Introduction
Coronary heart disease (CHD) is the most common
cause of cardiovascular associated death worldwide. Acute
myocardial infarction (AMI) is a critical and severe condition,
which can occur following CHD, and exhibits the second highest
mortality rate in the disease spectrum. The incidence of AMI has
increased, with treatment options including thrombolysis and
interventional therapy to restore blood perfusion, which can
prevent mortality in patients with AMI. However, the presence of
myocardial ischemia-reperfusion injury (MIR/I) affects the
prognosis of these patients (1). The
pathological mechanism of MIR/I is complex. However, oxidative
stress, secondary myocardial cell apoptosis and abnormal cardiac
function have been revealed to be associated with MIR/I
pathophysiology (2).
Numerous studies have demonstrated that MIR/I is
associated with the generation of reactive oxygen species (ROS),
which impair the structure and function of the cell membrane
(3,4). Phospholipids are rich in
polyunsaturated fatty acids and constitute an essential part of
cell membranes. Phospholipids are also susceptible to ROS and may
result in a decrease in membrane phospholipids, increased membrane
cholesterol and ratio of cholesterol/phosphate, thereby reducing
membrane fluidity (5). Decreased
membrane fluidity can affect membrane proteins via cross-linking or
polymerization, promoting membrane damage (6,7). An
imbalance between the production of free radicals during MIR/I and
the inability to counteract or detoxify their harmful effects
results in inflammation and oxidative stress (8).
The microbiota has been revealed to protect innate
immunity and decrease inflammation and oxidative stress (9). Previous studies have demonstrated that
bacterial preparations serve protective functions against
inflammation (10,11). Chai et al (12) reported that inactivated pseudomonas
aeruginosa (IPA) attenuated pulmonary hypertension, right ventricle
hypertrophy and pulmonary vascular remodeling in rats by
restraining the hypoxia-induced overactive transforming growth
factor-β1/Smad signaling (12). The
current study aimed to investigate the role of IPA in
anti-oxidative stress, endothelial protection and the subsequent
protective effects of IPA against MIR/I.
Materials and methods
Animal models
A total of 40 specific pathogen-free male
Sprague-Dawley rats (weight, 250-300 g) were equally divided into
five groups. Rats were provided by The Experimental Animal Center
of General Hospital of Central Theater Command (Wuhan, China). The
current study was performed in strict accordance with the
recommendations in The Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health of China. The protocol
was also approved by the Institutional Animal Care and Use
Committees of Wuhan Army General Hospital (permit no.
SCXK-20080047). Rats were provided with food and water ad
libitum, 12-h light/dark cycle and constant temperature (25˚C)
and humidity (50%). Rats were acclimatized to these conditions for
~1 week. All rats were then divided into five groups of 8 rats
under the same housing conditions: A control group, a model group,
a MIR/I+IPA (low; 106 cfu/kg) group, a MIR/I+IPA
(medium; 107 cfu/kg) group and a MIR/I+IPA (high;
108 cfu/kg) group. Rats were then anaesthetized with 2%
sodium pentobarbital (40 mg/kg; intraperitoneal injection) and
fixed on an operating Table in supine position with rubber bands to
fix limbs. The trachea was separated and tracheal intubation was
performed, whilst connected to a DHX-150 animal ventilator (Chengdu
Instrument Factory). The respiratory rate was adjusted to 60-80/min
and ventilation volume to 20 ml/kg. The chest was opened 3 mm away
from left margin of the chest bone and the heart was exposed.
Ligation of the left anterior descending coronary artery was
performed for 30 min. This was then released for 2 h. This surgical
protocol was performed on all of model and the three IPA treatment
groups. Sham surgery with no coronary artery ligation was performed
in the control group. IPA was intravenously administered once per
day for 1 week prior to the surgery in the groups with various IPA
treatments. An equal volume of 0.5% saline was administered to the
control and model groups once per day for a week. Model reliability
was assessed by continuously monitoring rat electrocardiograms. ST
segment elevation was considered to indicate the presence of
myocardial ischemia, with 1/2 drop of ST indicating reperfusion
success. The heart ejection fraction (EF), fractional shortening
(FS) and left ventricle inner diameter at systole (LVIDs) were
obtained using Philips iU22 Color Doppler Ultrasound Diagnostic
Instrument (Philips Healthcare) prior to and after coronary artery
ligation. A total of 25% of the rats (n=2) were euthanized with
sodium pentobarbital following ligation procedure owing to acute
heart failure or malignant arrhythmia (13).
Determination of serum and heart
catalase (CAT), superoxide dismutase (SOD), lactate dehydrogenase
(LDH) and malondialdehyde (MDA) protein expression
After reperfusion, blood was extracted from the
femoral artery of all groups and left for 30 min. To obtain serum,
blood was subsequently centrifuged at 300 x g for 15 min. Total
protein was extracted from myocardial tissues of the infarct area
using RIPA buffer according to the manufacturer's protocol (Beijing
Dingguo Changsheng Biotechnology Co., Ltd.) and protein
concentrations were determined using the bicinchoninic acid method.
CAT (cat. no. A007), LDH (cat. no. M002), SOD (cat. no. A001-2)
activities and MDA (cat. no. A003-2) contents were determined using
kits in accordance with the manufacturer's protocol (Nanjing
Jiancheng Bioengineering Institute).
Myocardial infarction area
After sacrifice, rat hearts were obtained and rinsed
in PBS buffer. Ventricular tissues were subsequently obtained and
half of the ventricular tissues were cut into five sections of
equal thickness (2-3 mm). The pieces were added into 0.1% NBT
solution (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) at
37˚C for 15 min. Normal myocardium was indicated by a blue color,
while the infarcted myocardium was colorless. Digital camera
imaging and ImagePro plus 7.0 analysis software (Media Cybernetics,
Inc.) were used to calculate the ventricular infarction area and
ventricular total area. The myocardial infarction rate was the
ratio of infarcted myocardium area compared with the total
myocardium area of five sections (%).
Apoptosis assay
Myocardial tissues from the infarct area were
collected by digestion with a pancreatic enzyme. Cells were then
resuspended in PBS at a concentration of 1x106 cells/ml.
Apoptosis was assessed via Annexin V-FITC and propidium iodide (PI)
staining using the Annexin V-FITC/PI Apoptosis Detection kit
(Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol followed by flow cytometry analysis, as previously
described (14). A total of
1x105 cells were added to a 5 ml culture tube and 5 µl
Annexin V-FITC and 10 µl of PI were added. The tube was vortexed
and incubated for 15 min at room temperature in the dark. A total
of 400 µl 1X binding buffer was added to each tube. The stained
cells were analyzed using flow cytometry (BD Accuri C6 cytometer
and BD Accuri C6 Software; BD Biosciences).
Western blot analysis
The total protein from infarct areas of myocardial
tissues was extracted using RIPA buffer. A total of 75 µg protein
was mixed with 5X loading buffer and separated using SDS-PAGE. The
protein was then transferred to PVDF membranes and incubated with
5% skim milk powder at 37˚C for 1 h and rabbit anti-rat GAPDH (cat.
no. BM1623; 1:1,000), nuclear factor erythroid 2-related factor 2
(Nrf2; cat. no. AB76026; 1:500) and heme oxygenase 1 (HO-1; cat.
no. M00253-2; 1:500) antibodies at 4˚C overnight (all from Boster
Biological Technology). After washing with TBST three times, the
membrane was incubated with horseradish peroxidase-labeled goat
anti-rabbit secondary antibodies (cat. no. BA1056; 1:1,000; Boster
Biological Technology) at room temperature for 1 h. Signals were
subsequently detected using ECL-0012 chemiluminescence detection
kit (Beijing Dingguo Changsheng Biotechnology Co., Ltd.) and
analyzed using a ChemiDocXRS chemiluminescence imaging system
(Bio-Rad Laboratories, Inc). Densitometry analysis was performed
using Quantity One software (version 4.6.2; Bio-Rad Laboratories,
Inc.).
Immunofluorescence
Myocardial tissues from the infarct area were
collected by digestion with pancreatic enzyme. The cells were fixed
with 4% paraformaldehyde for 15 min at room temperature,
permeabilized by 3% H2O2 for 10 min, blocked
with normal goat serum (Wuhan Boster Biological Technology, Ltd.)
for 20 min at 37˚C, and then incubated with rabbit anti-rat Nrf2
antibodies (cat. no. AB76026; Boster Biological Technology)
overnight at 4˚C and washed with 0.2% Tween 20/PBS prior to and
following incubation with goat anti-rabbit secondary antibodies,
Cy3 conjugate (cat. no. BA1032; Boster Biological Technology) for 1
h at room temperature. The sections were counterstained with DAPI
(100 ng/ml) at room temperature for 15 min. The slides were sealed
with the anti-fluorescence quenching agent (cat. no. AR1109; Wuhan
Boster Biological Technology, Ltd.). and subsequently analyzed
using fluorescence microscopy (magnification, x200). The integral
optical density values of Nrf2 were measured using the CMIAS-8
color pathological image analysis system (purchased from Institute
of Biological Engineering, PLA Air Force General Hospital).
Statistical analysis
SPSS 15.0 (SPSS, Inc.) software was used for
statistical analysis. The experimental data are presented as the
mean ± standard deviation (x±s). Statistical comparisons were
performed using a Student's t-test for comparison between two
groups and a one-way ANOVA for comparison among multiple groups,
followed by a Duncan's multiple range test. P<0.05 was
considered to indicate a statistically significant result.
Results
IPA alleviates myocardial infarction
and inhibits myocardial apoptosis
EF, FS and LVIDs were examined prior to and after
coronary artery ligation. As presented in Table I, EFs and FS in the IPA group were
significantly higher compared with the model group. However, LVID
values in all IPA treatment groups revealed a significant reduction
compared with the model group. No statistical differences were
exhibited among groups treated with different concentrations of
IPA.
 | Table IEchocardiographic analysis. |
Table I
Echocardiographic analysis.
| Groups | EF (%) | FS (%) | LVIDs (mm) |
|---|
| Control | 72.44±3.65 | 41.56±2.87 | 3.92±0.68 |
| MIR/I |
41.23±3.21a |
15.32±3.16a |
6.23±0.57a |
| MIR/I +106
IPA |
56.37±4.67b |
25.57±2.65b |
4.93±0.89b |
| MIR/I
+107 IPA |
55.78±3.09b |
28.62±1.98b |
4.42±0.53b |
| MIR/I
+108 IPA |
58.79±4.98b |
28.49±2.62b |
4.11±0.25b |
The results demonstrated that the myocardial
infarction area in the model group was 33.67±4.53% and that this
was reduced to 30.07±2.99% in MIR/I+IPA (low), 14.88±3.12% in
MIR/I+IPA (medium) and 18.69±3.41% in MIR/I+IPA (high) groups
(Fig. 1A). The results presented in
Fig. 1B demonstrated that apoptosis
was significantly increased in the model group compared with the
control group while apoptosis in all IPA groups were decreased
significantly. Moreover, IPA treatments of 107 and
108 cfu/kg exhibited better protective effects against
myocardial infarction and apoptosis than IPA treatment of
106 cfu/kg.
IPA reduces oxidative stress
After 30 min of myocardial ischemia and 2 h of
reperfusion, the level of MDA and LDH in the serum and myocardial
tissues of the model group increased significantly and the
activities of CAT and SOD decreased significantly compared with the
control group. Compared with the model group, all IPA groups
revealed a significant decrease in serum MDA and LDH at various
concentrations (Fig. 2A). However,
in myocardial tissues of infarct area, only IPA at 106
and 107 cfu/kg exhibited a significant reduction
(Fig. 2B; P<0.05). Furthermore,
serum CAT significantly increased in all IPA treatment groups and
SOD activities significantly increased at 106 cfu/kg in
serum (Fig. 2A). However, CAT and
SOD only increased at 106 and 107 cfu/kg IPA
in myocardial tissues of infarct area compared with the model group
(Fig. 2B).
 | Figure 2Activities of MDA, LDH, CAT and SOD in
serum and in myocardial tissues assayed using biochemical kits. (A)
The activities of MDA, LDH, CAT and SOD in serum. (B) The
activities of MDA, LDH, CAT and SOD in myocardial tissues. n=6 for
model group/n=8 for IPA groups. **P<0.01 and
*P<0.05 vs. the control; #P<0.05 vs.
MIR/I. MDA, malondialdehyde; LDH, lactate dehydrogenase; CAT,
catalase; SOD, superoxide dismutase; MIR/I. MIR/I, myocardial
ischemia reperfusion injury; IPA, pseudomonas
aeruginosa. |
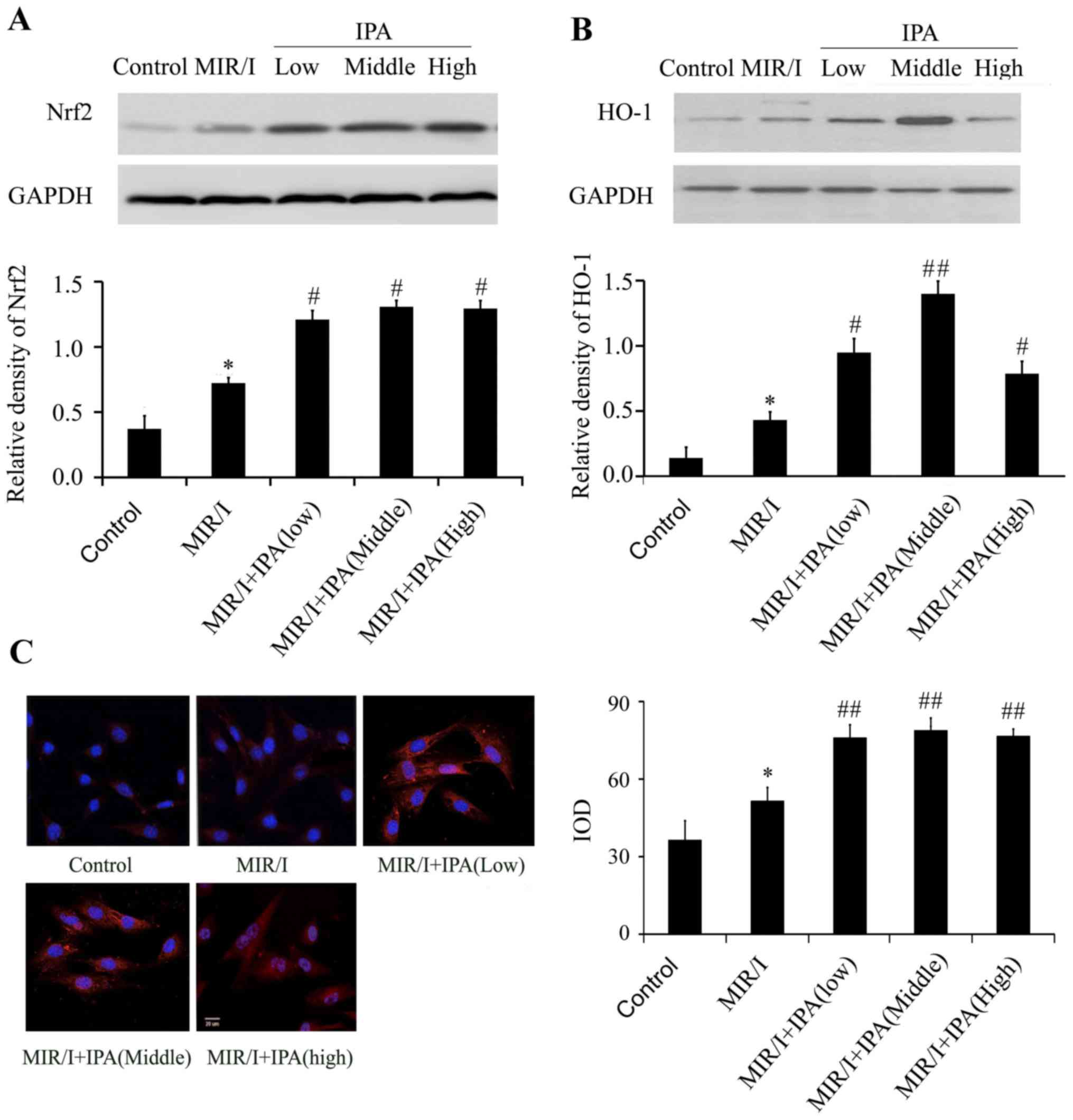
IPA increases the expression of Nrf2
and HO-1
When compared with the control group, the model
group Nrf2 protein expression increased significantly (P<0.05;
Fig. 3A), and nuclear translocation
of Nrf2 also increased (Fig. 3C).
Compared with the model group, Nrf2 protein expression in all IPA
groups significantly increased (Fig.
3A), and Nrf2 nuclear translocation also increased (Fig. 3C). No significant differences in Nrf2
protein expression levels and nuclear translocation were determined
among groups treated with different concentrations of IPA. HO-1
protein expression in the model group significantly increased
compared with the control group (Fig.
3B). Compared with the model group, HO-1 protein expression in
all treatment groups significantly increased (Fig. 3B) in all IPA treatment groups and IPA
at 107 exhibited stronger promoting effects.
Discussion
CHD is currently more prevalent in the elderly,
causing increased rates of morbidity and mortality rates among
these patients and implicating the use of medical resources in CHD
treatment (15). Repeated MIRI can
cause further damage to the ischemic myocardium (16). A number of studies have suggested
that the excessive generation of ROS serves a central role in
myocardial injury (17,18). A free radical scavenging system
exists, which includes antioxidant enzymes such as SOD, peroxidase,
CAT and antioxidants including vitamins C and E (19). The excessive production of free
radicals, particularly ROS, often impairs the structure and
function of cells (20). Although
the use of antioxidants in the treatment of MIR/I has been the
focus of a variety of studies (21),
an effective, clinically useful treatment is yet to be
determined.
Myocardial apoptosis intervention could be used in
the recovery of MIR/I (22,23). The present study demonstrated that
during MIR/I, EFs and FS decreased and LVID and apoptosis
increased. IPA treatments of 107 and 108
cfu/kg exhibited increased protective effects and improved heart
function, endothelial cell injury and apoptosis. These results are
consistent with a previous report, which demonstrated that IPA
promoted cell proliferation and inhibited apoptosis in bronchial
epithelial cells (7). Inactivated
IPA at treatments of 107 and 108 cfu/kg
exhibited increased protective effects on heart function and anti
apoptotic effects compared with IPA treatment of 106
cfu/kg.
MDA and LDH, which are biological products of ROS
and lipid peroxidation, indicate the degree of lipid peroxidation
in the body and subsequently indirectly reflect the degree of
myocardial injury (24). SOD and CAT
are the main antioxidant enzymes responsible for the mobilization
of free radicals (25). The present
study revealed that IPA decreased LDH and MDA levels and increased
SOD and CAT activities in myocardial tissues and serum samples,
confirming the myocardial protection of IPA through the inhibition
of lipid peroxidation and increased ROS removal. However, these
results demonstrated that, in myocardial tissues of infarct area,
IPA at 106 and 107 cfu/kg exhibited increase
effects against oxidative stress. Oxidative stress refers to the
process of oxidative damage caused by excessive ROS production,
reduced scavenging capacity and an imbalance between the oxidation
and antioxidant system (26). Nrf2
is an important transcription factor that is associated with
regulating oxidative stress response. In oxidative stress, Nrf2 is
translocated into the nucleus and binds with the antioxidant
response element, initiating the transcription of detoxifying
enzymes and antioxidant enzyme gene expression, including HO-1,
which protects the body from ROS (27,28). The
continuous expression of HO-1 in endothelial cells can reduce cell
inflammatory damage and apoptosis that is caused by ischemia
(29,30). Therefore, Nrf2 and HO-1 expression
indicate the protective effects against cell injury. Inactivated
IPA can significantly promote Nrf2 and HO-1 expression in rats
exhibiting MIR/I, indicating that IPA induces protection against
apoptosis and oxidative stress.
IPA treatments of 107 and 108
cfu/kg in the current study exhibited an increased protective
effect against myocardial damage and apoptosis. IPA at
106 and 107 cfu/kg exhibited an increased
effect against oxidative stress. Although IPA at 107 and
108 cfu/kg exhibited a stronger effect on the promotion
of Nfr2 and HO-1, the activation of Nrf2 and HO-1 expression began
to decrease at 108 cfu/kg. Combined with the results of
anti-apoptosis and antioxidant effects, the highest protective
effect of IPA was exhibited by the 107 cfu/kg treatment
group. IPA exhibited a protective effect in the concentration range
that was selected in the current study. However, further studies
are still required to further investigate the effective treatment
doses and detailed underlying mechanisms of IPA protection against
MIR/I.
In conclusion, IPA pretreatment improves heart
function, reduces endothelial apoptosis and oxidative stress, which
is induced by MIR/I, via the Nrf2/HO-1 pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by Key Project of
Hubei Natural Science Foundation (grant. no. 2014C FA066).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZZ, ZT, WZ and JL performed the experiments. BL
designed the current study and performed statistical analysis. ZZ
drafted the manuscript for critical and intellectual content. SD
made substantial contributions to study conception and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments and procedures were approved
by The Institutional Animal Care and Use Committees of Wuhan Army
General Hospital (permit no. SCXK-20080047).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lønborg JT: Targeting reperfusion injury
in the era of primary percutaneous coronary intervention: Hope or
hype? Heart. 101:1612–1618. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Park ES, Kang DH, Kang JC, Jang YC, Lee
MJ, Chung HJ, Yi KY, Kim DE, Kim B and Shin HS: Cardioprotective
effect of KR-33889, a novel PARP inhibitor, against oxidative
stress-induced apoptosis in H9c2 cells and isolated rat hearts.
Arch Pharm Res. 40:640–654. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang P, Lu Y, Yu D, Zhang D and Hu W:
TRAP1 provides protection against myocardial ischemia-reperfusion
injury by ameliorating mitochondrial dysfunction. Cell Physiol
Biochem. 36:2072–2082. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu L, Tan JL, Wang ZH, Chen YX, Gao L, Liu
JL, Shi YH, Endoh M and Yang HT: ROS generated during early
reperfusion contribute to intermittent hypobaric hypoxia-afforded
cardioprotection against postischemia-induced Ca(2+) overload and
contractile dysfunction via the JAK2/STAT3 pathway. J Mol Cell
Cardiol. 81:150–161. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Silva LND, Pessoa MTC, Alves SLG,
Venugopal J, Cortes VF, Santos HL, Villar JAFP and Barbosa LA:
Differences of lipid membrane modulation and oxidative stress by
digoxin and 21-benzylidene digoxin. Exp Cell Res. 359:291–298.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
White CR, Giordano S and Anantharamaiah
GM: High-density lipoprotein, mitochondrial dysfunction and cell
survival mechanisms. Chem Phys Lipids. 199:161–169. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ross T, Szczepanek K, Bowler E, Hu Y,
Larner A, Lesnefsky EJ and Chen Q: Reverse electron flow-mediated
ROS generation in ischemia-damaged mitochondria: Role of complex I
inhibition vs. depolarization of inner mitochondrial membrane.
Biochim Biophys Acta. 1830:4537–4542. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu X, Zhang L, Qin H, Han X, Zhang Z,
Zhang Z, Qin SY and Niu J: Inhibition of TRAF3 expression
alleviates cardiac ischemia reperfusion (IR) injury: A mechanism
involving in apoptosis, inflammation and oxidative stress. Biochem
Biophys Res Commun. 506:298–305. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang L, Wu G, Qin X, Ma Q, Zhou Y, Liu S
and Tan Y: Expression of Nodal on bronchial epithelial cells
influenced by lung microbes through DNA methylation modulates the
differentiation of T-helper cells. Cell Physiol Biochem.
37:2012–2022. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tan Y, Liu H, Yang H, Wang L and Qin X: An
inactivated Pseudomonas aeruginosa medicament inhibits airway
allergic inflammation and improves epithelial functions. J Physiol
Sci. 63:63–69. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kerber-Momot T, Leemhuis D, Lührmann A,
Munder A, Tümmler B, Pabst R and Tschernig T: Beneficial effects of
TLR-2/6 ligation in pulmonary bacterial infection and immunization
with Pseudomonas aeruginosa. Inflammation. 33:58–64.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chai SD, Liu T, Dong MF, Li ZK, Tang PZ,
Wang JT and Ma SJ: Inactivated Pseudomonas aeruginosa inhibits
hypoxia-induced pulmonary hypertension by preventing TGF-β1/Smad
signaling. Braz J Med Biol Res. 49(e5526)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hua Y, Chen H, Zhao X, Liu M, Jin W, Yan
W, Wu Y, Tan Z, Fan H, Wu Y, et al: Alda-1, an aldehyde
dehydrogenase-2 agonist, improves long-term survival in rats with
chronic heart failure following myocardial infarction. Mol Med Rep.
18:3159–3166. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li L, Liu M, Kang L, Li Y, Dai Z, Wang B,
Liu S, Chen L, Tan Y and Wu G: HHEX: A crosstalker between HCMV
infection and proliferation of VSMCs. Front Cell Infect Microbiol.
6(169)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hodzic E: Potential anti-inflammatory
treatment of ischemic heart disease. Med Arch. 72:94–98.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wojcik M, Burzynska-Pedziwiatr I and
Wozniak LA: A review of natural and synthetic antioxidants
important for health and longevity. Curr Med Chem. 17:3262–3288.
2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou Z, Zhang S, Ding S, Abudupataer M,
Zhang Z, Zhu X, Zhang W, Zou Y, Yang X, Ge J and Hong T: Excessive
neutrophil extracellular trap formation aggravates acute myocardial
infarction injury in apolipoprotein E deficiency mice via the
ROS-dependent pathway. Oxid Med Cell Longev.
2019(1209307)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schriewer JM, Peek CB, Bass J and
Schumacker PT: ROS-mediated PARP activity undermines mitochondrial
function after permeability transition pore opening during
myocardial ischemia-reperfusion. J Am Heart Assoc.
2(e000159)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Huang Z, Zhuang X, Xie C, Hu X, Dong X,
Guo Y, Li S and Liao X: Exogenous hydrogen sulfide attenuates high
glucose-induced cardiotoxicity by inhibiting NLRP3 inflammasome
activation by suppressing TLR4/NF-κB pathway in H9c2 cells. Cell
Physiol Biochem. 40:1578–1590. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gao S, Yang Z, Shi R, Xu D, Li H, Xia Z,
Wu QP, Yao S, Wang T and Yuan S: Diabetes blocks the
cardioprotective effects of sevoflurane postconditioning by
impairing Nrf2/Brg1/HO-1 signaling. Eur J Pharmacol. 779:111–121.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Venardos KM, Perkins A, Headrick J and
Kaye DM: Myocardial ischemia-reperfusion injury, antioxidant enzyme
systems, and selenium: A review. Curr Med Chem. 14:1539–1549.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun J, Yu X, Huangpu H and Yao F:
Ginsenoside Rb3 protects cardiomyocytes against
hypoxia/reoxygenation injury via activating the antioxidation
signaling pathway of PERK/Nrf2/HMOX1. Biomed Pharmacother.
109:254–261. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Qian W, Wang Z, Xu T and Li D:
Anti-apoptotic effects and mechanisms of salvianolic acid A on
cardiomyocytes in ischemia-reperfusion injury. Histol Histopathol.
34:223–231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu X, Yu Z, Huang X, Gao Y, Wang X, Gu J
and Xue S: Peroxisome proliferator-activated receptor γ (PPARγ)
mediates the protective effect of quercetin against myocardial
ischemia-reperfusion injury via suppressing the NF-κB pathway. Am J
Transl Res. 8:5169–5186. 2016.PubMed/NCBI
|
|
25
|
Charlagorla P, Liu J, Patel M, Rushbrook
JI and Zhang M: Loss of plasma membrane integrity, complement
response and formation of reactive oxygen species during early
myocardial ischemia/reperfusion. Mol Immunol. 56:507–512.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tejero J, Shiva S and Gladwin MT: Sources
of vascular nitric oxide and reactive oxygen species and their
regulation. Physiol Rev. 99:311–379. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cheng L, Jin Z, Zhao R, Ren K, Deng C and
Yu S: Resveratrol attenuates inflammation and oxidative stress
induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE
pathway. Int J Clin Exp Med. 8:10420–10428. 2015.PubMed/NCBI
|
|
28
|
Tong G, Zhang B, Zhou X, Zhao J, Sun Z,
Tao Y, Pei J and Zhang W: Kappa-opioid agonist U50,488H-mediated
protection against heart failure following myocardial
ischemia/reperfusion: Dual roles of heme oxygenase-1. Cell Physiol
Biochem. 39:2158–2172. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zuidema MY, Peyton KJ, Fay WP, Durante W
and Korthuis RJ: Antecedent hydrogen sulfide elicits an
anti-inflammatory phenotype in postischemic murine small intestine:
Role of heme oxygenase-1. Am J Physiol Heart Circ Physiol.
301:H888–H894. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jazwa A, Stepniewski J, Zamykal M,
Jagodzinska J, Meloni M, Emanueli C, Jozkowicz A and Dulak J:
Pre-emptive hypoxia-regulated HO-1 gene therapy improves
post-ischaemic limb perfusion and tissue regeneration in mice.
Cardiovasc Res. 97:115–124. 2013.PubMed/NCBI View Article : Google Scholar
|