/P21 signaling
pathway promotes proliferation and migration of human osteosarcoma
cells, and activation of the Akt/P53/P21 signaling pathway induced
the downregulation of DEC1 (21);
however, the direct effect of DEC1 on regulating the Akt/P53/P21
signaling pathway remains unknown. Further studies are required to
verify the interaction between DEC1 and the Akt signaling pathway
in human osteosarcoma cells.
In conclusion, the present study showed that DEC1
was upregulated in human osteosarcoma tissues and cell lines. Using
gain-of- and loss-of-function approaches, the present study
suggested that DEC1 may exert its effect on osteosarcoma
progression by promoting cell proliferation, adhesion and invasion
in vitro. Furthermore, it was found that DEC1 could promote
aggressiveness of osteosarcoma cell lines by regulating the
expression levels of genes involved in EMT. This preliminary study
suggested that DEC1 may represent a novel molecular target for the
treatment of osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data sets generated and analyzed during the
study are available from the corresponding author, on reasonable
request.
Authors' contributions
SL and DP conceived, designed and supervised the
study. SL, ZY, WZ, XH and CL performed the experiments. SL and ZY
analyzed and interpreted the data. SL drafted the manuscript. DP
gave final approval of the version to be published. All authors
read and approved the manuscript.
Ethics approval and consent to
participate
All patients provided informed written consent, and
the study was approved by the Ethics Committee of The Second
Xiangya Hospital of Central South University (Changsha, Hunan,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao H, Yao Y, Wang Z, Lin F, Sun Y and
Chen P: Therapeutic effect of pirarubicin-based chemotherapy for
osteosarcoma patients with lung metastasis. J Chemother.
22:119–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He A, Yang X, Huang Y, Feng T, Wang Y, Sun
Y, Shen Z and Yao Y: CD133(+) CD44(+) cells mediate in the lung
metastasis of osteosarcoma. J Cell Biochem. 116:1719–1729. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Ma L, Wei G; Comment on Fu, ; et
al: A systematic review of p53 as a biomarker of survival in
patients with osteosarcoma. Tumour Biol. 35:5049–5050. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shin SH, Jeong HJ, Han I, Cho HS and Kim
HS: Osteosarcoma and chondrosarcoma of the shoulder: Site-specific
comparative analysis. Orthopedics. 36:e179–e185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turley H, Wykoff CC, Troup S, Watson PH,
Gatter KC and Harris AL: The hypoxia-regulated transcription factor
DEC1 (Stra13, SHARP-2) and its expression in human tissues and
tumours. J Pathol. 203:808–813. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kato Y, Kawamoto T, Fujimoto K and Noshiro
M: DEC1/STRA13/SHARP2 and DEC2/SHARP1 coordinate physiological
processes, including circadian rhythms in response to environmental
stimuli. Curr Top Dev Biol. 110:339–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato F, Bhawal UK, Yoshimura T and
Muragaki Y: DEC1 and DEC2 crosstalk between circadian rhythm and
tumor progression. J Cancer. 7:153–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miyazaki K, Kawamoto T, Tanimoto K,
Nishiyama M, Honda H and Kato Y: Identification of functional
hypoxia response elements in the promoter region of the DEC1 and
DEC2 genes. J Boil Chem. 277:47014–47021. 2002. View Article : Google Scholar
|
|
10
|
Yamada K and Miyamoto K: Basic
helix-loop-helix transcription factors, BHLHB2 and BHLHB3; Their
gene expressions are regulated by multiple extracellular stimuli.
Front Biosci. 10:3151–3171. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marczak MM and Yan B: Circadian
rhythmicity: A functional connection between differentiated
embryonic chondrocyte-1 (DEC1) and small heterodimer partner (SHP).
Arch Biochem Biophys. 631:11–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iwata T, Kawamoto T, Sasabe E, Miyazaki K,
Fujimoto K, Noshiro M, Kurihara H and Kato Y: Effects of
overexpression of basic helix-loop-helix transcription factor Dec1
on osteogenic and adipogenic differentiation of mesenchymal stem
cells. Eur J Cell Boil. 85:423–431. 2006. View Article : Google Scholar
|
|
13
|
Wu Y, Sato F, Yamada T, Bhawal UK,
Kawamoto T, Fujimoto K, Noshiro M, Seino H, Morohashi S, Hakamada
K, et al: The BHLH transcription factor DEC1 plays an important
role in the epithelial-mesenchymal transition of pancreatic cancer.
Int J Oncol. 41:1337–1346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
You J, Lin L, Liu Q, Zhu T, Xia K and Su
T: The correlation between the expression of differentiated
embryo-chondrocyte expressed gene l and oral squamous cell
carcinoma. Eur J Med Res. 19:212014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Miao Y, Wang J, Lin X, Wang L, Xu
HT and Wang EH: DEC1 is positively associated with the malignant
phenotype of invasive breast cancers and negatively correlated with
the expression of claudin-1. Int J Mol Med. 31:855–860. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Q, Ma P, Hu C, Chen L, Xue L, Wang Z,
Liu M, Zhu H, Xu N and Lu N: Overexpression of the DEC1 protein
induces senescence in vitro and is related to better survival in
esophageal squamous cell carcinoma. PLoS One. 7:e418622012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Wang L, Lin XY, Wang J, Yu JH, Miao
Y and Wang EH: The transcription factor DEC1
(BHLHE40/STRA13/SHARP-2) is negatively associated with TNM stage in
non-small-cell lung cancer and inhibits the proliferation through
cyclin D1 in A549 and BE1 cells. Tumour Biol. 34:1641–1650. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia Y, Hu R, Li P, Zheng Y, Wang Y and Ma
X: DEC1 is required for anti-apoptotic activity of gastric cancer
cells under hypoxia by promoting Survivin expression. Gastric
Cancer. 21:632–642. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma W, Shi X, Lu S, Wu L and Wang Y:
Hypoxia-induced overexpression of DEC1 is regulated by HIF-1α in
hepatocellular carcinoma. Oncol Rep. 30:2957–2962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng Q, Wang C, Wang L, Zhang D, Liu N,
Ming X, Zhou H, Guli Q and Liu Y: Interaction with SP1, but not
binding to the E-box motifs, is responsible for
BHLHE40/DEC1-induced transcriptional suppression of CLDN1 and cell
invasion in MCF-7 cells. Mol Carcinog. 57:1116–1129. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou L, Yu Y, Sun S, Zhang T and Wang M:
Cry 1 regulates the clock gene network and promotes proliferation
and migration via the akt/P53/P21 pathway in human osteosarcoma
cells. J Cancer. 9:2480–2491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jia YF, Xiao DJ, Ma XL, Song YY, Hu R,
Kong Y, Zheng Y, Han SY, Hong RL and Wang YS: Differentiated
embryonic chondrocyte-expressed gene 1 is associated with
hypoxia-inducible factor 1α and Ki67 in human gastric cancer. Diagn
Pathol. 8:372013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng Y, Jia Y, Wang Y, Wang M, Li B, Shi
X, Ma X, Xiao D and Sun Y: The hypoxia-regulated transcription
factor DEC1 (Stra13, SHARP-2) and its expression in gastric cancer.
OMICS. 13:301–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi XH, Zheng Y, Sun Q, Cui J, Liu QH, Qü
F and Wang YS: DEC1 nuclear expression: A marker of differentiation
grade in hepatocellular carcinoma. World J Gastroenterol.
17:2037–2043. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Reiser-Erkan C, Michalski CW,
Raggi MC, Quan L, Yupei Z, Friess H, Erkan M and Kleeff J: Hypoxia
inducible BHLHB2 is a novel and independent prognostic marker in
pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun.
401:422–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chakrabarti J, Turley H, Campo L, Han C,
Harris AL, Gatter KC and Fox SB: The transcription factor DEC1
(stra13, SHARP2) is associated with the hypoxic response and high
tumour grade in human breast cancers. Br J Cancer. 91:954–958.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Giatromanolaki A, Koukourakis MI, Sivridis
E, Turley H, Wykoff CC, Gatter KC and Harris AL: DEC1 (STRA13)
protein expression relates to hypoxia-inducible factor 1-alpha and
carbonic anhydrase-9 overexpression in non-small cell lung cancer.
J Pathol. 200:222–228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Xie M, Yang J, Yang D, Deng R, Wan Y
and Yan B: The expression of antiapoptotic protein survivin is
transcriptionally upregulated by DEC1 primarily through multiple
sp1 binding sites in the proximal promoter. Oncogene. 25:3296–3306.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thin TH, Li L, Chung TK, Sun H and Taneja
R: Stra13 is induced by genotoxic stress and regulates
ionizing-radiation-induced apoptosis. EMBO Rep. 8:401–407. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sgourakis G, Gockel I, Lyros O, Hansen T,
Mildenberger P and Lang H: Detection of lymph node metastases in
esophageal cancer. Expert Rev Anticancer Ther. 11:601–612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Samant RS and Shevde LA: NMI and EMT.
Oncoscience. 1:476–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Turley EA, Veiseh M, Radisky DC and
Bissell MJ: Mechanisms of disease: Epithelial-mesenchymal
transition-does cellular plasticity fuel neoplastic progression?
Nat Clin Pract Oncol. 5:280–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kon N, Hirota T, Kawamoto T, Kato Y,
Tsubota T and Fukada Y: Activation of TGF-beta/activin signalling
resets the circadian clock through rapid induction of Dec1
transcripts. Nat Cell Boil. 10:1463–1469. 2008. View Article : Google Scholar
|
|
36
|
Bhawal UK, Ito Y, Tanimoto K, Sato F,
Fujimoto K, Kawamoto T, Sasahira T, Hamada N, Kuniyasu H, Arakawa
H, et al: IL-1β-mediated up-regulation of DEC1 in human gingiva
cells via the Akt pathway. J Cell Biochem. 113:3246–3253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamada K, Kawata H, Shou Z, Mizutani T,
Noguchi T and Miyamoto K: Insulin induces the expression of the
SHARP-2/Stra13/DEC1 gene via a phosphoinositide 3-kinase pathway. J
Boil Chem. 278:30719–30724. 2003. View Article : Google Scholar
|
|
38
|
Ivanova AV, Ivanov SV,
Danilkovitch-Miagkova A and Lerman MI: Regulation of STRA13 by the
von Hippel-Lindau tumor suppressor protein, hypoxia, and the
UBC9/ubiquitin proteasome degradation pathway. J Biol Chem.
276:15306–15315. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu Z, Wang YW, Ge DH, Lu M, Liu W, Xiong
J, Hu G, Li XP and Yang J: Downregulation of DEC1 contributes to
the neurotoxicity induced by MPP(+) by suppressing PI3K/Akt/GSK3β
pathway. CNS Neurosci Ther. 23:736–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu J, Mao Z, He S, Zhan Y, Ning R, Liu W,
Yan B and Yang J: Icariin protects against glucocorticoid induced
osteoporosis, increases the expression of the bone enhancer DEC1
and modulates the PI3K/Akt/GSK3β/β-catenin integrated signaling
pathway. Biochem Pharmacol. 136:109–121. 2017. View Article : Google Scholar : PubMed/NCBI
|