Introduction
Natural products are small molecules produced by
organisms as primary and secondary metabolites and they exert
various useful properties including anti-oxidant,
anti-inflammatory, cancer preventive, cardiovascular,
anti-Alzheimer's, platelet modulatory and pain relief effects
(1-5).
These compounds are considered new therapeutic tools
for the management of cardiovascular diseases, and they have
received considerable attention. In this context, increasing number
of reports indicated beneficial effects of foods with high levels
of polyphenols (fruits, tea and cocoa) against cardiovascular
diseases (6-9)
in humans and similar favorable effects were noted for isolated
flavonoids in animal models (10,11).
Crocin (CRO), a carotenoid from the tetraterpenes
family, is responsible for the unique color of saffron, stigmas of
Crocus sativus (12).
Considerable amount of evidence has confirmed that CRO exhibits
antioxidant, radical scavenging, cytotoxic and antitumor (12,13),
renoprotective (14) and
anti-hyperlipidemic (15) effects.
Several studies have shown the beneficial effects of this compound
against cardiovascular conditions as it could alleviate blood
biomarkers associated with obesity (16), had protective effects against
angiotensin II-hypertension (17)
and normalized the blood pressure of rats chronically administered
with desoxycorticosterone acetate (18).
In a study, CRO reduced mean arterial blood pressure
(MAP) in rats and its effects were more marked in hypertensive
animals compared to normotensive ones (19). It was noted that CRO inhibitory
effects on the extracellular Ca2+ influx along with
Ca2+ cytosolic release from intracellular
Ca2+ supplies in the endoplasmic reticulum, might
underlie its hypotensive effects induced by relaxing blood vessels
(20,21).
Citrus fruits contain flavanone glycosides
hesperidin (HES) and naringin which are members of the bigger
family of flavonoids that comprises a large number of phenolic
compounds. Flavanone glycosides were shown to have higher
bioavailability compared to other flavonoid compounds. HES has
shown various therapeutic properties such as anti-oxidant,
anti-inflammatory, and anti-aging effects (22-26).
Although the beneficial effects of HES on the cardiovascular system
were shown by the significant reductions observed in the blood
pressure and serum lipids following its administration (27-29),
there is still debate regarding its value in this field since there
are reports indicating that HES has no significant effect on the
said cardiovascular parameters (30,31).
It was reported that 25-week administration of HES
reduced blood pressure and heart rate (HR) in spontaneously
hypertensive rats but in normotensive Wistar-Kyoto rats, no such
effect was observed (32). Moreover,
a randomized, controlled, crossover study in healthy volunteers
showed that supplementation with HES during a 4-week period, led to
a significant reduction in diastolic blood pressure (DBP) (29).
In another study, glucosyl HES (G-HES), a
water-soluble derivative of HES, and hesperetin, aglycone HES, were
given to spontaneously hypertensive rats (SHR); both compounds
reduced systolic blood pressure (SBP) which was significantly
inhibited by a nitric oxide synthase inhibitor,
N(G)-nitro-L-arginine methyl ester (33). Nevertheless, a recent meta-analysis
of randomized controlled trials revealed that HES supplementation
had no effect on SBP or DBP (28).
In the present study, effects of CRO and HES
supplementation, alone and in combination, on MAP, SBP, DBP, and HR
were assessed in rats treated with a high-fat diet (HFD) for 7
weeks.
Materials and methods
Animals.
In this study, 40 male Wistar rats (aged 8-10 weeks;
200-250 g body weight, obtained from Animal Center of Zabol
University of Medical Sciences, Zabol, Iran) were kept under
standard conditions (at 25˚C with 12/12 h light/dark cycles) and
they had free access to food and water, ad libitum. All
animal experiments were approved by the animal research Ethics
Committee of Zabol University of Medical Sciences (Ethics committee
approval no. IR.ZBMU.REC.1398.091) and performed in accordance with
National Institute of Health Guide for the Care and Use of the
Laboratory Animals.
Animals were randomly assigned to the following five
groups (8 animals in each group): control group [rats received
standard chow diet (CD; Harlan TD.7012) for 7 weeks + normal saline
on day 50], HFD control group [received HFD containing 32% kcal of
fat and 0.1% cholesterol (Harlan TD.88137) for 7 weeks and normal
saline on day 50(34)], and three
groups of HFD-treated animals that received a single dose of either
CRO (20 mg/kg), HES (20 mg/kg), or CRO + HES (20 + 20 mg/kg) on day
50.
Materials.
HES and CRO were purchased from Merck KGaA. Ketamine
(Alfasan) and xylazine (Pantex Holland B.V.) were employed for
induction of anesthesia. For measurement of SBP, DBP, HR and MAP,
Power Lab (AD Instruments) was used (35,36).
Animal diets.
Animals were assigned into the two diet groups. The
control group received purified low-fat diet (containing 10.6% kcal
as fat) for 7 weeks. The rest of the animals received HFD diet
containing 32% kcal as fat and 0.1% cholesterol, for 7 weeks
(37). HFD was provided from Razi
Institute, Tehran, Iran. The HFD animals were divided into HFD
control group and CRO or HES or CRO + HES groups.
Methods.
After the 7-week period of HFD, animals were fasted
overnight (minimum period of 8-10 h), and anesthetized using
ketamine/xylazine. The reflexes of the animals were checked, and
they were placed on a suitable rodent surgical Table with
electrocardiogram (ECG) recording. The ventral side of the neck,
right hind leg, and chest of the animals were carefully disinfected
and shaved. A small incision (1.5-2 cm) was made in the neck of the
rats for the tracheostomy procedure and carotid artery cannulation.
Tracheostomy was performed using a small piece of pediatric Ryle's
tube or rodent tracheal intubation tube (36). The carotid artery was identified and
the cardiac end of the blood vessel was clamped by a bulldog clamp
for cannulation (36). The blood
vessel was cannulated employing a cannula pre-filled with
heparinized normal saline (0.5 IU/ml). After cannulation, the
bulldog clamp at the cardiac end of the blood vessel, was released
slowly (9).
Three hours before cannulation, CRO and HES alone or
in combination in the respective treatment groups or normal saline
in the control and HFD-hypertensive group, were injected
intraperitoneally (ip). After cannulation, the sensor was connected
to the Power Lab instrument and the blood pressure, HR and ECG were
recorded and analyzed using Lab Chart 8 software (36).
Statistical analysis.
Data analysis was performed using SPSS version 11.5
(SPSS, Inc.). Differences among the groups were assessed using
One-way ANOVA followed by Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
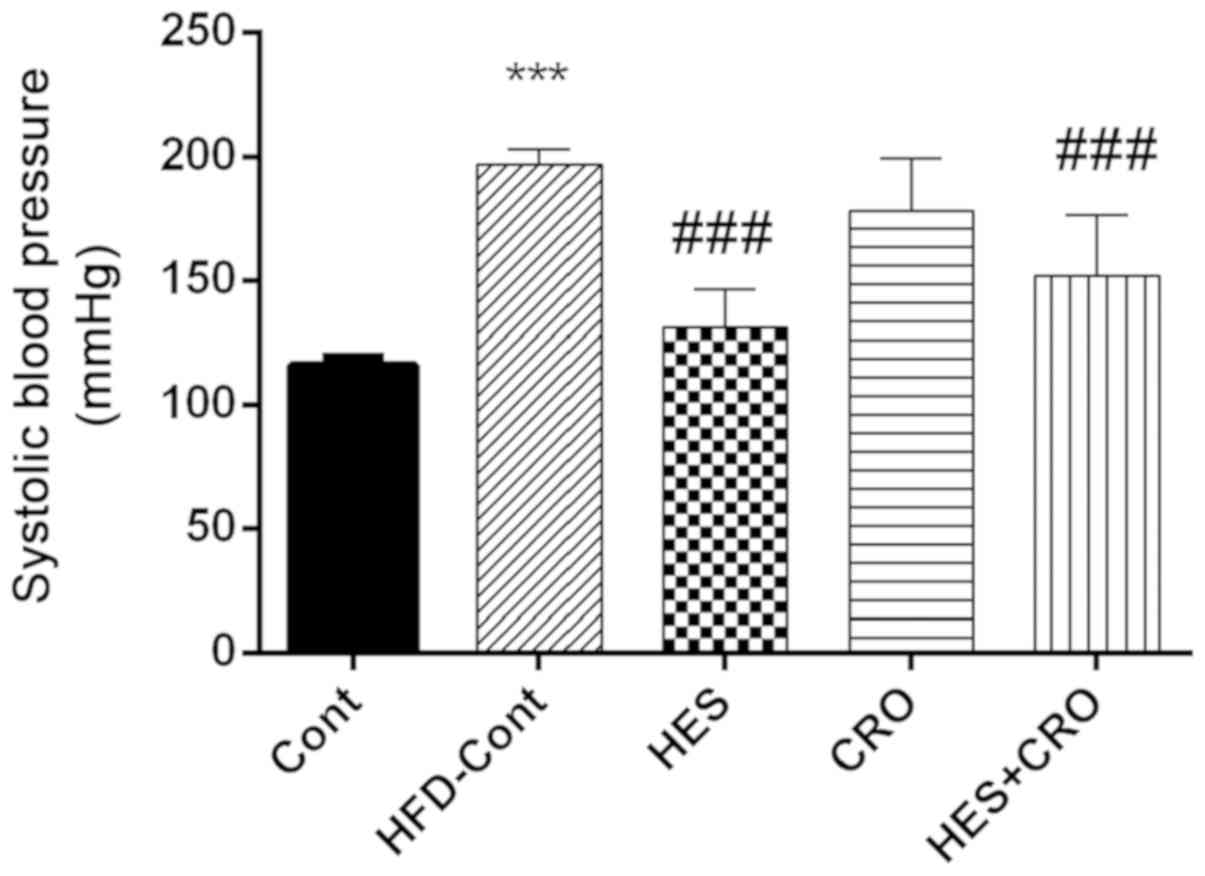
Effects of HES and CRO on SBP in
HFD-hypertensive rats.
As presented in Fig.
1, HFD-treated animals showed significant increases in SBP
compared to the control group (P<0.001). In the HES and HES+CRO
groups, SBP was reduced significantly in comparison to the HFD
control group (for both cases P<0.001). HES treatment resulted
in a significant decrease of SBP compared to the HFD control group.
On the other hand, treatment of hypertensive animals with CRO alone
did not reduce SBP.
Effects of HES and CRO on DBP in
HFD-hypertensive rats.
As shown in Fig. 2,
DBP did not show significant variations among the different
groups.
Effects of HES and CRO on HR in
HFD-hypertensive rats.
Fig. 3 shows that HFD
rats did not present a significant difference in HR compared to the
control group which did not receive HFD. Nonetheless, HES+CRO
treated animals showed a significant reduction in HR in comparison
to the HFD control group (P<0.001).
Effects of HES and CROC on MAP in
HFD-hypertensive rats.
7-week treatment of rats with HFD led to a
significant increase in MAP compared to the control group
(P<0.001). Furthermore, our results showed that HES+CRO
administration in HFD rats significantly attenuated MAP compared to
the HFD control group (P<0.001); however, treatment with HES or
CRO alone did not result in significant alterations in MAP compared
to the HFD control group (Fig.
4).
Discussion
In the current study, 7-week administration of HFD
in rats induced systolic hypertension and increased MAP.
Administration of a single dose of HES reduced high SBP. HES and
the combination of HES and CRO significantly decreased SBP. HFD did
not affect DBP or HR; however, it significantly increased MAP,
which in turn, was significantly decreased by treatment with the
combination of HES and CRO. To the best of our knowledge, this is
the first experiment investigating the effect of co-administration
of two natural products, HES and CRO, in a rat model of HFD-induced
hypertension and reports their effects on blood pressure and HR.
Co-administration of these compounds seems to have synergistic
effects.
It was reported that hypotensive effects of HES are
induced through nitric oxide (NO)-mediated vasodilation (33). In a study by Yamamoto et al
(33), it was shown that short-term
administration of HES reduces SBP by NO-induced vascular
relaxation. Several studies similarly confirmed the aforementioned
mechanism of action of HES (31,38,39).
Another report confirmed that HES acutely stimulates
phosphorylation of endothelial NO synthase to produce NO and cause
vasodilation (31) and these results
are in agreement with the data obtained in the present study on the
short-term antihypertensive effects of HES (31). We found that HES alone reduced SBP,
but HES in combination with CRO reduced simultaneously SBP, MAP and
HR confirming the synergistic effects of HES and CRO in reduction
of high blood pressure.
Previous studies showed that CRO can reduce
hypertension in animal models (18,40).
Besides, there are other reports on the antihypertensive effects of
other active ingredients and extracts of Crocus sativus
(41-43).
Some of these studies examined possible acute antihypertensive
effects of CRO and crocetin (40,42,43). In
this context, Shafei et al (40) showed that CRO 50, 100 and 200 mg/kg
attenuated MAP, SBP and HR induced by angiotensin II. Our results
showed that CRO 20 mg/kg did not alleviate hypertension induced by
HFD. In HES + CRO (20 + 20 mg/kg)-treated animals, a significant
decrement of SBP and MAP was observed. In the HES (20 mg/kg)-only
treated group, though SBP was decreased, no changes in DBP, HR and
MAP were found. Our results point to a potentially interesting
synergistic effect for these natural products that might act via
similar pathways (i.e., via NO-associated mechanisms) (33,40).
In our study, the co-administration of HES and CRO
exhibited favorable antihypertensive effects. The beneficial
effects of these nutraceuticals could be further tested in clinical
settings for the prevention and treatment of hypertension,
especially in patients presenting with borderline stage 1
hypertension.
Acknowledgements
The present study was part of a Pharm. D. thesis and
authors wish to thank Zabol University of Medical Sciences for
support.
Funding
This work was funded by Zabol University of Medical
Sciences and Students Research Committee of Zabol University of
Medical Sciences.
Availability of data and materials
Not applicable.
Authors' contributions
MH and RR conceived and designed, and supervised the
study. MN performed the experiments and collected the data and TKN
analyzed the data. GL, KT, AT, JS and DAS interpreted the data and
prepared the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the animal
research Ethics Committee of Zabol University of Medical Sciences
(Ethics committee approval no. IR.ZBMU.REC.1398.091).
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Hashemzaei M, Abdollahzadeh M, Iranshahi
M, Golmakani E, Rezaee R and Tabrizian K: Effects of luteolin and
luteolin-morphine co-administration on acute and chronic pain and
sciatic nerve ligated-induced neuropathy in mice. J Complement
Integr Med. 14(20160066)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hashemzaei M, Imen Shahidi M, Moallem SA,
Abnous K, Ghorbani M and Mohamadpour AH: Modulation of JAK2, STAT3
and Akt1 proteins by granulocyte colony stimulating factor
following carbon monoxide poisoning in male rat. Drug Chem Toxicol.
39:375–379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hashemzaei M, Karami SP, Delaramifar A,
Sheidary A, Tabrizian K, Rezaee R, Shahsavand S, Arsene AL,
Tsatsakis AM and Taghdisi SM: Anticancer effects of
co-administration of daunorubicin and resveratrol in MOLT-4, U266
B1 and Raji cell lines. Farmacia. 64:36–42. 2016.
|
|
4
|
Tabeshpour J, Hashemzaei M and Sahebkar A:
The regulatory role of curcumin on platelet functions. J Cell
Biochem. 119:8713–8722. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tabrizian K, Yaghoobi NS, Iranshahi M,
Shahraki J, Rezaee R and Hashemzaei M: Auraptene consolidates
memory, reverses scopolamine-disrupted memory in passive avoidance
task, and ameliorates retention deficits in mice. Iran J Basic Med
Sci. 18:1014–1019. 2015.PubMed/NCBI
|
|
6
|
Buijsse B, Weikert C, Drogan D, Bergmann M
and Boeing H: Chocolate consumption in relation to blood pressure
and risk of cardiovascular disease in German adults. Eur Heart J.
31:1616–1623. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Di Castelnuovo A, Rotondo S, Iacoviello L,
Donati MB and De Gaetano G: Meta-analysis of wine and beer
consumption in relation to vascular risk. Circulation.
105:2836–2844. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mink PJ, Scrafford CG, Barraj LM, Harnack
L, Hong CP, Nettleton JA and Jacobs DR Jr: Flavonoid intake and
cardiovascular disease mortality: A prospective study in
postmenopausal women. Am J Clin Nutr. 85:895–909. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Peters U, Poole C and Arab L: Does tea
affect cardiovascular disease? A meta-analysis. Am J Epidemiol.
154:495–503. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Auclair S, Milenkovic D, Besson C, Chauvet
S, Gueux E, Morand C, Mazur A and Scalbert A: Catechin reduces
atherosclerotic lesion development in apo E-deficient mice: A
transcriptomic study. Atherosclerosis. 204:e21–e27. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Norata GD, Marchesi P, Passamonti S,
Pirillo A, Violi F and Catapano AL: Anti-inflammatory and
anti-atherogenic effects of cathechin, caffeic acid and
trans-resveratrol in apolipoprotein E deficient mice.
Atherosclerosis. 191:265–271. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Rezaee R, Jamialahmadi K, Riahi Zanjani B,
Mahmoudi M, Abnous K, Zamani Taghizadeh Rabe S, Tabasi N, Zali M,
Rezaee M, Amin B, et al: Crocin effects on human myeloma cells
regarding intracellular redox state, DNA fragmentation, and
apoptosis or necrosis profile. Jundishapur J Nat Pharm Prod.
9(e20131)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rezaee R, Mahmoudi M, Abnous K, Zamani
Taghizadeh Rabe S, Tabasi N, Hashemzaei M and Karimi G: Cytotoxic
effects of crocin on MOLT-4 human leukemia cells. J Complement
Integr Med. 10:105–112. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yaribeygi H, Mohammadi MT, Rezaee R and
Sahebkar A: Crocin improves renal function by declining Nox-4,
IL-18, and p53 expression levels in an experimental model of
diabetic nephropathy. J Cell Biochem. 119:6080–6093.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lee IA, Lee JH, Baek NI and Kim DH:
Antihyperlipidemic effect of crocin isolated from the fructus of
Gardenia jasminoides and its metabolite Crocetin. Biol Pharm Bull.
28:2106–2110. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mashmoul M, Azlan A, Mohtarrudin N, Nisak
B, Yusof M and Khaza’ai H: Saffron extract and crocin reduced
biomarkers associated with obesity in rats fed a high-fat diet. Mal
J Nutr. 23:117–127. 2017.
|
|
17
|
Anaeigoudari A, Faramarzi A, Abbasnezhad A
and Shafei M: Effect of intrapertonal injection of crocin on
cardiovascular parameters in Angiotensin II-induced hypertensive
rats. Horizon Med Sci. 24:309–315. 2018.
|
|
18
|
Imenshahidi M, Razavi BM, Faal A,
Gholampoor A, Mousavi SM and Hosseinzadeh H: Effects of chronic
crocin treatment on desoxycorticosterone acetate (doca)-salt
hypertensive rats. Iran J Basic Med Sci. 17:9–13. 2014.PubMed/NCBI
|
|
19
|
Imenshahidi M, Hosseinzadeh H and
Javadpour Y: Hypotensive effect of aqueous saffron extract
(Crocus sativus L.) and its constituents, safranal and
crocin, in normotensive and hypertensive rats. Phytother Res.
24:990–994. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
He SY, Qian ZY and Tang FT: Effect of
crocin on intracellular calcium concentration in cultured bovine
aortic smooth muscle cells. Yao Xue Xue Bao. 39:778–781. 2004.(In
Chinese):PubMed/NCBI
|
|
21
|
Williams BA, Liu C, Deyoung L, Brock GB
and Sims SM: Regulation of intracellular Ca2+ release in
corpus cavernosum smooth muscle: Synergism between nitric oxide and
cGMP. Am J Physiol Cell Physiol. 288:C650–C658. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iranshahi M, Rezaee R, Parhiz H,
Roohbakhsh A and Soltani F: Protective effects of flavonoids
against microbes and toxins: The cases of hesperidin and
hesperetin. Life Sci. 137:125–132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Parhiz H, Roohbakhsh A, Soltani F, Rezaee
R and Iranshahi M: Antioxidant and anti-inflammatory properties of
the citrus flavonoids hesperidin and hesperetin: An updated review
of their molecular mechanisms and experimental models. Phytother
Res. 29:323–331. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Rezaee R, Sheidary A, Jangjoo S, Ekhtiary
S, Bagheri S, Kohkan Z, Dadres M, Oana Docea A, Tsarouhas K,
Sarigiannis DA, et al: Cardioprotective effects of
hesperidin on carbon monoxide poisoned in rats. Drug Chem Toxicol:
Aug 14, 2019 (Epub ahead of print).
|
|
25
|
Roohbakhsh A, Parhiz H, Soltani F, Rezaee
R and Iranshahi M: Neuropharmacological properties and
pharmacokinetics of the citrus flavonoids hesperidin and hesperetin
- a mini-review. Life Sci. 113:1–6. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Roohbakhsh A, Parhiz H, Soltani F, Rezaee
R and Iranshahi M: Molecular mechanisms behind the biological
effects of hesperidin and hesperetin for the prevention of cancer
and cardiovascular diseases. Life Sci. 124:64–74. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Haidari F, Heybar H, Jalali MT, Ahmadi
Engali K, Helli B and Shirbeigi E: Hesperidin supplementation
modulates inflammatory responses following myocardial infarction. J
Am Coll Nutr. 34:205–211. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Homayouni F, Haidari F, Hedayati M,
Zakerkish M and Ahmadi K: Blood pressure lowering and
anti-inflammatory effects of hesperidin in type 2 diabetes; a
randomized double-blind controlled clinical trial. Phytother Res.
32:1073–1079. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Morand C, Dubray C, Milenkovic D, Lioger
D, Martin JF, Scalbert A and Mazur A: Hesperidin contributes to the
vascular protective effects of orange juice: A randomized crossover
study in healthy volunteers. Am J Clin Nutr. 93:73–80.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Demonty I, Lin Y, Zebregs YE, Vermeer MA,
van der Knaap HC, Jäkel M and Trautwein EA: The citrus flavonoids
hesperidin and naringin do not affect serum cholesterol in
moderately hypercholesterolemic men and women. J Nutr.
140:1615–1620. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rizza S, Muniyappa R, Iantorno M, Kim JA,
Chen H, Pullikotil P, Senese N, Tesauro M, Lauro D, Cardillo C, et
al: Citrus polyphenol hesperidin stimulates production of nitric
oxide in endothelial cells while improving endothelial function and
reducing inflammatory markers in patients with metabolic syndrome.
J Clin Endocrinol Metab. 96:E782–E792. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ohtsuki K, Abe A, Mitsuzuwi H, Kondo M,
Uemura K, Iwasaki Y and Kondo Y: Effects of long-term
administration of hesperidin and glucosyl hesperidin to
spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo).
48:420–422. 2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yamamoto M, Suzuki A and Hase T:
Short-term effects of glucosyl hesperidin and hesperetin on blood
pressure and vascular endothelial function in spontaneously
hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 54:95–98.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Asgharpour A, Cazanave SC, Pacana T,
Seneshaw M, Vincent R, Banini BA, Kumar DP, Daita K, Min H-K,
Mirshahi F, et al: A diet-induced animal model of non-alcoholic
fatty liver disease and hepatocellular cancer. J Hepatol.
65:579–588. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lin HT, Shiou YL, Jhuang WJ and Lee HC:
Simultaneous electrocardiography recording and invasive blood
pressure measurement in rats. J Vis Exp. 143(e59115)2019.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Parasuraman S and Raveendran R:
Measurement of invasive blood pressure in rats. J Pharmacol
Pharmacother. 3:172–177. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dobrian AD, Davies MJ, Prewitt RL and
Lauterio TJ: Development of hypertension in a rat model of
diet-induced obesity. Hypertension. 35:1009–1015. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ikemura M, Sasaki Y, Giddings JC and
Yamamoto J: Preventive effects of hesperidin, glucosyl hesperidin
and naringin on hypertension and cerebral thrombosis in
stroke-prone spontaneously hypertensive rats. Phytother Res.
26:1272–1277. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Wunpathe C, Potue P, Maneesai P, Bunbupha
S, Prachaney P, Kukongviriyapan U, Kukongviriyapan V and
Pakdeechote P: Hesperidin suppresses renin-angiotensin system
mediated NOX2 over-expression and sympathoexcitation in 2K-1C
hypertensive rats. Am J Chin Med. 46:751–767. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Shafei MN, Faramarzi A, Khajavi Rad A and
Anaeigoudari A: Crocin prevents acute angiotensin II-induced
hypertension in anesthetized rats. Avicenna J Phytomed. 7:345–352.
2017.PubMed/NCBI
|
|
41
|
Imenshahidi M, Razavi BM, Faal A,
Gholampoor A, Mousavi SM and Hosseinzadeh H: The effect of chronic
administration of saffron (Crocus sativus) Stigma aqueous
extract on systolic blood pressure in rats. Jundishapur J Nat Pharm
Prod. 8:175–179. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Llorens S, Mancini A, Serrano-Díaz J,
D'Alessandro AM, Nava E, Alonso GL and Carmona M: Effects of
crocetin esters and crocetin from Crocus sativus L. on
aortic contractility in rat genetic hypertension. Molecules.
20:17570–17584. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mancini A, Serrano-Díaz J, Nava E,
D'Alessandro AM, Alonso GL, Carmona M and Llorens S: Crocetin, a
carotenoid derived from saffron (Crocus sativus L.),
improves acetylcholine-induced vascular relaxation in hypertension.
J Vasc Res. 51:393–404. 2014.PubMed/NCBI View Article : Google Scholar
|