Introduction
Laparoscopy was first described in the beginning of
the 20th century as a valuable adjunct to the diagnosis of diseases
of the abdominal cavity. At present, laparoscopic surgery is
reputed as one of the most effective diagnostic and therapeutic
tools in general surgery. The benefits of laparoscopy versus open
surgery include several advantages such as less post-operative
pain, shorter recovery time and less post-operative scarring
(1). However, it must also be
pointed out, that there are a number of complications, which may
occur after laparoscopy. It is known that pneumoperitoneum, which
is created for adequate visualization of the organs during
laparoscopy, increases intra-abdominal pressure. The latter reduces
organ perfusion and this is associated with splanchnic
ischemia/reperfusion (I/R) injury and oxidative stress (2-6).
The level of injury is dependent on the magnitude of the pressure
and application time (7). It has
been confirmed that increasing intra-abdominal pressure from 10 to
15 mmHg, decreases blood flow in the stomach by 40%, the duodenum
by 11%, the jejunum by 32%, the colon by 44%, the liver by 39% and
the parietal peritoneum by 60% (2).
Numerous reports are available in the literature
concerning the formation of excessive amounts of oxygen-derived
free radicals in ischemic reperfused tissues. Many vital organs
including the brain, lung, heart, liver, kidney, intestine, gastric
mucosa and stomach can be subjected to I/R injury; and it seems
that oxygen free radicals play a major role in the pathogenesis of
the cellular injury (8,9).
Several strategies for the prevention of
pneumoperitoneum-induced oxidative stress, such as preconditioning
and postconditioning, Trendelenburg positioning and insufflation
with helium, have been introduced in recent years, showing
promising results (5,7,10-12).
Postconditioning, which comprises short ischemia periods applied at
the time of reperfusion was first described in a canine model
(13) and demonstrated protective
effect against reperfusion injury. Despite the positive scientific
reports regarding the effects of postconditioning, there is still
limited understanding of the application and different regimens. In
addition, prevention of the negative effects of pneumoperitoneum
remains a problematic issue in laparoscopic surgery. Recently
authors reported the effect of a 10-min ischemia postconditioning
regimen (with 5 min of each-desufflation and insufflation), after
pneumoperitoneum (7). The effect of
postconditioning was evaluated by blood level analysis of oxidative
stress markers and assessment of tissue morphology in animal
models, which were initially established by our group (14). In the present study, we used a
similar animal model and comparatively assessed two 10-min
postconditioning regimens: a) Desufflation/insufflation for 5 min
each; b) desufflation/insufflation for 2.5 min each-repeated twice
(2 cycles). Furthermore, we analyzed levels of blood oxidative
stress markers at three different time points after
pneumoperitoneum (30 min, 2 and 6 h).
The aim of the present experimental study was to
characterize the effects of two different postconditioning regimens
in transvaginally created pneumoperitoneum in a rat animal
model.
Materials and methods
Animals and study design Animals
Sixty adult female Wistar rats each weighing 300±50
g were acquired from the Ilia State University animal house and
housed in cages (under normal atmosphere, standard room temperature
and 12-h light/dark cycle conditions) where standard rodent chow
and water were available. The rats went without food for 12 h
before any procedures. Animals were anesthetized by intraperitoneal
(i.p.) injection of ketamine (37.5 mg/kg) and seduxen (3.75 mg/kg).
All animal experiments were in compliance with the ARRIVE
guidelines and were carried out in accordance with the U.K. Animals
(Scientific Procedures) Act, 1986 and associated guidelines. These
studies were approved by the Local Institutional Committee on
Animal Research of Pecs University, Hungary.
Study design
The animals were divided into four equal groups-each
consisting of 15 rats, which also were divided into three subgroups
according to the time of blood taken (n=5) (explained below). In
all animals other than the sham group, the pneumoperitoneum was
created by CO2 insufflation under a pressure of 10 mmHg;
because of this, the Veress needle was placed into the abdominal
cavity from the vaginal orifice as previously described (14), which was connected to an insufflator
(Karl Storz GmbH & Co. KG). In two cases bleeding was observed
from the outer part of the vaginal orifice after the placement of
the Veress needle. No other complications were noted during the
surgical procedures.
Study groups
Group 1: Sham-operation group. A Veress needle was
placed into the vaginal orifice without any other surgical
procedure or gas insufflation. Group 2: Transvaginal
pneumoperitoneum (TV/PP) group was subjected to pneumoperitoneum
for 60 min followed by 30 min of desufflation. Group 3:
Postconditioning with 5 min. (Post-5) was subjected to
pneumoperitoneum for 60 min, plus 5 min of desufflation and 5 min
of insufflation followed by 30 min of desufflation. Group 4:
Post-conditioning with cycle (Post-2.5) was subjected to
pneumoperitoneum for 60 min plus 2.5 min of desufflation and 2.5
min of insufflation repeated in two cycles and followed by 30 min
of desufflation.
Animals were sacrificed after 30 min, 2 and 6 h from
the last desufflation. The blood samples were collected in each
group at the aforementioned times from all animals by cardiac
puncture. Oxidative stress markers such as malondialdehyde (MDA),
superoxide dismutase (SOD), reduced glutathione (GSH) and
sulfhydryl group (SH) levels as well as inflammatory cytokine TNF-α
concentrations were measured in all samples.
Biochemical tests
For detection of MDA concentration, 4.5 ml
thiobarbituric acid (TBA) and trichloroacetic acid (TCA) mixture
was added to 0.5 ml diluted blood sample or its plasma. The sample
was incubated at 100˚C (in boiling water) for 20 min and cooled in
icy water. Afterwards, the sample was centrifuged for 15 min at
1,400 x g, and measurement with a spectrophotometer was performed
at 532 nm. The MDA concentration was calculated in nmol/ml
(15).
For detection of reduced GSH and SH concentrations,
1 ml quintuple diluted blood sample and 4 ml trichloroacetic acid
(TCA) mixture were used for determination. The mixture was
centrifuged for 15 min at 18 x g. Two milliliters of the
supernatant was added to 4 ml Tris buffer (0.4 M, pH 8.7), and 100
µl of 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB) was added to the
mixture immediately before the measurement was taken. Measurement
with a spectrophotometer was made at 412 nm with concentrations
calculated in nmol/ml (16).
For detection of SOD activity, 1 ml blood mixed with
EDTA was used. Nine milliliters of Hartman's solution was added to
the blood sample and centrifuged for 5 min at 380 x g. This washing
procedure was repeated after the discarding of the supernatant. One
milliliter of a chloroform and ethanol (2:1) mixture was added to 1
ml of hemolyzed erythrocytes and centrifuged for 4 min at 12,000 x
g. The supernatant was then separated, and adrenalin (16.488 mg
adrenalin diluted in 10 ml 0.1 N hydrochloric acid) was added to
the supernatant with concentrations measured at 480 nm by a
spectrophotometer. Concentrations were calculated in U/ml (17).
For the TNF-α concentration, detection was carried
out using an ELISA kit (Quantikine® Rat TNF-α/TNFSF1A
Immunoassay, R&D Systems; cat. no. RTA00) according to the
manufacturer's instructions. Concentration was calculated in
pg/ml.
Statistical analysis
All data are presented as mean values ± standard
error of mean (SEM), which were compared within the various groups
using one-way analysis of variance followed by Bonferroni post hoc
tests and the non-parametric Mann-Whitney U test as appropriate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
After successful modeling of pneumoperitoneum in the
experimental animals (all rats survived until the end of the
experiment), we measured blood oxidative stress markers upon
different postconditioning settings.
Analysis of MDA levels after 30 min showed
significant upregulation in all experimental groups compared to
sham group (Fig. S1A). At 2 and 6
h, the levels of MDA in the post-5 group were significantly
decreased (72.11±2.85 nmol/ml after 2 h and 74.43±1.24 nmol/ml
after 6 h) compared with the TV/PP group at both 2 and 6 h, and
post-2.5 group at 6 h (Fig. 1A and
B; P<0.05). In the TV/PP group,
the level of MDA was significantly increased compared to the Sham
and Post-5 groups (Fig. 1A and
B; P<0.05), and maximum level of
89.58±7.87 nmol/ml was shown after 6 h (Fig. 1C; P<0.05).
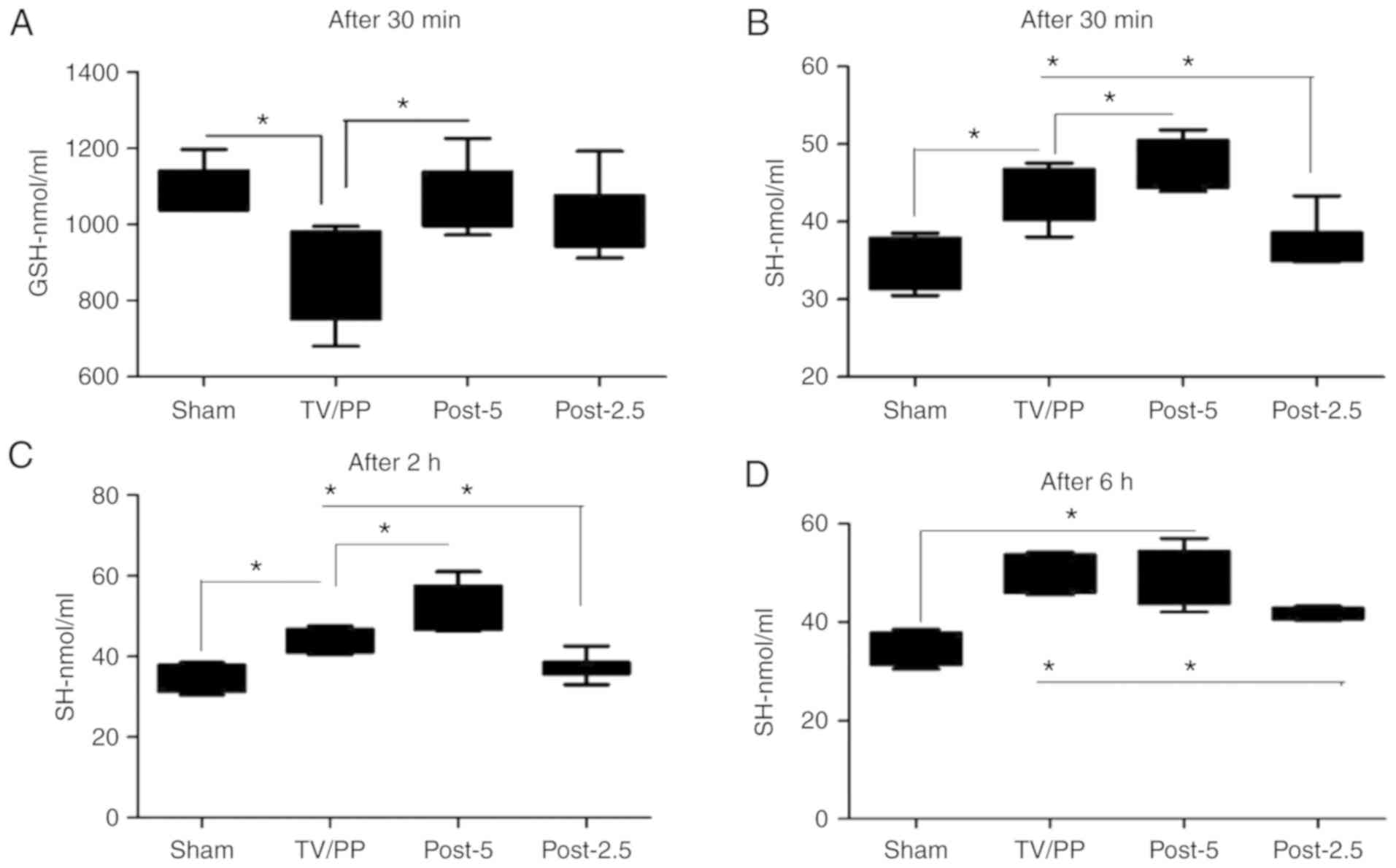
Next, we sought to analyze levels of GSH in the
studied animals. Our experiment demonstrated that GSH blood levels
were decreased in the TV/PP group (847.25±86.17 nmol/ml) compared
to the Sham (1,075.86±48.92 nmol/ml), Post-5 (1,051.13±92.14
nmol/ml) and Post-2.5 (1,008.32±94.67 nmol/ml) groups after 30 min
(Fig. 2A; P<0.05 for TV/PP vs.
Sham or Post-5). After 2 and 6 h there were no marked differences
in GSH level between the groups (Fig.
S1B and C). Additionally,
analysis of the levels of SH demonstrated that levels of blood SH
were markedly increased in Post-5 groups (47.30±4.81 nmol/ml after
30 min and 54.22±5.33 nmol/ml after 2 h) in comparison to the Sham,
TV/PP and Post-2.5 groups (Fig.
2B-D). Animals in the Post-2.5 group demonstrated significant
reduction of blood SH levels after 30 min (36.75±6.5), 2 h
(38.21±2.7) and 6 h (44.52±4.1) compared to the TV/PP and Post-5
groups (Fig. 2B-D; P<0.05).
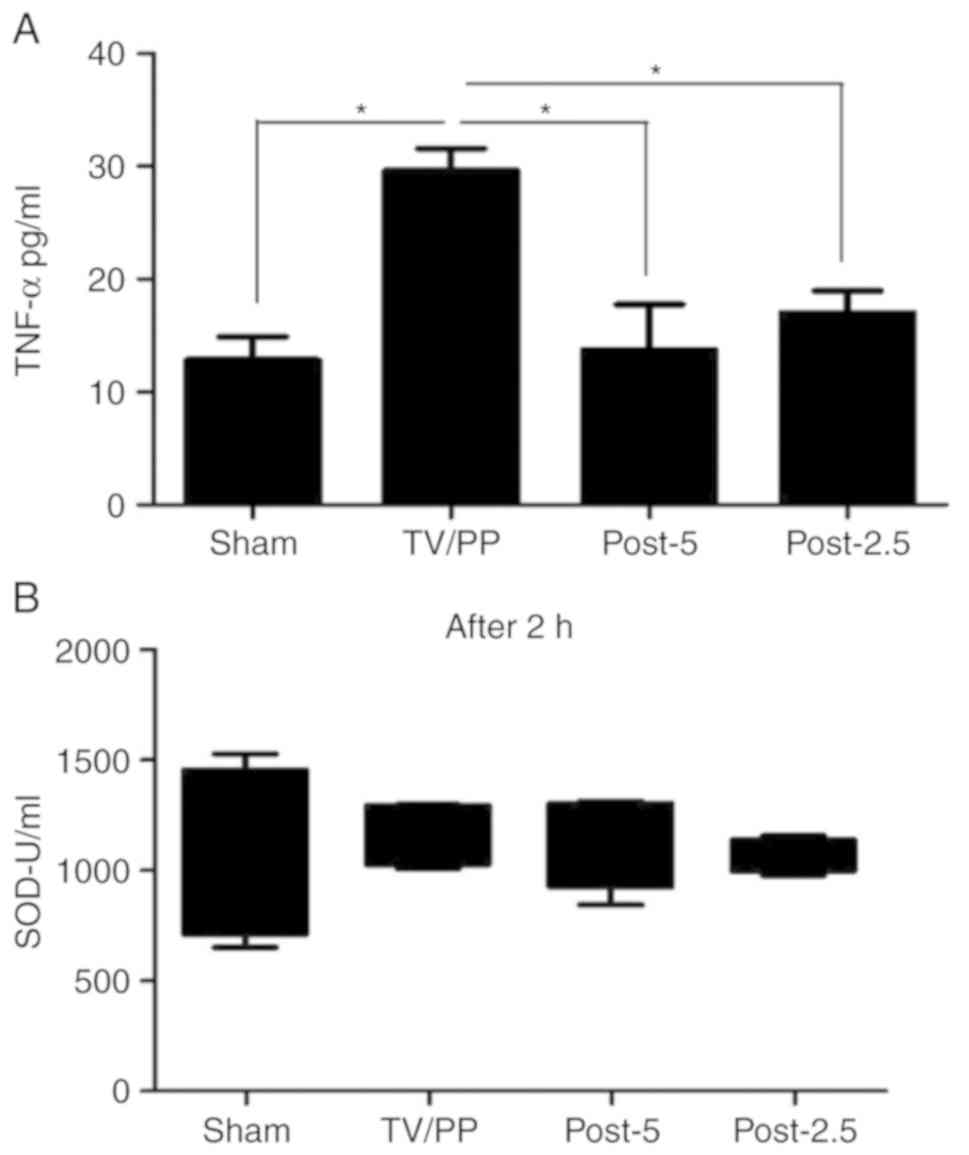
We also analyzed inflammatory cytokine TNF-α
concentrations in the blood of the experimental animals. The
postconditioning groups (Post-5/Post-2.5) displayed significantly
reduced level of TNF-α (13.11±4.49 and 17.08±2.15 pg/ml,
respectively) compared to the TV/PP animals (29.89±1.78 pg/ml)
after pneumoperitoneum (Fig. 3A;
P<0.05). Remarkably, no differences between the groups regarding
SOD activity were found at any time point (Figs. 3B and S2A
and B).
Discussion
It is known that during pneumoperitoneum there is a
cascade of chemical release occurring after peritoneal tissue
stress, insult and damage (18). The
proceeding hypoperfusion caused by insufflation of the abdominal
cavity (under pressure of 10 mmHg) may induce ischemia/reperfusion
(I/R) injury.
Authors have reported experimental results
demonstrating diverse stress conditions of different gases and
high/low-pressure settings for the establishment of
pneumoperitoneum (19,20). The conditioning of pneumoperitoneum
has been suggested to be valuable and is an I/R protective tool
(10). There are few reports
concerning the use of the conditioning model to prevent the
negative effects related to the pneumoperitoneum associated with
respiratory, homeostatic and physiologic consequences (5,7,11,12,21). It
was concluded that conditioning is more effective than pre-ischemic
administration of erythropoietin in reducing the negative effects
of oxidative injury (22).
Ischemic postconditioning is a new, simple
manipulation that can protect organs from I/R injury. It has been
shown that much of the post-ischemic injury occurs during the early
moments of reperfusion, and that manipulation of this early
reperfusion phase reduces I/R injury (10). We may assume that repetitive blood
flow interruptions of tissues and organs applied immediately after
a period of ischemia make tissues more resistant/tolerant to
subsequent ischemic injury.
In our experiments, we created pneumoperitoneum
conditions using a transvaginal approach due to the advantages of
NOTES (Natural Orifice Transluminal Endoscopic Surgery) procedures,
which are associated with less traumatic stress (14,23).
Simultaneously, however, we wanted to achieve substantial oxidative
stress, and therefore stressful levels of pneumoperitoneum pressure
were applied (10 mmHg) (24,25). Indeed, this approach resulted in
extensive elevation of oxidative stress parameters (TV/PP animal
group). In order to effectively reduce negative signs of
pneumoperitoneum, we decisively chose to use two different regimens
of postconditioning in this transvaginally created pneumoperitoneum
model.
Recently, the effects of a one cycle 5-min ischemic
postconditioning has been reported in an animal model, which
included 5 min of desufflation and 5 min of insufflation after
pneumoperitoneum (7). In the present
study, a similar animal model was used and we performed comparative
analysis of the above mentioned regimen (Post-5) with two shorter
cycled 2.5-min desufflation/insufflation regimen (Post-2.5).
Duration of total postconditioning time was the same in both groups
(10 min).
To ascertain the extent of oxidative stress, levels
of several markers, malondialdehyde (MDA), superoxide dismutase
(SOD), reduced glutathione (GSH) and sulfhydryl group (SH) levels,
as well as inflammatory cytokine TNF-α were determined. Free
radicals have been seen to generate the lipid peroxidation process
in organisms. MDA is one of the final products of lipid
peroxidation in the cells, and an increase in free radicals causes
overproduction of MDA (26). It is
also known that GSH is a major endogenous antioxidant produced by
cells, and that it participates directly in the neutralization of
free radicals and reactive oxygen compounds. Reactive oxygen
species overproduced as a result of oxidative stress are potent
oxidizing and reducing agents that can directly damage cellular
membranes by lipid peroxidation (8).
SH is considered a major plasma antioxidant in vivo; and
most of the SH-groups are present over albumin and are major
reducing groups present in body fluids (27). Cytokines, such as TNF-α, play a key
role in mediating the host inflammatory response. TNF-α is
considered to be an initial mediator of the cytokine cascade and
appears to be the first cytokine into the blood stream. The release
of TNF-α is inhibited following hypoxia and re-oxygenation periods
(28).
As expected, pneumoperitoneum caused upregulation of
blood MDA and TNF-α levels. Introduction of the postconditioning
method was found to significantly reduce lipid peroxidation process
and cytokine release in the body of experimental animals, as well
as increased levels of antioxidant factors such as GSH and SH.
Notably, based on blood MDA level results, we can assume, that
Post-5 regimen of postconditioning can more effectively reduce
lipid peroxidation compared to the Post-2.5 regimen. Moreover,
based on analysis of the SH results we can suggest that the
Post-2.5 postconditioning setup markedly reduced release of
antioxidant factors. Assessment of GSH, TNF-α and SOD showed more
or less similar effect among these postconditioning groups. Based
on the results it can be suggested that both a single cycle
postconditioning regimen (Post-5) as well as the cycled
postconditioning (Post-2.5) method can reduce oxidative stress and
subsequently may decrease pneumoperitoneum-caused complications.
However, according to our experiments it is also suggested that the
single cycle postconditioning regimen (Post-5) can be more
effective in this regard. Further basic and clinical investigations
are necessary to better characterize the postconditioning regimens
and their effects although any of these approaches may already
provide important clinical implications.
To conclude, pneumoperitoneum used during
laparoscopic surgery induces elevation of oxidative stress markers.
Postconditioning can normalize levels of oxidative stress markers
after pneumoperitoneum. Furthermore, the method of postconditioning
without cycles seems to be rather effective compared with
postconditioning with cycles.
Supplementary Material
(A) Blood levels of MDA in the Sham,
TV/PP, Post-5 and Post-2.5 groups after 30 min of pneumoperitoneum.
(B and C) Blood levels of GSH in the Sham, TV/PP, Post-5 and
Post-2.5 groups after (B) 2 h and (C) 6 h of pneumoperitoneum.
*P<0.05 and **P<0.01. MDA,
malondialdehyde; GSH, reduced glutathione; TV/PP, transvaginal
pneumoperitoneum.
(A and B) Activity of SOD after (A) 30
min and (B) 6 h of pneumoperitoneum. SOD, superoxide dismutase;
TV/PP, transvaginal pneumoperitoneum.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
KS, SJ and VK designed and performed the animal
studies. KS and SJ performed and analyzed the biochemical
experiments. GW, SJ and IA supervised and aided the experimental
research, and participated in the data collection and analysis. KS,
SJ, IA and GW drafted the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experiments were approved by the local ethics
committee of the University of Pecs, Hungary.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clarke HC: History of endoscopic and
laparoscopic surgery. World J Surg. 25:967–968. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sammour T, Mittal A, Loveday BP, Kahokehr
A, Phillips AR, Windsor JA and Hill AG: Systematic review of
oxidative stress associated with pneumoperitoneum. Br J Surg.
96:836–850. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Liu KX, Li YS, Huang WQ, Chen SQ, Wang ZX,
Liu JX and Xia Z: Immediate postconditioning during reperfusion
attenuates intestinal injury. Intensive Care Med. 35:933–942.
2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Polat C, Yilmaz S, Serteser M, Koken T,
Kahraman A and Dilek ON: The effect of different intraabdominal
pressures on lipid peroxidation and protein oxidation status during
laparoscopic cholecystectomy. Surg Endosc. 17:1719–1722.
2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ates E, Yilmaz S, Ihtiyar E, Yasar B and
Karahuseyinoglu E: Preconditioning-like amelioration of
erythropoietin against laparoscopy-induced oxidative injury. Surg
Endosc. 20:815–819. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hatipoglu S, Akbulut S, Hatipoglu F and
Abdullayev R: Effect of laparoscopic abdominal surgery on
splanchnic circulation: Historical developments. World J
Gastroenterol. 20:18165–18176. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Veres TG, Petrovics L, Sárvári K,
Vereczkei A, Jancsó G, Farkas KB and Takács I: The effect of
laparoscopic pre- and postconditioning on pneumoperitoneum induced
injury of peritoneum. Clin Hemorheol Microcirc, May 27, 2019 (Epub
ahead of print).
|
|
8
|
Loubele ST, ten Cate H and Spronk HM:
Anticoagulant therapy in critical organ ischaemia/reperfusion
injury. Thromb Haemost. 104:136–142. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lipton P: Ischemic cell death in brain
neurons. Physiol Rev. 79:1431–1568. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Oksuz H, Bulbuloglu E, Senoglu N, Ciralik
H, Yuzbasioglu MF, Kilinc M, Dogan Z, Goksu M, Yildiz H, Ozkan OV
and Atli Y: Re-protective effects of pre- and post-laparoscopy
conditioning, zinc, pentoxifylline, and N-acetylcysteine in an
animal model of laparoscopy-induced ischemia/reperfusion injury of
the kidney. Ren Fail. 31:297–302. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yilmaz S, Ates E, Polat C, Koken T, Tokyol
C, Akbulut G and Gokce O: Ischemic preconditioning decreases
laparoscopy-induced oxidative stress in small intestine.
Hepatogastroenterology. 50:979–982. 2003.PubMed/NCBI
|
|
12
|
Yilmaz S, Koken T, Tokyol C, Kahraman A,
Akbulut G, Serteser M, Polat C, Gokce C and Gokce O: Can
preconditioning reduce laparoscopy-induced tissue injury? Surg
Endosc. 17:819–824. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F,
Wang NP, Guyton RA and Vinten-Johansen J: Inhibition of myocardial
injury by ischemic postconditioning during reperfusion: Comparison
with ischemic preconditioning. Am J Physiol Heart Circ Physiol.
285:H579–H588. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jávor S, Shanava K, Hocsák E, Kürthy M,
Lantos J, Borsiczky B, Takács I, Horváth S, Balatonyi B, Ferencz S,
et al: Preconditioning is a method that may reduce the negative
side-effect of pneumoperitoneum. Interv Med Appl Sci. 2:115–120.
2010.
|
|
15
|
Placer ZA, Cushman LL and Johnson BC:
Estimation of product of lipid peroxidation (malonyl dialdehyde) in
biochemical systems. Anal Biochem. 16:359–364. 1966.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sedlak J and Lindsay RH: Estimation of
total, protein-bound, and nonprotein sulfhydryl groups in tissue
with Ellman's reagent. Anal Biochem. 25:192–205. 1968.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Misra HP and Fridovich I: The role of
superoxide anion in the autoxidation of epinephrine and a simple
assay for superoxide dismutase. J Biol Chem. 247:3170–3175.
1972.PubMed/NCBI
|
|
18
|
Ott DE: The peritoneum and the
pneumoperitoneum: A review to improve clinical outcome. Gynecol
Surg. 1:101–106. 2004.
|
|
19
|
Lee JY and Choi SH: Evaluation of total
oxidant and antioxidant status in dogs under different CO2
pneumoperitoneum conditions. Acta Vet Scand. 57(23)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ypsilantis P, Lambropoulou M, Tentes I,
Chryssidou M, Georgantas T and Simopoulos C: Room air versus carbon
dioxide pneumoperitoneum: Effects on oxidative state, apoptosis and
histology of splanchnic organs. Surg Endosc. 30:1388–1395.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thuret R, Saint Yves T, Tillou X,
Chatauret N, Thuillier R, Barrou B and Billault C: Ischemic pre-
and post-conditioning: Current clinical applications. Prog Urol. 24
(Suppl 1):S56–S61. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Trunzo JA, McGee MF, Cavazzola LT,
Schomisch S, Nikfarjam M, Bailey J, Mishra T, Poulose BK, Lee YJ,
Ponsky JL and Marks JM: Peritoneal inflammatory response of natural
orifice translumenal endoscopic surgery (NOTES) versus laparoscopy
with carbon dioxide and air pneumoperitoneum. Surg Endosc.
24:1727–1736. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bingener J, Krishnegowda NK and Michalek
JE: Immunologic parameters during NOTES compared with laparoscopy
in a randomized blinded porcine trial. Surg Endosc. 23:178–181.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Berguer R, Cornelius T and Dalton M: The
optimum pneumoperitoneum pressure for laparoscopic surgery in the
rat model. A detailed cardiorespiratory study. Surg Endosc.
11:915–918. 1997.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al-Saeedi M, Nickkholgh A, Schultze D,
Flechtenmacher C, Zorn M, Liang R, Gutt CN and Schemmer P: Glycine
protects the liver from reperfusion injury following
pneumoperitoneum. Eur Surg Res. 59:91–99. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mateos R, Lecumberri E, Ramos S, Goya L
and Bravo L: Determination of malondialdehyde (MDA) by
high-performance liquid chromatography in serum and liver as a
biomarker for oxidative stress. Application to a rat model for
hypercholesterolemia and evaluation of the effect of diets rich in
phenolic antioxidants from fruits. J Chromatogr B Analyt Technol
Biomed Life Sci. 827:76–82. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kolagal V, Karanam SA, Dharmavarapu PK,
D'Souza R, Upadhya S, Kumar V, Kedage V, Muttigi MS, Shetty JK and
Prakash M: Determination of oxidative stress markers and their
importance in early diagnosis of uremia-related complications.
Indian J Nephrol. 19:8–12. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sack M: Tumor necrosis factor-alpha in
cardiovascular biology and the potential role for anti-tumor
necrosis factor-alpha therapy in heart disease. Pharmacol Ther.
94:123–135. 2002.PubMed/NCBI View Article : Google Scholar
|