Introduction
Sepsis is a systemic inflammatory response syndrome
caused by infection with clinical confirmation or a suspected
bacterial infection reaching the blood (1). Sepsis is a serious social problem
threatening human health and life. The global incidence of sepsis
is 1.5-8.0% per year, and the mortality rate can reach 30-70%
(2,3). At present, to the best of the authors'
knowledge, there has been no fundamental breakthrough in the
treatment of sepsis because the underlying mechanisms of sepsis are
still unclear. Evidence has shown that immunosuppression plays an
important role in sepsis (4). The
initial immune response of patients with sepsis is triggered by a
high inflammatory state (a large number of cytokines are produced),
but the high inflammatory state soon changes to an
immunosuppressive state (4). The
immunosuppressive state can increase the likelihood of a secondary
opportunistic infection or reactivate the latent infection
(5,6). Therefore, restoring the
immunosuppressive state of patients plays an important role in the
treatment of sepsis.
The maintenance of immune suppression involves the
participation of various immunoregulatory cells. In many cells with
an immunomodulatory function, regulatory T cells (Tregs) have been
shown to be crucial in maintaining immune balance and tolerance.
Tregs are a relatively early cell to appear with immunosuppressive
function (7,8). Tregs have been demonstrated to be
associated with the pathogenesis, prognosis and drug treatment of
patients with sepsis (9,10). Wan (9)
have demonstrated that in the early stages of sepsis, there is a
significant proportion of abnormal Treg cells, which are mainly
manifested as an increase in proportion and enhancement of
immunosuppressive function. Huang et al (11) identified a significant increase in
the proportion of CD39+ Tregs in the peripheral blood of
patients with sepsis. The increase in the proportion of
CD39+ Tregs in the peripheral blood of patients with
sepsis was closely related to prognosis (11). Shao et al (12) demonstrated that drug therapy can play
a therapeutic role by inhibiting the function of CD4+
CD25+ Tregs. In addition to Tregs, regulatory B cells
have also been demonstrated to play an important role in the
pathogenesis of neonatal sepsis (10).
γδ T cells are the main effector cells involved in
the innate immune response of the host, and are the bridge
connecting innate immunity and adaptive immunity. γδ T cells appear
early in the immune response and efficiently produce inflammatory
cytokines, such as interferon-γ (IFN-γ) and tumor necrosis factor
(TNF) (13). It has been observed in
literature that γδ T cells can inhibit the differentiation of Tregs
by secreting the soluble cytokine IFN-γ and increase the
transformation of antigen-specific Treg cells (14). γδ T cells have been documented to be
associated with disease activity and survival in patients with
sepsis (15). γδ T cells can be
further divided into two types of cell subtypes, in vivo Vδ1
T cells and Vδ2 T cells. These two cell subtypes have different
functions; specifically, Vδ1 T cells have an immunosuppressive
function and participate in the immune escape process of tumors;
while Vδ2 T cells are inflammatory cells and inhibit tumor
occurrence (16-19).
Therefore, the functional changes of Vδ1 T cells in patients with
sepsis may be consistent with Tregs, but further data are required
to verify the changes in Vδ2 T cells in patients with sepsis. The
changes in total γδ T cells in patients with sepsis, and the
changes in Vδ1 and Vδ2 T cells were observed to provide new insight
for the study of sepsis.
Patients and methods
Patients
Between December 2016 and December 2017, 30 patients
with sepsis (14 patients with sepsis, 9 patients with severe sepsis
and 7 patients with septic shock) and 30 healthy control (HC)
patients at the same time were enrolled from the intensive care
unit of Yueqing People's Hospital.
The inclusion criteria were as follows: Patients
aged >18 years and met the sepsis diagnostic criteria
established by The International Conference on Sepsis in
Washington, DC in December 2001(20). The following were exclusion criteria:
Autoimmune diseases, acute stroke, myocardial infarction, viral
hepatitis, HIV infection and use of hormone or immunosuppressive
agents in March before admission. The age and sex of patients with
sepsis matched the data of the HCs (P>0.05). The Sequential
Organ Failure Assessment (SOFA) score, which can dynamically
reflect changes in organ function, was assessed for the patients
(20). The daily difference score
was taken daily; the higher the score, the worse the prognosis. The
SOFA score is based on each indicator in the SOFA score table. The
detailed clinical data of patients with sepsis are presented in
Table I. All patients signed
informed consent. The present study was approved by The Ethics
Committee of Yueqing People's Hospital.
 | Table IClinical characteristics of
patients. |
Table I
Clinical characteristics of
patients.
| Characteristic | Healthy controls | Patients with
sepsis |
|---|
| Number | 30 | 30 |
| Age, year | 39.4.2±13.7 | 38.9±14.6 |
| Sex,
male/female | 15/15 | 16/14 |
| SOFA score | - | 11.5±4.0 |
| Source of
infection | | |
|
Lung | - | 15 (50.0%) |
|
Abdomen | - | 6 (20%) |
|
Urinary
tract infection | - | 1 (3.3%) |
|
Pathogen | - | 8 (26.7%) |
|
Gram-negative
bacillus | - | 16.1±6.7 |
|
Gram-positive
bacillus | - | 5.5±4.8 |
|
Fungus | - | 10 (33.3%) |
|
Negative | - | 28.2±9.1 |
|
White blood
cell count, x109/l | - | 56.7% (17/13) |
|
PCT,
ng/ml | 30 | 30 |
|
Mechanical
ventilation | 39.4.2±13.7 | 38.9±14.6 |
|
Renal
transplantation | 15/15 | 16/14 |
|
ICU
hospitalization, days | - | 11.5±4.0 |
|
Mortality,
survival/death | - | - |
Main reagents
The reagents and sources used were as follows:
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) and FBS
(Gibco; Thermo Fisher Scientific, Inc.); FITC-anti human T cell
receptor (TCR) Vδ1 antibody (1:1.000; cat. no. 331208; BioLegend,
Inc.), phycoerythrin (PE)-anti-human-CD3 antibody (1:1,000; cat.
no. 300408; BioLegend, Inc.), FITC-anti human TCR Vδ2 antibody
(1:1,000; cat. no. 331410; BioLegend, Inc.), allophycocyanin
(APC)-anti-human cytotoxic T-lymphocyte-associated protein 4
(CTLA-4) antibody (1:1,000; cat. no. 369612; BioLegend, Inc.),
Pecy5-anti-human T cell immunoglobulin and mucin-domain
containing-3 (TIM-3) antibody (1:1,000; cat. no. 318308; BioLegend,
Inc.); CellTrace™ carboxyfluorescein succinimidyl ester
(CFSE) cell proliferation kit (Invitrogen; Thermo Fisher
Scientific, Inc.); amplification of purified anti-human CD3
antibody (1:1,000; cat. no. 561806; BD Biosciences); and purified
anti-human CD28 antibody (1:1,000; cat. no. 553295; BD
Biosciences); purified anti-human Vδ1 antibody (1:1,000; cat. no.
B49309; Beckman Coulter, Inc.); anti-human phosphorylated (p)-Erk
antibody (1:1,000; cat. no. 4370; Cell Signaling Technology, Inc.)
and HRP goat anti-rat secondary antibody (1:1,000; cat. no. 7077;
Cell Signaling Technology, Inc.); and Supersignal West Femto/Pico
HRP sensitive chemiluminescence substrate (Saiser World Science and
Technology Co., Ltd.).
Peripheral blood mononuclear cell
acquisition and flow cytometry staining
Peripheral blood mononuclear cells (PBMCs) were
extracted from the patients and healthy subjects as previously
described (21). The concentration
of PBMCs in the RPMI-1640 medium containing 10% FBS was
1x107 (10 µl PBMC in an Eppendorf tube, 3 µl PE-anti-CD3
antibody, 3 µl FITC-anti-TCR Vδ1 antibody/3 µl FITC-anti-TCR Vδ2
antibody, and 3 µl APC-anti-human CTLA-4 antibody/3 µl
Pecy5-Pecy5-anti-human TIM-3 antibody). The solution was incubated
at 4˚C for 30 min. After two passages of RPMI-1640 culture with 10%
FBS, the cells were suspended in 0.1 ml 4% polyformaldehyde fixing
solution at room temperature for 10 min and examined by flow
cytometry. Cell surface antigens were evaluated by flow cytometry
with a FACSCalibur flow cytometer or BD LSRFortessa (BD
Biosciences). Data were analyzed using FlowJo 7.6.1 (Tree Star,
Inc).
Peripheral blood Vδ1T cell surface
immunosuppressive molecules (CTLA-4 and TIM-3) expression and Vδ2T
cell inflammatory factors (IFN-γ and TNF-α) secretion
detection
The concentration of PBMCs in RPMI-1640 medium
containing 10% FBS was 1x107 (10 µl PBMC was obtained
from an Eppendorf tube and 1 ml RPMI-1640 medium containing 10% FBS
was added). In the Eppendorf tube, Cell Activation Cocktail (cat.
no. 423304; BioLegend, Inc,) was added for incubation for 6 h at
37˚C with 5% CO2. The cells were collected, and the
advanced cell surface molecules were stained with Vδ2 TCR at 4˚C
for 30 min, then 0.5 ml cell membrane was added to immobilize the
permeation fluid to suspend the cells, while avoiding light for 30
min at the room temperature. The cells were twice-washed with
permeation liquid, and the cells were suspended in 0.1 ml 4%
paraformaldehyde fixative at room temperature for 10 min and
detected by flow cytometry, then 5 µl IFN-γ antibody/TNF-α antibody
was added while avoiding light for 30 min at room temperature. The
cells were twice-washed in 0.1 ml 1% paraformaldehyde fixative,
then detected by flow cytometry.
When Vδ1T cells were used to inhibit the secretion
of cytokines in Vδ2T cells, the two cell subtypes were co-cultured
for 72 h at a 1:5 ratio, then the aforementioned steps were
repeated.
Detection of CFSE cell
proliferation
The cells were washed once with 10 ml serum-free
RPMI-1640 medium, followed by CFSE dye at a final concentration of
5 mmol/l, and placed in an incubator containing 5% CO2
at 37˚C for 10 min. Then, 5 ml pre-cooled CFSE staining terminator
was immediately added to the cells, including 5% FBS RPMI-1640, and
placed on ice for 5 min to stop the dyeing. The cells were then
centrifuged at 400 x g for 8 min at room temperature, and washed
with 10 ml RPMI-1640 medium. After the cells were suspended with
RPMI-1640 complete medium, the Vδ1 and naïve CD4 T cells were added
at a 1:5 ratio into a 48-well plate containing 1 µg/ml CD3 antibody
and 2 µg/ml CD28 antibody. After 5 days of incubation, the cells
were harvested, and the cell suspension was added to a 5 ml flow
tube, 100 µl per tube, blocked with 5% BSA at room temperature for
1 h, and the dead cells were removed, and 1:500 dilutions of
FITC-anti human TCR Vδ1 antibody and FITC-anti human TCR Vδ2
antibody was added, gently mixed, and incubated at 4˚C for 30 min
in the dark. The cells were then centrifuged (350 x g, 4˚C, 5 min)
and washed twice with PBS. The supernatant was discarded; the cells
were resuspended and tested by flow cytometry.
Western blotting detection of p-Erk
expression in Vδ2 T cells
Vδ2 T cells with purity >90% were obtained by
sorting, and after 0, 5, 10 and 15 min stimulation, they were
placed in an Eppendorf tube containing RIPA buffer (cat. no. 9806;
Cell Signaling Technology, Inc.), 1% PMSF solution was added to
prevent protein degradation, homogenized, left to stand for 3 h,
centrifuged (400 x g; 5 min; 4˚C) and then the supernatant was
removed for dispensing. The protein concentration of the extracted
samples was determined using a BCA kit. A total of 30 µg
protein/lane was separated by SDS-PAGE on 8% gels. The separated
proteins were subsequently transferred to PDVF membranes and
incubated for 2 h at room temperature in 5% BSA. Erk antibody
(1:500; cat. no. 9102; Cell Signaling Technology, Inc) and
anti-p-Erk antibody (1:500; cat. no. 4370; Cell Signaling
Technology, Inc) was added and incubated overnight at 4˚C. The next
day, the membrane was washed three times with TBS with 0.1%
Tween-20 (TBST; 5 min each time) and the corresponding goat
anti-mouse HRP-labeled secondary antibody (1:1,000; cat. no. 7077;
Cell Signaling Technology, Inc.) was added and incubated at room
temperature for 1 h. After washing in 0.1% TBST, the Supersignal
West Femto/Pico HRP-sensitive chemiluminescent substrate was used
to color the bands. Actin was used as an internal reference. ImageJ
software (v2.1.4.7; National Institutes of Health) scans the image
to yield gray values that reflect the intensity of protein
expression.
Statistical analysis
SPSS 16.0 (SPSS, Inc.) was used to analyze the
data. Counting data are presented as percentages and
measurement data are presented as the mean ± SD. Experiments were
repeated five times. A t-test was used to compare the measurement
data between the two groups. Comparisons of experimental groups
were evaluated by one-way ANOVA, followed by Bonferroni analysis.
Spearman rank correlation analysis was used to determine the
correlation between the ratio of Vδ1 and Vδ2 T cells in the
peripheral blood of patients with sepsis and patient condition.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Detection of peripheral blood γδ T
cells and the different subtypes
The proportion of γδ T cells (Fig. 1A-a and b) in the peripheral blood of the HCs was
6.01±1.42% compared with 5.81±1.94% in patients with sepsis.
Compared with the HCs, the proportion of peripheral blood γδ T
cells in patients with sepsis did not significantly change
(P>0.05). The proportion of Vδ1 T cells (Fig. 1B-a and b) in the peripheral blood of the HC group
was 1.33±1.19% and the proportion of Vδ1 T cells in the peripheral
blood of patients with sepsis was 4.22±2.38%. Compared with the
HCs, the proportion of Vδ1 T cells in the peripheral blood of the
patients with sepsis was significantly increased (P<0.01). The
proportion of Vδ2 T cells (Fig. 1C-a
and b) in the peripheral blood of
the HC group was 4.65±1.67%, and the proportion of Vδ2 T cells in
the peripheral blood of the patients with sepsis was 1.94±1.15%.
Compared with the HCs, the proportion of Vδ2 T cells in the
peripheral blood of the patients with sepsis was significantly
decreased (P<0.01).
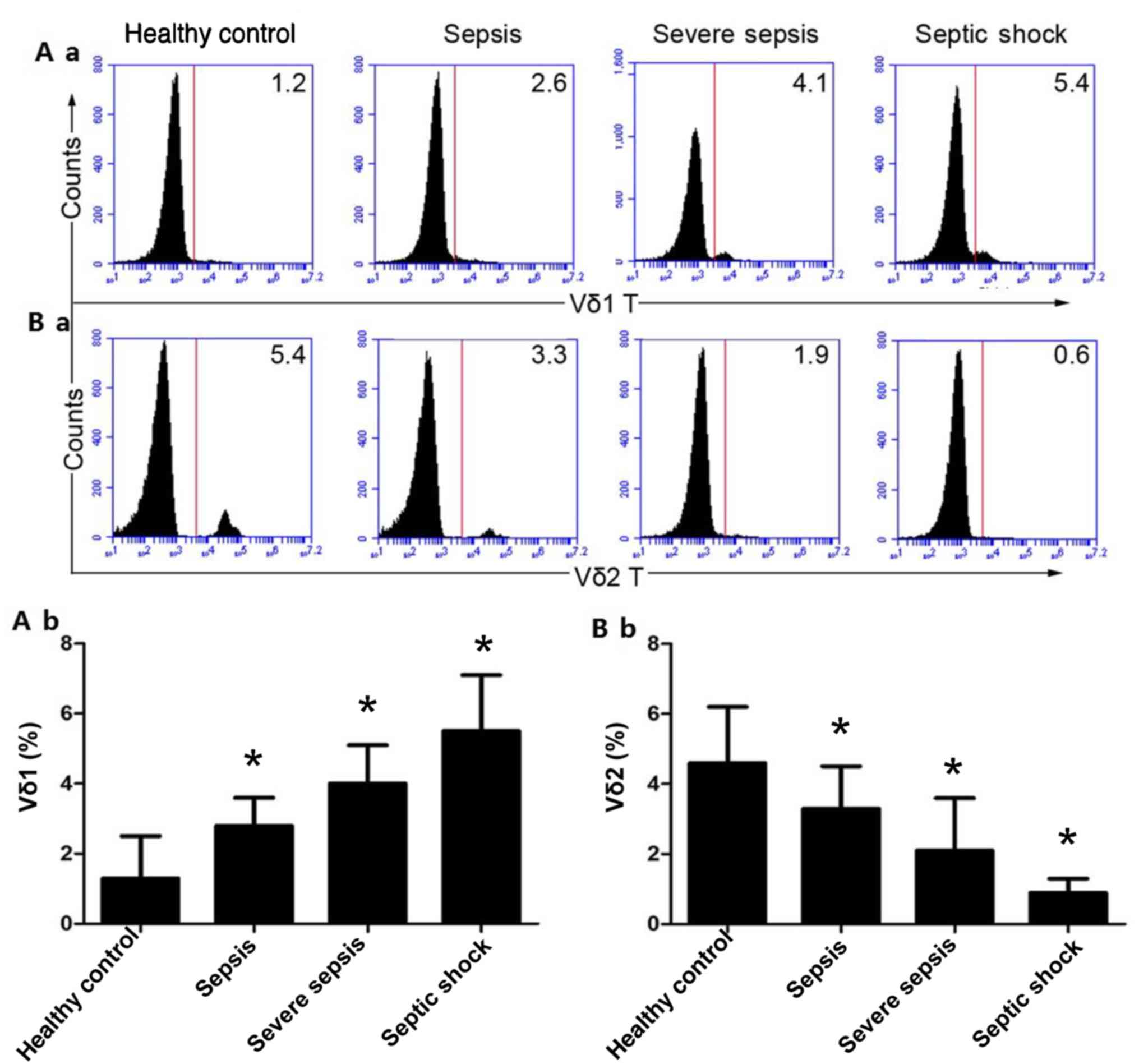
Proportion of Vδ1 and Vδ2 T cells in
different types of patients with sepsis
The proportion of Vδ1 T cells in the peripheral
blood of the HCs was 1.33±1.19%. The proportion of Vδ1 T cells in
the peripheral blood of patients with mild sepsis was 2.84±0.81%,
and the proportion of Vδ1 T cells in the peripheral blood of severe
sepsis patients was 4.04±1.19%, while the percentage of Vδ1 T cells
in the peripheral blood of the septic shock group was 5.51±1.65%.
In addition, with the exacerbation of sepsis, the proportion of Vδ1
T cells in the peripheral blood increased gradually (P<0.01;
Fig. 2A-a and b). The proportion of Vδ2 T cells in the
peripheral blood of the HCs was 4.65±1.67%, and the proportion of
Vδ2 T cells in the peripheral blood of patients with mild sepsis
was 3.34±1.28%. The proportion of Vδ2 T cells in the peripheral
blood of patients with severe sepsis was 2.09±1.54%, and the
proportion of Vδ2 T cells in the peripheral blood of patients in
the septic shock group was 0.92±1.38%. Moreover, with the
exacerbation of sepsis, the proportion of Vδ2T cells in the
peripheral blood decreased gradually (P<0.01; Fig. 2B-a and b).
Correlation between the ratio of V δ1
and Vδ 2 T cells in the peripheral blood of patients with sepsis
and patient condition
The proportion of Vδ1 T cells (Fig. 3A-a) in patients with sepsis was
positively correlated with the SOFA score (r=0.4535; P<0.05) and
the proportion of Vδ2 T cells (Fig.
3B-a) was negatively correlated with the SOFA score (r=-0.3629;
P<0.05). A higher Vδ1 T cell ratio correlated with lower patient
survival rate (Fig. 3A-b), while a
lower Vδ2 T cell ratio correlated with lower patient survival rate
(Fig. 3B-b).
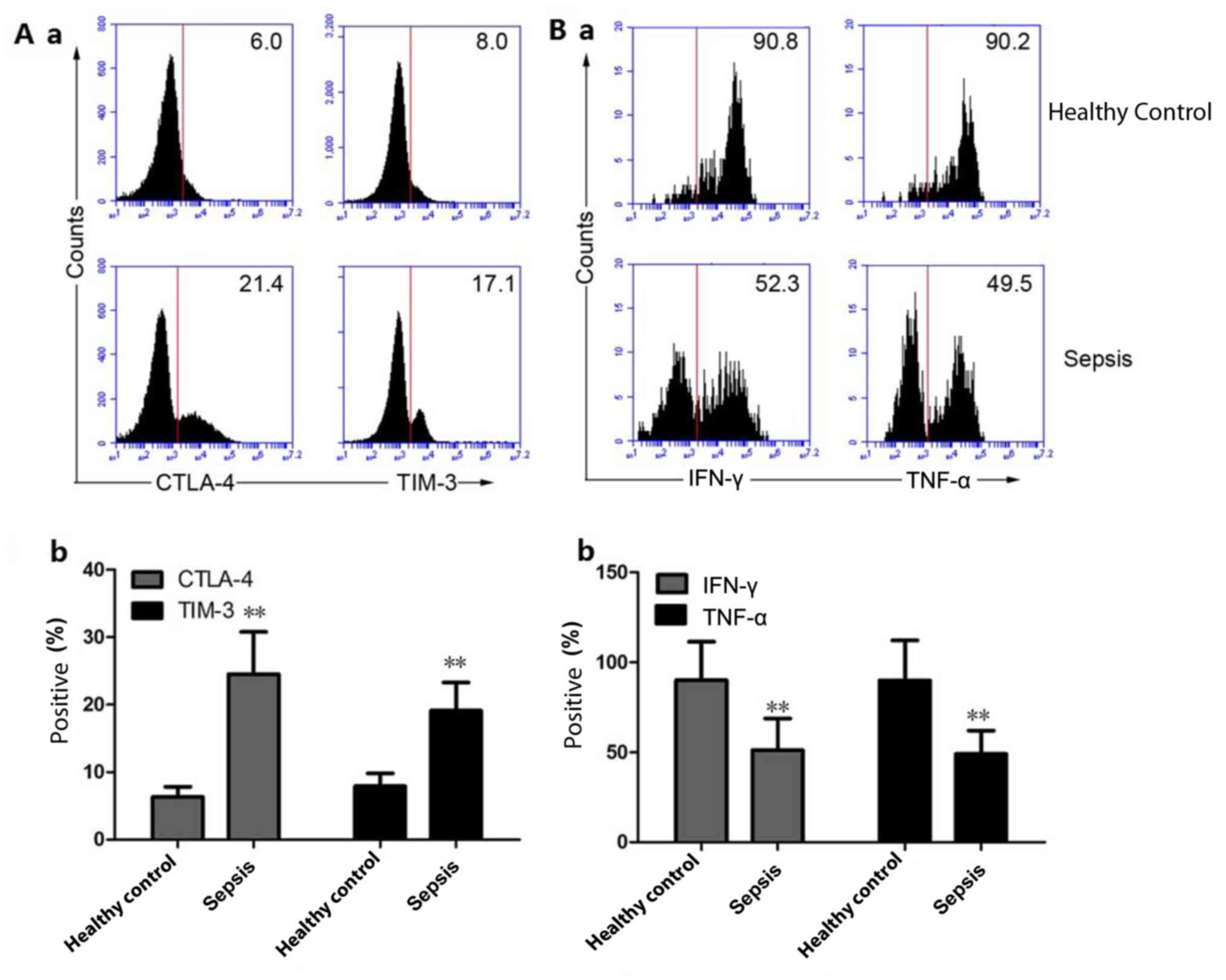
Expression of immunosuppressive
molecules on Vδ1 T cells and secretion of inflammatory cytokines
from Vδ2 T cells
The percentage of CTLA-4- and TIM-3-positive Vδ1 T
cells in the peripheral blood of the HC group was 6.32±1.52 and
7.78±1.91%, respectively, while the percentage of CTLA-4- and
TIM-3-positive Vδ1 T cells in the peripheral blood of patients with
sepsis was 24.8±6.31 and 19.1±4.19%, respectively. Compared with
the HCs, the percentage of CTLA-4- and TIM-3-positive Vδ1 T cells
in the peripheral blood of patients with sepsis increased
significantly (P<0.01; Fig. 4A-a
and b). The percentage of IFN-γ and
TNF-α positive Vδ2 T cells in the peripheral blood of the HC group
was 90.1±21.3 and 89.9±22.3%, respectively, and the percentage of
IFN-γ and TNF-α Vδ2 T cells in the peripheral blood of patients
with sepsis was 51.2±17.6 and 49.1±12.9%, respectively. Compared
with the HC group, the percentage of IFN-γ and TNF-α positive Vδ2 T
cells in the peripheral blood of patients with sepsis was
significantly decreased (P<0.01; Fig.
4B-a and b).
Functional detection of Vδ1 and Vδ2 T
cells
The level of CD4 T cell proliferation in the
peripheral blood of the healthy control Vδ1 T and Vδ2 T cell
co-incubation group was 90.1±18.5%. The level of CD4 T cell
proliferation in the peripheral blood of the healthy control Vδ1 T
and Vδ2 T cell co-incubation group was 67.9±16.4%, and the level of
Vδ1 and CD4 T cell proliferation in the peripheral blood of
patients with sepsis was 45.3±14.3% (Fig. 5A-a and b). The IFN-γ secretory capacity of Vδ2 T
cells in the peripheral blood of the healthy control Vδ1 T and Vδ2
T cell co-incubation group was 88.5±20.6%, and the IFN-γ secretion
ability of Vδ2 T cells was 60.3±17.5% after incubation of Vδ1 T
cells and Vδ2 T cells in the healthy control Vδ1 T and Vδ2 T cell
co-incubation group. The IFN-γ secretory ability of Vδ1 T and Vδ2 T
cells in the peripheral blood of patients with sepsis was
41.8±14.6% (Fig. 5B-a and b). These results suggested that the
immunosuppressive function of peripheral blood Vδ1 T cells in
patients with sepsis was significantly higher than the HCs
(P<0.01). The western blotting results showed that the level of
Erk signaling pathway phosphorylation associated with Vδ2 T cells
in patients with sepsis was significantly lower than the control
group (P<0.01; Fig. 5C-a and
b).
 | Figure 5Functional detection of Vδ1 and Vδ2 T
cells. (A-a) Peripheral blood CD4 T proliferation was detected
using CFSE staining by flow cytometry. (A-b) Quantitative analysis
of proliferation rate. **P<0.01 vs. HC Vδ1 T cells +
HC CD4 T cells. (B-a) IFN-γ secretion was detected by flow
cytometry. (B-b) Quantitative analysis of IFN-γ-positive cells.
**P<0.01 vs. HC Vδ1 T cells + HC CD4 T cells. (C-a)
Western blotting was used to detect Erk and p-Erk1/2 expression.
Actin was used as an internal reference protein. (C-b) Quantitative
analysis of p-Erk1/2 expression. **P<0.01 vs. the
respective HC group. IFN-γ, interferon-γ; p, phosphorylated; CFSE,
carboxyfluorescein succinimidyl ester; HC, healthy control; a, HC
CD4 T cells; b, HC Vδ1 T cells + HC CD4 T cells; c, sepsis Vδ1 T
cells + HC CD4 cells; i, HC Vδ2 T cells; ii, HC Vδ1 T cells + HC
Vδ2 T cells; iii, sepsis Vδ1 T cells + HC Vδ2 T cells. |
Discussion
Even with standard treatment, sepsis remains a major
cause of death worldwide. One of the reasons for the lack of
effective treatment for sepsis is the complexity and incomplete
understanding of the underlying mechanism of sepsis. In sepsis, the
body destroys the immune homeostasis by inducing an initial strong
systemic inflammatory response, followed by a rapid negative
feedback of the modern compensatory anti-inflammatory response, and
decreased function of T cells (22).
γδ T cells are a group of T cells expressing the γδ chain, which
accounts for 0.5-5% of T cells in peripheral blood, and play an
important role in anti-infection and immune regulation (23). The present study identified that the
number of Vδ1 T and Vδ2 T cells in the peripheral blood of patients
with sepsis. Vδ1 T cells in patients with sepsis was significantly
increased compared with the healthy controls, and the expression
rate was highest in the septic shock group. The proportion of Vδ1 T
cells was positively correlated with the SOFA score.
Andreu-Ballester et al (24) demonstrated that the percentage of
peripheral blood γδ T cells is reduced in patients with sepsis. The
present results showed that the proportion of γδ T cells in the
peripheral blood of patients with sepsis did not change
significantly, which is in contrast from previously reported
results (24). Analysis of the
difference between the present study and the literature revealed
that the average age of the patients in the literature was 66.3
years, while the average age of the patients in the present study
was 38.9 years. Another previous study published by
Andreu-Ballester et al (25)
identified that there is a correlation between the number of γδ T
cells in the peripheral blood and age, and the proportion of γδ T
cells in the peripheral blood decreased with age. Therefore, it was
hypothesized that the age difference is the basis for the
differences in the study results, which suggested that the number
of γδ T cells in the peripheral blood of patients with sepsis in
different ages should also be further investigated in the future.
CD3 staining was additionally conducted; the present study first
measured γδ T cell levels, followed by measurement of γδ T cell
levels. This may also be the reason for the difference between the
two studies.
A review by Wan (9)
stated that in the early stages of sepsis, Tregs showed significant
abnormal proportions. Specifically, there was an increase in the
proportion and enhancement of immunosuppressive function (9). The present study also detected changes
in the proportion and function of Vδ1 T cells in patients with
sepsis, and showed that the proportion of Vδ1 T cells was
increased. The level of Vδ1 T cell expression in patients with
sepsis was also higher than the controls, while the immunization
inhibition test also confirmed that the function of Vδ1 T cells was
enhanced. This finding is consistent with the changes in the number
and function of Tregs cells reported in patients with sepsis.
Previous studies reported that Treg cells exert an
immunosuppressive function via two pathways (26,27):
Direct cell contact, mainly through the expression of related
immunosuppressive receptors; and secreting cytokines with
immunosuppressive functions, such as IL-10 and TGF-β. It has been
demonstrated that Vδ1 T cells play an immunosuppressive role mainly
through cell contact, and play an important role in the
pathogenesis of systemic lupus erythematous (28). In the present study, it was detected
that the level of Vδ1 T cell expression was associated with contact
immunosuppression, and it was demonstrated that the expression of
Vδ1 T cells in the peripheral blood of patients with sepsis was
increased. A limitation of the present study is that it was not
possible to study the immunosuppressive effect of Vδ1 T cells in
patients with sepsis.
CTLA-4 plays an important role in inhibiting T cell
activation and maintaining immune tolerance. Moderate regulation of
CTLA-4 expression can balance the inhibition signal and the
stimulation signal to enhance protective immunity reaction
(29). TIM-3 is a key molecule that
regulates the T cell immune response and is involved in the
induction of immune tolerance, and its sustained expression leads
to depletion of T cell function (30). T cells regulate immune responses
mainly by secreting INF-γ and TNF-α (30). The results of the present study
showed that the expression of CTLA-4 and TIM-3 on the surface of
Vδ1 T cells in peripheral blood of patients with sepsis was
significantly increased, and the levels of IFN-γ and TNF-α secreted
by Vδ2 T cells were significantly decreased. The immunosuppressive
function of Vδ1 T cells was significantly enhanced, and the
function of Vδ2 T cells was significantly reduced.
Erk1/2 is an important member of the Erk family.
When members of the Erk family are activated by signals such as the
external environment or cytokines, they can transmit signals to the
nucleus to regulate biological behavior, such as cell proliferation
and differentiation (31). The
Erk1/2 signaling pathway plays a significant regulatory role in the
activation of γδ T cells (31). In
the present study, western blot analysis showed that the expression
of p-Erk1/2 in γδ T cell subset Vδ2 T cells in the peripheral blood
of patients with sepsis was significantly downregulated, which
further impaired the function of γδ T cells contributing to the
development of sepsis. The present study provided novel insight for
the mechanism underlying sepsis.
The present study examined the expression level of
surface molecules associated with Vδ1 T cells in contact with
immunosuppression, suggesting that the expression of
immunosuppressive molecules in peripheral blood Vδ1 T cells is
elevated in patients with sepsis. However, a limitation of the
present study was that it was not possible to study the pathway by
which Vδ1 T cells exert immunosuppressive effects in patients with
sepsis. The present study also detected changes in Vδ2 T cells in
patients with sepsis. The present study first observed changes in
the proportion and function of Vδ2 T cells in the peripheral blood
of patients with sepsis, which promoted the study of the mechanism
underlying sepsis.
In conclusion, the results of the present study
suggested that the proportion of Vδ1 and Vδ2 T cells in the
peripheral blood of patients with sepsis is out of balance, the
immune inhibition function of Vδ1 T cells is significantly
enhanced, and the level of inflammatory factors secreted by Vδ2 T
cells is significantly reduced. As a result, the immune function of
patients with sepsis is inhibited. This change may be closely
associated with the prognosis of patients with sepsis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and XC conceptualized and designed the study and
performed statistical analysis. WL performed literature research.
DZ, HZ and PC performed experimental studies and data acquisition.
XW, WL, DZ and HZ analyzed the data. XW, WL and XC prepared, edited
and reviewed the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Huangshi Central University/The Affiliated Hospital of
Hubei Polytechnic University. All patients signed informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nam M, Son BH, Seo JE, Kim IR, Park CK and
Kim HK: Improved diagnostic and prognostic power of combined delta
neutrophil index and mean platelet volume in pediatric sepsis. Ann
Clin Lab Sci. 48:223–230. 2018.PubMed/NCBI
|
|
2
|
Jones BL and Smith SM: Choice of
crystalloid and mortality in sepsis-all in the timing? Crit Care
Med. 42(e796)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barochia AV, Cui X and Eichacker PQ: The
surviving sepsis campaign's revised sepsis bundles. Curr Infect Dis
Rep. 15:385–393. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hotchkiss RS, Monneret G and Payen D:
Sepsis-induced immunosuppression: From cellular dysfunctions to
immunotherapy. Nat Rev Immunol. 13:862–874. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Otto GP, Sossdorf M, Claus RA, Rödel J,
Menge K, Reinhart K, Bauer M and Riedemann NC: The late phase of
sepsis is characterized by an increased microbiological burden and
death rate. Crit Care. 15(R183)2011.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Limaye AP, Kirby KA, Rubenfeld GD,
Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes
TK, Corey L and Boeckh M: Cytomegalovirus reactivation in
critically ill immunocompetent patients. JAMA. 300:413–422.
2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shevach EM, DiPaolo RA, Andersson J, Zhao
DM, Stephens GL and Thornton AM: The lifestyle of naturally
occurring CD4+ CD25+ Foxp3+
regulatory T cells. Immunol Rev. 212:60–73. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sakaguchi S, Ono M, Setoguchi R, Yagi H,
Hori S, Fehervari Z, Shimizu J, Takahashi T and Nomura T:
Foxp3+ CD25+ CD4+ natural
regulatory T cells in dominant self-tolerance and autoimmune
disease. Immunol Rev. 212:8–27. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wan YY: Regulatory T cells: Immune
suppression and beyond. Cell Mol Immunol. 7:204–210.
2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pan X, Ji Z and Xue J: Percentage of
peripheral CD19+CD24hiCD38hi regulatory B cells in neonatal sepsis
patients and its functional implication. Med Sci Monit.
22:2374–2378. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang H, Xu R, Lin F, Bao C, Wang S, Ji C,
Li K, Jin L, Mu J, Wang , et al: High circulating CD39(+)
regulatory T cells predict poor survival for sepsis patients. Int J
Infect Dis. 30:57–63. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shao M, Liu B, Wang JQ, Tao XG, Zhou SS,
Jin K and Zhang CP: Clinical significance of
CD4+CD25+ T cell examination in sepsis
patients. J Hunan Chin Med Univ. 31:8–10. 2011.
|
|
13
|
Raga S, Julià MR, Crespí C, Figuerola J,
Martínez N, Milà J and Matamoros N: Gammadelta T lymphocytes from
cystic fibrosis patients and healthy donors are high TNF-alpha and
IFN-gamma-producers in response to Pseudomonas aeruginosa.
Respir Res. 4(9)2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gao L, Lu Q, Huang LJ, Ruan LH, Yang JJ,
Huang WL, ZhuGe WS, Zhang YL, Fu B, Jin KL and ZhuGe QC:
Transplanted neural stem cells modulate regulatory T, γδ T cells
and corresponding cytokines after intracerebral hemorrhage in rats.
Int J Mol Sci. 15:4431–4441. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Andreu-Ballester JC, Zamora V,
Garcia-Ballesteros C, Benet-Campos C, Lopez-Chuliá F,
Tormo-Calandín C and Cuéllar C: Anti-Anisakis sp. antibodies in
serum of patients with sepsis and their relationship with γδ T
cells and disease severity. Int J Parasitol. 48:483–491.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hayday AC: [gamma][delta] cells: A right
time and a right place for a conserved third way of protection.
Annu Rev Immunol. 18:975–1026. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Triebel F and Hercend T: Subpopulations of
human peripheral T gamma delta lymphocytes. Immunol Today.
10:186–188. 1989.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Haas W, Pereira P and Tonegawa S:
Gamma/delta cells. Annu Rev Immunol. 11:637–685. 1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kabelitz D, Wesch D and He W: Perspectives
of gammadelta T cells in tumor immunology. Cancer Res. 67:5–8.
2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Levy MM, Fink MP, Marshall JC, Abraham E,
Angus D, Cook D, Cohen J, Opal SM, Vincent JL and Ramsay G:
SCCM/ESICM/ACCP/ATS/SIS: 2001 SCCM /ESICM /ACCP/ATS/SIS
international sepsis definitions conference. Crit Care Med.
31:1250–1256. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Soyocak A, Kurt H, Ozgen M, Turgut Cosan
D, Colak E and Gunes HV: MiRNA-146a, miRNA-155 and JNK expression
levels in peripheral blood mononuclear cells according to grade of
knee osteoarthritis. Gene. 627:207–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kumar V: T cells and their
immunometabolism: A novel way to understanding sepsis
immunopathogenesis and future therapeutics. Eur J Cell Biol.
97:379–392. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ichinohe T, Ichimiya S, Kishi A, Tamura Y,
Kondo N, Ueda G, Torigoe T, Yamaguchi A, Hiratsuka H, Hirai I, et
al: T-cell receptor variable gamma chain gene expression in the
interaction between rat gammadelta-type T cells and heat-shock
protein 70-like molecule. Microbiol Immunol. 47:351–357.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Andreu-Ballester JC, Tormo-Calandín C,
Garcia-Ballesteros C, Pérez-Griera J, Amigó V, Almela-Quilis A,
Ruiz del Castillo J, Peñarroja-Otero C and Ballester F: Association
of γδ T cells with disease severity and mortality in septic
patients. Clin Vaccine Immunol. 20:738–746. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Andreu-Ballester JC, García-Ballesteros C,
Benet-Campos C, Amigó V, Almela-Quilis A, Mayans J and Ballester F:
Values for αβ and γδ T-lymphocytes and CD4+,
CD8+, and CD56+ subsets in healthy adult
subjects: Assessment by age and gender. Cytometry B Clin Cytom.
82:238–244. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sabbagh P, Karkhah A, Nouri HR, Javanian M
and Ebrahimpour S: The significance role of regulatory T cells in
the persistence of infections by intracellular bacteria. Infect
Genet Evol. 62:270–274. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cuende J, Liénart S, Dedobbeleer O, van
der Woning B, De Boeck G, Stockis J, Huygens C, Colau D, Somja J,
Delvenne P, et al: Monoclonal antibodies against GARP/TGF-β1
complexes inhibit the immunosuppressive activity of human
regulatory T cells in vivo. Sci Transl Med.
7(284ra56)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li X, Kang N, Zhang X, Dong X, Wei W, Cui
L, Ba D and He W: Generation of human regulatory gammadelta T cells
by TCRgammadelta stimulation in the presence of TGF-beta and their
involvement in the pathogenesis of systemic lupus erythematosus. J
Immunol. 186:6693–6700. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jain N, Nguyen H, Chambers C and Kang J:
Dual function of CTLA-4 in regulatory T cells and conventional T
cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci USA.
107:1524–1528. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang F, Hou H, Xu L, Jane M, Peng J, Lu Y,
Zhu Y and Sun Z: Tim-3 signaling pathway as a novel negative
mediator in lipopolysaccharide-induced endotoxic shock. Hum
Immunol. 75:470–478. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lafont V, Liautard J, Sable-Teychene M,
Sainte-Marie Y and Favero J: Isopentenyl pyrophosphate, a
mycobacterial non-peptidic antigen, triggers delayed and highly
sustained signaling in human gamma delta T lymphocytes without
inducing eown-modulation of T cell antigen receptor. J Biol Chem.
276:15961–15967. 2001.PubMed/NCBI View Article : Google Scholar
|