Introduction
Most of the surgical procedures involve an incision
in the skin to allow the surgeon to gain access to the deeper
organs or tissues. Most of these wounds are fully closed (primary
closure) at the end of the surgery (1). These wounds are covered by the surgeon
using either adhesive tape or dressing (2-4).
Use of dressing can act as a barrier from infection and protect the
wound until the restoration of skin continuity (5). It can also absorb the exudate from the
wound, keeps it dry, clean, and prevents bacterial contamination
from the external environment (6-8).
Ideally, surgeons should select appropriate dressings in order to
ensure the wound remains free of excessive slough, toxic chemicals,
and infection and remain at optimal temperature and pH for
healing.
Post-surgical wound dressings are usually left at
the site for at least 48 h or until suture removal (delayed removal
of dressing), irrespective of contamination level of the wounds, or
other factors such as antibiotic administration. Previous findings
have shown that the moist environment formed by the dressings
accelerates the wound healing process (9). However, other findings suggest that
this can be a disadvantage, as excess exudate causes softening and
deterioration of wound and surrounding healthy tissues (10). Delayed removal can cause
inconvenience and dissatisfaction among the patients and increase
length of nursing time, which ultimately lead to an increase in
economic burden (11-13).
In addition, dressing increases the chance of local sweating and
reduces the moisture evaporation. This results in higher dampness
which potentially acts as a point of entry for microorganisms for
infection (14).
The usefulness of dressing a surgical wound beyond
the first 24-48 h of surgery is therefore controversial. Thus, the
aim of this meta-analysis was to assess the comparative efficacy,
in terms of surgical site infection, wound dehiscence and patient
perception, as well as satisfaction between early removal and
delayed removal of dressing following primary closure in the
management of clean and contaminated surgical wounds.
Materials and methods
Inclusion criteria for the review
Type of studies to be included
We included parallel arm individual randomized,
quasi-randomized or cluster-randomized controlled trials for the
present review. Studies reported as full text were included while
studies published with only the abstract or unpublished data were
excluded.
Type of participants
We included studies conducted among patients with
clean and contaminated surgical wound, irrespective of the type of
surgery.
Type of intervention
We included studies that directly compared the early
and delayed removal of dressing following primary closure
irrespective of the duration of early or delay in removal.
Type of outcome measure
Outcome measures were then assessed: Surgical site
infection, wound dehiscence, patient satisfaction and patient's
perception on safety, comfort, dehiscence. We included the studies
reporting any of the outcomes mentioned above in both the arms.
Search strategy
We conducted an extensive electronic search in the
following databases: Medline, Scopus, Embase and Cochrane Central
Register of Controlled Trials (CENTRAL), clinical trial registries
such as the ClinicalTrials.gov and WHO International Clinical
Trials Registry Platform (ICTRP). Combination of medical subject
heading (MeSH) and free text terms were used for carrying out a
literature search. The MeSH terms ‘dressing wounds’, ‘clean and
contaminated wound’, ‘dressing removal’, ‘early dressing removal’,
‘delayed dressing removal’, ‘surgical wounds’ and ‘randomized
controlled trial’ along with free text terms were used in all
search engines for the above-mentioned databases in various
combinations. The search was conducted in all the databases from
January, 1964 to October, 2019 with publication language restricted
to English.
Searching other resources
We checked the reference list of primary trials
obtained through electronic search, and relevant articles were
included in the review and analysis. We contacted the authors of
the published trials in case clarification or additional
information was required for the methodological assessment of the
studies included.
Data collection and analysis
Selection of studies
Two independent investigators independently
performed a literature search and screened the title, abstract and
keywords of all the studies identified for possible inclusion in
the review. Full-text articles were obtained for studies considered
to be relevant. Further screening of the abstract and full text of
the retrieved articles was performed independently by primary and
secondary investigators to select the studies that satisfied the
eligibility criteria of the present review. Any disagreements
during the entire selection process between two investigators were
resolved either through consensus or consultation with a third
investigator. Quality of the overall review process was monitored
by the third investigator. The preferred Reporting Items for
Systematic Review and Meta-Analysis (PRISMA) check list were used
for reporting of the present review (http://prisma-statement.org/prismastatement/Checklist.aspx).
Data extraction and management
The primary investigator extracted the relevant
study characteristics for the review from the included studies. The
data extracted were: General information such as date of
extraction; study title and authors; details under methods section
such as study design, participants and study setting; details under
participants' section such as total number of participants in each
arm, baseline and endline outcome measures, inclusion and exclusion
criteria; details under interventions section such as details of
intervention group, details of comparison group and follow up
duration; details under outcome section such as primary and
secondary outcomes captured in the study and time of outcome
assessment and other details necessary for assessing the quality of
studies.
Primary and secondary investigators independently
extracted data related to outcome measure from the included
studies. When studies report multiple arms in a single trial, only
the relevant arms were included for the analysis. The primary
investigator transferred the obtained data into the statistical
software RevMan (ver 5.3). Data entry was double checked for
correct entry by the third investigator through comparison of data
presented in review and included study reports.
Risk of bias assessment in included
studies
Two independent investigators assessed the risk of
bias for included studies using Cochrane risk of bias tool for RCTs
(https://handbook-51.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm).
The domains used for assessing the risk of bias were: Random
sequence generation, allocation concealment, blinding of the
participants, incomplete outcome data, blinding of outcome
assessment and selective reporting of outcome. For each of the
abovementioned domains, risk of bias was graded as low (if adequate
information was provided), high (if the information was inadequate
or not performed) and unclear (if the information was missing).
Statistical analysis
Meta-analysis was performed with the selected
studies using RevMan 5·3 (Copenhagen: The Nordic Cochrane Centre,
The Cochrane Collaboration, 2014). Since all the outcomes were
dichotomous, the number of events and participants in each group
were obtained and entered into the Revman software to estimate the
pooled effect size in terms of relative risk. We used the random
effects model with inverse variance. In case of missing data, the
author of the included trial was contacted and the necessary data
could still not be retrieved, the imputation method was
followed.
Assessment of heterogeneity
Evidence of between-study variance due to
heterogeneity was assessed through the Chi-square test of
heterogeneity and I2 statistics to quantify the
inconsistency. I2<25% was mild, 25-75% was moderate
and >75% was considered as substantial heterogeneity. Study
specific and pooled estimates were graphically represented through
forest plot.
Assessment of reporting biases
Reporting bias was assessed by checking whether the
included trial is registered in a trial registry or full protocol
is available. If available, list of outcomes in the protocol were
compared with the list of outcomes mentioned in the full published
trial. Publication bias was assessed using Egger's test and
graphically represented by the funnel plot.
Subgroup analysis and investigation of
heterogeneity
There was no significant heterogeneity for primary
outcome on surgical site infection, while rest of the outcomes did
not have sufficient number of studies to perform subgroup analysis
or meta regression.
Results
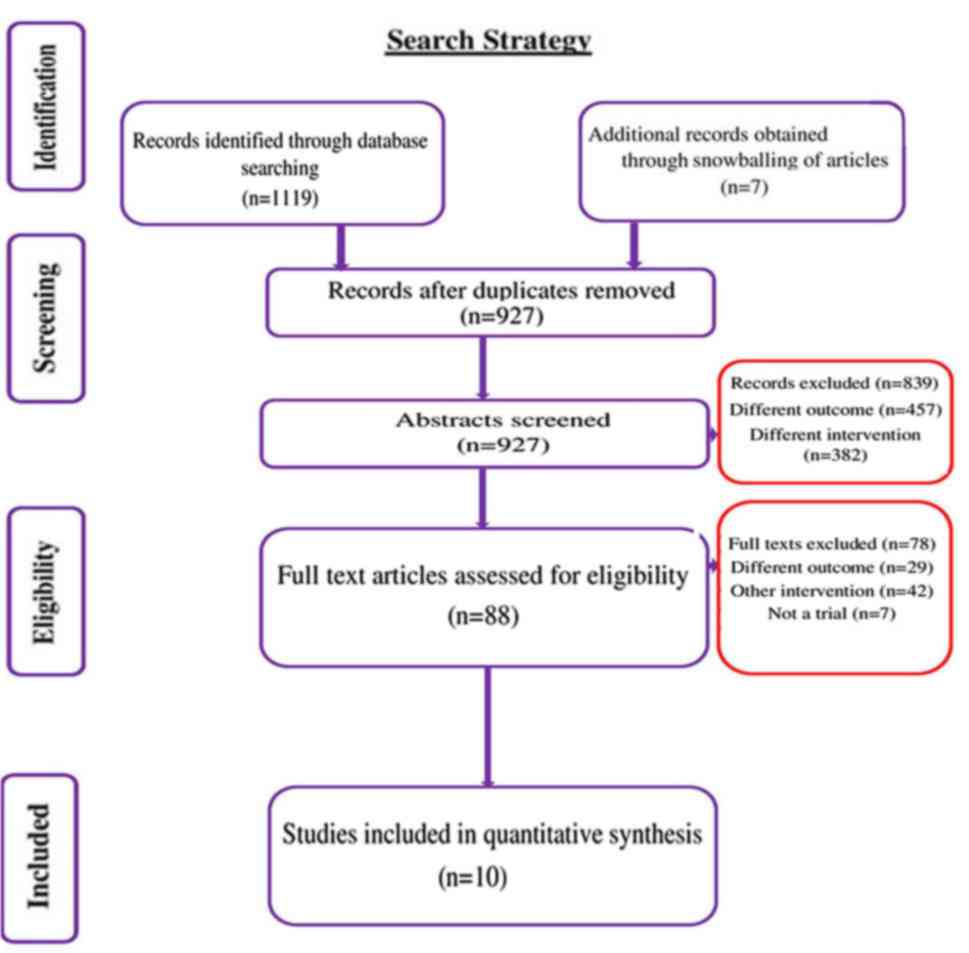
Selection of studies
We conducted a systematic search to identify studies
that directly compared the early vs. delayed removal of dressing
following primary closure of surgical wound from January, 1964
until October, 2019. In total, 1,119 citations were identified, of
which 522 trials were retrieved from Medline, 242 from Scopus, 232
from Embase, 111 from CENTRAL, 7 from ClinicalTrials.gov and 5 from
WHO ICTRP. After the first stage of screening (title, abstract and
keywords), 88 relevant studies were retrieved. Full text of these
studies was reviewed for eligibility criteria. Bibliographies of
the retrieved articles were reviewed and 7 relevant studies were
retrieved. Finally, 10 studies with 1,708 participants satisfying
the inclusion criteria were included (Fig. 1) (13,15-23).
Characteristics of studies
included
Characteristics of the studies are described in
Table I. All the included studies
were RCTs. Most the studies (7 out of 10) were conducted in
countries belonging to American regions including the USA and
Brazil. In total, 1,708 participants were found in the included
studies with 853 participants in the early dressing removal arm and
855 participants in the delayed removal arm. Sample size (both arms
together) varied from 70 to 602 while sample size in intervention
arm varied from 35 to 300 and in control arm varied from 35 to 302.
Among the 10 studies included, all the studies reported on surgical
site infection, 4 studies reported on wound dehiscence, 2 studies
reported on patient satisfaction, patient's perception on safety,
comfort and convenience.
 | Table ICharacteristics of the included
studies, n=10. |
Table I
Characteristics of the included
studies, n=10.
| No | Author, year
(Refs.) | Country | Study design | Type of Surgery | Sample size in early
removal arm | Sample size in
delayed removal arm | Intervention | Mean age |
|---|
| 1 | Ajao 1997(15) | Nigeria | Randomized controlled
trial | Various surgical
procedures (all operations on the trunk) | 50 | 50 | Group 1 (n=50): Early
dressing removal, wound left open 24-36 h after suturing. Group 2
(n=50): Delayed dressing removal, dressing left for 7-10 days
unless infection suspected, when the wound was inspected and
dressing reapplied. | Not provided |
| 2 | Dosseh et al,
2008(13) | Togo | Randomized controlled
trial | Abdominal surgery,
neck surgery and thoracic surgery and having clean or
clean-contaminated wounds | 51 | 51 | Group 1 (n=51): Early
dressing removal,wound left open 48 h after surgery. Group 2
(n=51): Delayed dressing removal, dressing changed every 48 h until
suture removal. | 36 years Group A=26
years Group B=27.5 years |
| 3 | Mendes et al,
2018(16) | Brazil | Randomized controlled
trial | Breast augmentation
surgery | 40 | 38 | Group 1 (n=40): Early
dressing removal, wound left open 24 h after surgery. | Group 2 (n=38):
Delayed dressing removal, ressing left for 6 postoperative
days. |
| 4. | Nesrallah et
al, 2017(17) | USA | Randomized controlled
trial | Caesarean
section | 300 | 302 | Group 1 (n=300):
Early dressing removal, wound left open 12-30 h after surgery.
Group 2 (n=302): Delayed dressing removal, dressing left for 30-48
h after surgery | Not provided |
| 5 | Peleg et al,
2016(18) | USA | Randomized controlled
trial | Caesarean
section | 160 | 160 | Group 1 (n=160):
Early dressing removal, wound left open 6 h after surgeryGroup 2
(n=160): Delayed dressing removal, dressing left for 24 h after
surgery | Group A=32.9 years
Group B=31.6 years |
| 6 | Ramkumar et
al, 2006(19) | UK | Randomized
controlled trial | Unilateral or
bilateral primary correction of prominent ears | 39 | 39 | Group 1 (n=39):
Early dressing removal, head bandage was removed the next day.
Group 2 (n=39): Delayed dressing removal, head bandage was removed
after 10 days. Both groups received Tubigrip bandage at night-time
for 4-6 weeks | 10 years |
| 7 | Ritting et
al, 2012(20) | USA | Randomized
controlled trial | Mini-open carpal
tunnel release | 45 | 49 | Group 1 (n=45):
Early dressing removal, within 48-72 h. Group 2 (n=49): Delayed
dressing removal, after 2 weeks. | Group A = 46.3
years Group B = 44.8 years |
| 8 | Veiga et al,
2016(21) | Brazil | Randomized
controlled trial | Breast
reconstruction surgery | 94 | 92 | Group 1 (n=94):
Early dressing removal, within 24 h. Group 2 (n=92): Delayed
dressing removal, after 6 days. | Group A = 47.8
years Group B = 49.3 years |
| 9 | Veiga-Filho et
al, 2012(22) | Brazil | Randomized
controlled trial | Reduction
mammoplasty | 35 | 35 | Group 1 (n=94):
Early dressing removal, within 24 hours Group 2 (n=92): Delayed
dressing removal, after 6 days. | 34 years |
| 10 | Wipke-Tevis and
Stotts, 1998(23) | USA | Randomized
controlled trial | Coronary artery
bypass graft surgery with saphenous vein grafts | 39 | 39 | Group 1 (n=39):
Early dressing removal, wound left open 24 h after surgery Group 2
(n=39): Delayed dressing removal, dressing remained in place until
removal of sutures. | 62 years |
Methodological quality of the included
studies
Assessment of risk of bias is done for RCTs
(Table II). Most of the studies had
low risk of bias with respect to bias arising from randomization
process (random sequence generation and allocation concealment).
All included studies had either high or unclear risk of bias with
respect to blinding of participants. Three of 10 included studies
had low risk of bias with respect to blinding of outcome
assessment. All studies had high or unclear risk of bias with
respect to incomplete outcome data except Peleg et al
(18). All studies had high or
unclear risk of bias with respect to selective reporting of outcome
except Ramkumar et al (19).
 | Table IIRisk of bias assessment for the
included studies, n=10. |
Table II
Risk of bias assessment for the
included studies, n=10.
| No | Author year,
(Ref) | Random sequence
generation | Allocation
concealment | Blinding of the
participants | Blinding of outcome
assessment | Incomplete outcome
data | Selective reporting
of outcome |
|---|
| 1 | Ajao 1997,
(15) | Unclear risk | Unclear risk | High risk | Unclear risk | Unclear risk | High risk |
| 2 | Dosseh et
al, 2008(13) | Unclear risk | Unclear risk | High risk | Unclear risk | High risk | High risk |
| 3 | Mendes et
al, 2018(16) | Low risk | Low risk | Unclear risk | Unclear risk | High risk | Unclear risk |
| 4 | Nesrallah et
al, 2017(17) | Unclear risk | Unclear risk | High risk | High risk | Unclear risk | Unclear risk |
| 5 | Peleg et al,
2016(18) | Low risk | Low risk | High risk | High risk | Low risk | Unclear risk |
| 6 | Ramkumar et
al, 2006(19) | Unclear risk | Unclear risk | High risk | Unclear risk | High risk | Low risk |
| 7 | Ritting et
al, 2012(20) | Low risk | Unclear risk | High risk | Low risk | High risk | Unclear risk |
| 8 | Veiga et al,
2016(21) | Low risk | Low risk | High risk | Low risk | High risk | Unclear risk |
| 9 | Veiga-Filho et
al, 2012(22) | Low risk | Low risk | High risk | Low risk | High risk | Unclear risk |
| 10 | Wipke-Tevis and
Stotts, 1998(23) | Low risk | Low risk | High risk | Unclear risk | High risk | High risk |
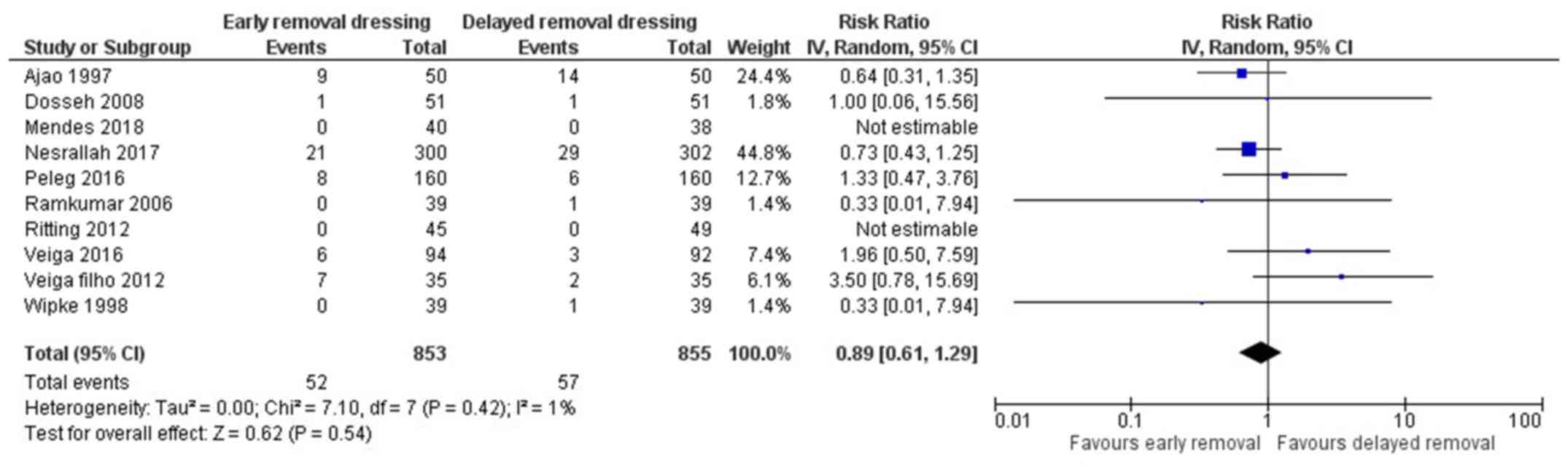
Surgical site infection (SSI)
All 10 included studies reported on the surgical
site infection in both arms (13,15-23).
The pooled RR was 0.89 (95% CI: 0.61 to 1.29) (Fig. 2). This indicates that the patients
having early removal of dressing have 11% less risk of having SSI
when compared to patients having delayed removal of dressing
following surgery. However, this association was not statistically
significant (P=0.54). There was no heterogeneity among the studies
reporting surgical site infections (I2=1%, P=0.42).
Funnel plot showed a symmetrical plot indicating absence of
publication bias (Fig. 3). Egger's
test also confirmed the finding with P-value of 0.30 indicating the
absence of small study effects.
Wound dehiscence
Four studies have reported on the wound dehiscence
in both the arms (13,18-20).
The pooled RR was 1.45 (95% CI: 0.56 to 3.75) (Fig. 4). This indicates that the patients
having early removal of dressing have 1.45-fold higher risk of
having wound dehiscence when compared to patients having delayed
removal of dressing following surgery. However, this association
was not statistically significant (P=0.44). There was no
heterogeneity among the studies reporting the wound dehiscence
(I2=0%, P=0.66).
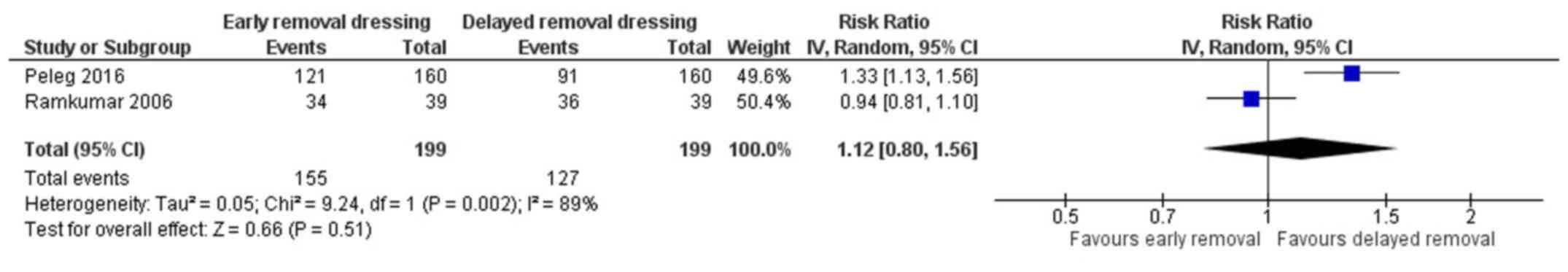
Patient satisfaction
Two studies have reported on the patient
satisfaction in both the arms (18,19). The
pooled RR was 1.12 (95% CI: 0.80 to 1.56) (Fig. 5). This indicates that the patients
having early removal of dressing have 1.12 times higher chance of
being satisfied when compared to patients having delayed removal of
dressing following surgery. However, this association was not
statistically significant (P=0.51). There was a significant
heterogeneity among the studies reporting patient satisfaction
(I2=89%, P=0.002).
Patient's perception on safety
Two studies have reported on the patient's
perception on safety following early or delayed removal of dressing
for surgical wounds (21,22). The pooled RR was 0.60 (95% CI: 0.48
to 0.76) (Fig. 6). This indicates
that the patients having early removal of dressing have 40% higher
perception of being safe when compared to patients having delayed
removal of dressing following surgery. This association was found
to be statistically significant (P<0.001). There was no
heterogeneity among the studies reporting patient's perception on
safety (I2=14%, P=0.28).
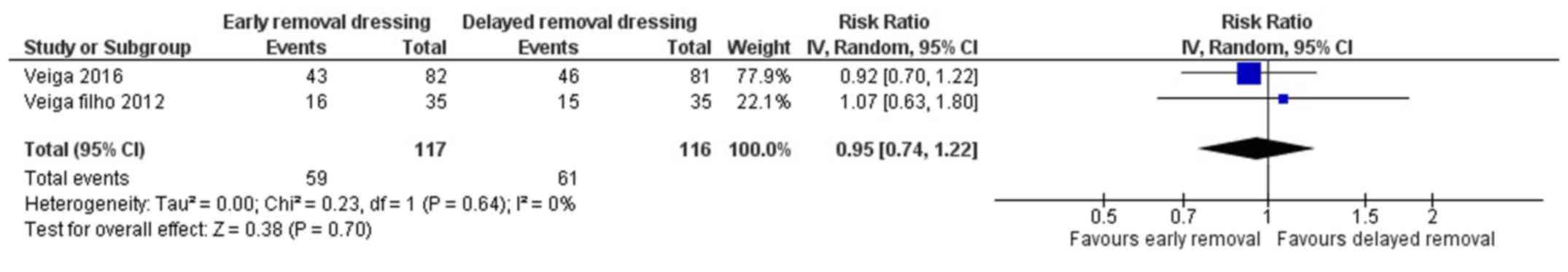
Patient's perception on comfort
Two studies have reported on the patient's
perception on comfort following early or delayed removal of
dressing for surgical wounds (21,22). The
pooled RR was 0.95 (95% CI: 0.74 to 1.22) (Fig. 7). This shows that there is no
significant difference in patient's perception on comfort following
early or delayed removal of dressing (P=0.70). There was no
heterogeneity among the studies reporting patient's perception on
comfort (I2=0%, P=0.64).
Patient's perception on
convenience
Two studies have reported on the patient's
perception on convenience following early or delayed removal of
dressing for surgical wounds (21,22). The
pooled RR was 1.14 (95% CI: 0.83 to 1.57) (Fig. 8). This shows that there is no
significant difference in patient's perception on convenience
following early or delayed removal of dressing (P=0.43). There was
moderate heterogeneity among the studies reporting patient's
perception on convenience (I2=37%, P=0.21).
Discussion
Dressings put on surgical wounds following primary
closure can be removed early or delayed (retained) until the suture
is removed or strips. However, we could not find any review
comprehensively assessing the effect of early or delayed removal of
dressing following surgery in reducing surgical site infection,
wound dehiscence or patient’s satisfaction or perception. Thus,
this review was conducted with an objective of comparing the early
with delayed removal of dressing following primary closure of
surgical wound in terms of clinical and quality outcomes among
patients undergoing surgery. We tried to compile the best possible
evidence currently available.
In total, we identified 10 studies with 1,708
participants for our analysis. All the included studies were RCTs.
The majority of the studies were conducted in countries of the
American regions including the USA and Brazil. Most of the included
studies had high or unclear risk of bias with respect to all the
domains except randomization process domains. We did not find any
substantial heterogeneity for most of the outcomes in the studies
except studies reporting patient satisfaction. Nonetheless, we did
not have an adequate number of studies to perform a subgroup
analysis or meta-regression to explore the source of heterogeneity
for the studies reporting patient satisfaction.
Clinical outcome such as SSI and patient perception
on safety and comfort favoured the early dressing removal arm while
outcomes such as wound dehiscence, patient satisfaction and patient
perception on convenience favoured the delayed dressing removal
arm. However, conclusive or significant evidence was found only for
patient perception on safety which favoured early removal of
dressing. For all other outcomes, we did not find conclusive or
significant evidence for any of these outcomes as the confidence
limit crossed the null value in all the outcomes assessed. This
shows that timing of removal of dressing following surgical wound
does not have significant impact on clinical outcomes or patient
perception or satisfaction. One similar review was conducted before
on this topic, a meta-analysis by Toon et al which compared
early and delayed removal of dressing, reported almost similar
findings to our review (24).
However, the previous review included only 4 trials and outcomes
related to patient satisfaction and patient perception were not
assessed. The present review includes 10 studies and reported these
additional outcomes. This should be useful in making better
decisions and judgement in choosing the timing of removal of
dressing for the patients following primary closure of surgical
wounds.
The major strengths of our study include the
comprehensive search of literature and the broad search strategy to
gather all the required publications up-to-date. Our review adds to
the limited evidence available on direct comparison of the early
and delayed removal of dressing for the management of patients with
postoperative surgical wounds. We only included RCTs in our review
which enables us to infer causal associations between intervention
and outcomes. We also included patient's perception and
satisfaction as this may provide added advantage while taking
informed decisions on timing of removal of dressing for surgical
wounds. We also assessed publication bias for the main outcome on
SSI and found almost symmetrical funnel plot. Nevertheless, there
are limitations to our review. We could not assess the source of
heterogeneity for outcomes that showed significant heterogeneity
due to the limited number of studies. Finally, most of the studies
included in our review were conducted in American regions, which
may limit the generalizability of our findings to other
geographical regions.
Our study has certain implications towards clinical
practice. There is a sense of uncertainty and inconsistency
revolving around the timing of removal of dressing following
primary closure in surgery. Our study may be useful in overcoming
this sense of inconsistency and across the findings and provided a
reliable pooled estimate for the same. We found that delayed
removal of dressing did not have significantly better clinical
outcomes when compared to early removal of dressing in the
management of surgical wounds. Early removal of dressing from
clean/clean contaminated surgical wounds seems to have no negative
effect on postoperative patients. Furthermore, patients seem to
have the perception that early removal of dressing is safer when
compared to delayed removal. However, these findings are based on
studies with high or unclear risk of bias and can be applied to
patients with surgical wounds closed by primary intention. In
addition, the surgical wound healing process is related to the type
of dressing applied (25). Evidences
have shown that honey dressing has significantly faster wound
healing compared to any other type of dressings (26,27).
Thus, the nature and type of dressing should also be taken into
account before deciding the timing of removal of dressings for any
surgical wounds. Application of these findings to patients with
accidental injuries or delayed primary closure is unclear. In
addition, none of the studies have reported on health-related
quality of life of the patients. Thus, further high-quality trials
with a focus on quality of life component have to be conducted in
the future.
In conclusion, delayed removal of dressing is not
superior to early removal following primary closure of clean or
clean-contaminated surgical wounds. However, more robust RCTs with
large sample size are required to derive conclusive evidence
towards health-related quality of life and applicability of these
findings for delayed primary closure or accidental injuries.
Acknowledgements
Not applicable
Funding
Not applicable
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ conceived and designed the study. TZ, FZ, ZC and
XC collected the data and performed the literature search. TZ wrote
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
García-Gubern CF, Colon-Rolon L and Bond
MC: Essential concepts of wound management. Emerg Med Clin North
Am. 28:951–967. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ahn HB, Shin DM, Roh MS, Jeung WJ, Park WC
and Rho SH: A comparison of 2-octyl cyanoacrylate adhesives versus
conventional suture materials for eyelid wound closure in rabbits.
Korean J Ophthalmol. 25:121–127. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Biancari F and Tiozzo V: Staples versus
sutures for closing leg wounds after vein graft harvesting for
coronary artery bypass surgery. Cochrane Database Syst Rev.
5(CD008057)2010.doi: 10.1002/14651858.CD008057. PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hasan Z, Gangopadhyay AN, Gupta DK,
Srivastava P and Sharma SP: Sutureless skin closure with isoamyl
2-cyanoacrylate in pediatric day-care surgery. Pediatr Surg Int.
25:1123–1125. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lawrence WT: Physiology of the acute
wound. Clin Plast Surg. 25:321–340. 1998.PubMed/NCBI
|
|
6
|
Hutchinson JJ and Lawrence JC: Wound
infection under occlusive dressings. J Hosp Infect. 17:83–94.
1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mertz PM, Marshall DA and Eaglstein WH:
Occlusive wound dressings to prevent bacterial invasion and wound
infection. J Am Acad Dermatol. 12:662–668. 1985.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ubbink DT, Vermeulen H, Goossens A, Kelner
RB, Schreuder SM and Lubbers MJ: Occlusive vs gauze dressings for
local wound care in surgical patients: A randomized clinical trial.
Arch Surg. 143:950–955. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dyson M, Young S, Pendle CL, Webster DF
and Lang SM: Comparison of the effects of moist and dry conditions
on dermal repair. J Invest Dermatol. 91:434–439. 1988.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cutting KF and White RJ: Maceration of the
skin and wound bed. 1: Its nature and causes. J Wound Care.
11:275–278. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Merei JM: Pediatric clean surgical wounds:
Is dressing necessary? J Pediatr Surg. 39:1871–1873.
2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chrintz H, Vibits H, Cordtz TO, Harreby
JS, Waaddegaard P and Larsen SO: Need for surgical wound dressing.
Br J Surg. 76:204–205. 1989.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dosseh Eacute Koué D, Doleaglenou A,
Fortey YK and Ayite AE: Randomized trial comparing dressing to no
dressing of surgical wounds in a tropical setting. J Chir (Paris).
145:143–146. 2008.(In French). PubMed/NCBI
|
|
14
|
Gwosdow AR, Cunningham JJ, Lydon M,
Rascati R and Berglund LG: Evaporative water losses through a
temporary wound dressing under simulated wound conditions. J Burn
Care Rehabil. 14:450–454. 1993.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ajao OG: Surgical wound infection: A
comparison between dressed and undressed wounds. J Trop Med Hyg.
80:192–196. 1977.PubMed/NCBI
|
|
16
|
Mendes DA, Veiga DF, Veiga-Filho J, Loyola
ABAT, Paiva LF, Novo NF, Sabino-Neto M and Ferreira LM: Influence
of dressing application time after breast augmentation on cutaneous
colonization: A randomized clinical trial. J Plast Reconstr Aesthet
Surg. 71:906–912. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nesrallah M, Cole P and Kiley K: The
effect of timing of removal of wound dressing on surgical site
infection rate after cesarean delivery. Obstet Gynecol.
129:148–149. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Peleg D, Eberstark E, Warsof SL, Cohen N
and Ben Shachar I: Early wound dressing removal after scheduled
cesarean delivery: A randomized controlled trial. Am J Obstet
Gynecol. 215(388.e1-388.e5)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ramkumar S, Narayanan V and Laing JHE:
Twenty-four hours or 10 days? A prospective randomised controlled
trial in children comparing head bandages following pinnaplasty. J
Plast Reconstr Aesthet Surg. 59:969–974. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ritting AW, Leger R, O'Malley MP,
Mogielnicki H, Tucker R and Rodner CM: Duration of postoperative
dressing after mini-open carpal tunnel release: A prospective,
randomized trial. J Hand Surg Am. 37:3–8. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Veiga DF, Damasceno CAV, Veiga-Filho J,
Paiva LF, Fonseca FE, Cabral IV, Pinto NL, Juliano Y and Ferreira
LM: Dressing wear time after breast reconstruction: A randomized
clinical trial. PLoS One. 11(e0166356)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Veiga-Filho J, Veiga DF, Sabino-Neto M,
Damasceno CA, Sales EM, Garcia ES, Oliveira IB, De Simoni LF,
Juliano Y and Ferreira LM: Dressing wear time after reduction
mammaplasty: A randomized controlled trial. Plast Reconstr Surg.
129(1e-7e)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wipke-Tevis DD and Stotts NA: Effect of
dressings on saphenous vein harvest incision pain, distress and
cosmetic result. Prog Cardiovasc Nurs. 13:3–13. 1998.PubMed/NCBI
|
|
24
|
Toon CD, Lusuku C, Ramamoorthy R, Davidson
BR and Gurusamy KS: Early versus delayed dressing removal after
primary closure of clean and clean-contaminated surgical wounds.
Cochrane Database Syst Rev. 3(CD010259)2015.doi:
10.1002/14651858.CD010259. PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sood A, Granick MS and Tomaselli NL: Wound
Dressings and Comparative Effectiveness Data. Adv Wound Care.
8:511–529. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gethin G and Cowman S: Manuka honey vs.
hydrogel -a prospective, open label, multicentre, randomized
controlled trial to compare desloughing efficacy and healing
outcomes in venous ulcers. J Clin Nurs. 18:466–474. 2009.
|
|
27
|
Gupta SS, Singh O, Bhagel PS, et al: Honey
dressings versus silver sulfadiazine dressing for wound healing in
burn patients: A retrospective study. J Cutan Aesthet Surg.
4:183–187. 2011.PubMed/NCBI View Article : Google Scholar
|