Introduction
Voltage-dependent potassium (Kv) channels have been
previously demonstrated to serve an important role in canine and
mouse coronary arterial vasomotor regulation (1,2).
Streptozotocin (STZ)-induced diabetes weakened rat coronary
arterial vasodilation responsiveness to the β-adrenoceptor agonist
isoproterenol and the adenylyl cyclase activator forskolin, in
addition to reducing Kv channel currents (1,2).
Mechanistically, diabetes has been shown to downregulate the
expression of Kv1.2 and Kv1.5 channels in arterial myocytes
(3). High glucose concentrations
have been previously revealed to weaken rat coronary arterial
responsiveness to vasodilators isoproterenol and forskolin whilst
augmenting arterial sensitivity to vasoconstrictors (4), in addition to inhibiting myocyte Kv
currents in porcine coronary arteries, human internal mammary
arteries, rat mesenteric and coronary arteries (5). Therefore, preserving the expression of
Kv channels in myocytes may serve as a promising therapeutic
strategy in protection against coronary arterial dysfunction under
diabetic and hyperglycemic conditions.
Hesperetin (HSP) is one of the most abundant
flavonoids found in citrus fruits (6). It was suggested that HSP may be of
benefit in treating a number of ailments, including capillary
fragility and hypertension (6).
Higher intake of HSP in the daily diet was shown to be associated
with a reduction in human mortality as a result of cardiovascular
diseases, lung cancer and asthma (7). HSP contributes to the protective
effects of orange juice to the vascular system (8,9). It was
reported that HSP possesses antioxidant (10-12),
hypolipidemic (13),
anti-inflammatory (14),
neuroprotective (15),
cardioprotective (16), hypotensive
(17) and anti-coagulation (18) properties, in addition to being
effective in attenuating airway hypersensitivity (8,19). HSP
has been demonstrated to exert vasculoprotective and
anti-angiogenic effects in diabetic rats (20), in addition to inhibiting rat aortic
smooth muscle cell proliferation (21). A previous study on isolated blood
vessels showed that HSP treatment can induce rat aorta relaxation
(22). However, the mechanism
underlying HSP vasorelaxation currently remains controversial.
Suggested mechanisms include the enhancement of endothelial nitric
oxide production (23), suppression
of reactive oxygen species production (24), inhibition of phosphodiesterases
(22) and activation of
tetraethylammonium-sensitive K+ channels (25). Review of these studies (19,22-25)
suggests that the effects mediated by HSP are likely to involve
multiple targets, where each mechanism may contribute additively to
the eventual vasculoprotective effect downstream. A previous study
showed that HSP relaxes rat coronary arteries (RCAs) isolated from
healthy rats through, at least in part, an increase of myocyte Kv
channel currents (26). Although
accumulating evidence have indicated that HSP is promising in
preventing and treating cardiovascular diseases (7-11,13),
little is known about its effects on diabetic coronary arteries.
Therefore, the present study was designed to examine the effects of
HSP on the contraction-relaxation responsiveness of diabetic RCAs.
In addition, the potential effects of HSP on Kv channel currents
and the expression of Kv channels in rat coronary arterial smooth
muscle cells (RCASMCs) following diabetes- or high glucose-induced
injury were also investigated.
Materials and methods
Drugs and chemicals
HSP (analytical standard), L-glucose, collagenase F,
collagenase H, dithiothreitol, acetylcholine chloride (Ach),
9,11-dideoxy-9a,11a-methanoepoxy prostaglandin F2α
(U46619), forskolin, 4-aminopyridine (4-AP), Na2ATP,
STZ, HEPES, potassium aspartate, sodium carboxmethylcellulose,
DMEM, albumin and KCl were all purchased from Sigma-Aldrich; Merck
KGaA. Papain was purchased from Worthington Biochemical
Corporation. Penicillin G and streptomycin were purchased from
Beijing SolarBio Science & Technology Co., Ltd.
Animals
The experimental protocols of the present study were
approved by the Animal Care and Use Committee of Shanxi Medical
University (approval no. 2018LL348; Taiyuan, China). The Animal
Research: Reporting In Vivo Experiments guidelines were
strictly adhered (27). A total of
48 male Sprague-Dawley rats (weight, 190-220 g; age, 7-8 weeks)
were maintained at 24±2˚C, 50% humidity in a 12:12 h light/dark
cycle. The rats had free access to a standard pellet diet and tap
water.
General experimental protocol
The rats were fasted overnight and diabetes was
induced by a single intraperitoneal injection of 60 mg/kg STZ
dissolved in 0.1 M citrate buffer (pH 4.5). Age-matched
non-diabetic rats were administered a single intraperitoneal
injection of 0.1 M citrate buffer, which served as the non-diabetic
control. One week after STZ administration, plasma glucose
concentrations were measured using a glucometer. Rats with plasma
glucose levels >250 mg/dl were designated as diabetic and
randomly divided into 2 groups (n=16 rats per group): The diabetic
control and the HSP-treated group (intragastric administration of
HSP 100 mg/kg/day). Diabetic rats were treated with subcutaneous
injection of ultralente insulin (Shanghai Fosun Pharmaceutical
Group Co., Ltd.) 1-3 U/day to maintain moderate hyperglycemia to
prevent ketoacidosis and severe weight loss (28). HSP dose and concentration were
selected with reference to previous reports (20,24,26,29,30). HSP
suspended in 0.1% sodium carboxymethyl cellulose was administered
intragastrically once daily using a gavage needle with a volume of
2 ml/kg maintained throughout the experimental period for 8 weeks.
In the same manner, 2 ml/kg vehicle (0.1% sodium carboxymethyl
cellulose without HSP) was administered to the rats in the
non-diabetic control and diabetic control groups. Body weight, food
consumption and water intake were recorded once daily. By the end
of 8 weeks following STZ administration, the rats were fasted
overnight, anesthetized (intraperitoneal injection of 40 mg/kg
sodium pentobarbital) and sacrificed by exsanguination from the left
cephalic artery. Following sacrifice, the rat hearts were removed
and the coronary arteries (inner diameter, 150-280 µm) were
carefully isolated for myography, patch clamping, reverse
transcription-quantitative PCR (RT-qPCR) and western blot
analyses.
Measurement of isometric force
RCAs were cut into 2-mm long rings in 4˚C HEPES
solution composed of the following: i) NaCl, 128 mM; ii) KCl, 4.7
mM; iii) CaCl2, 2.5 mM; iv) MgCl2, 1.2 mM; v)
KH2PO4, 1.2 mM; vi) NaHCO3 10 mM;
vii) HEPES 10 mM; and viii) D-glucose 11.0 mM; pH 7.4. The rings
were mounted on a wire myograph (DMT-610 M; Danish Myo Technology
A/S) using two 40 µm tungsten wires in a tissue chamber containing
5.0 ml HEPES solution bubbled with 95% O2/5%
CO2 at 37˚C. The rings were stretched to a vascular tone
equivalent to ~80 mmHg according to the manufacturer's protocols
and equilibrated for 2 h. Following equilibration, the rings were
stimulated with 60 mM KCl for 20 min repeatedly. The ring was then
allowed to recover for 40 min after each stimulation. When the
contraction responses become reproducible,
concentration-contraction curves or concentration-relaxation curves
were constructed. In experiments of KCl-induced contraction,
equivalent concentrations of NaCl were replaced with KCl to exclude
the effect of osmolality.
The concentration-contraction curves of KCl
(20,28,39,55 and 77 mM) were constructed through the cumulative
addition of the KCl HEPES solution into the chamber. In a similar
manner, curves for U46619 (10-8-10-6 M) were
also constructed. The contraction response to each concentration of
an agonist was allowed to reach a relative tone plateau.
Vasodilator concentration-relaxation curves for acetylcholine
(3x10-8-10-5 M) and forskolin
(10-8-3x10-6 M) were constructed by the
cumulative addition of vasodilator to the chamber when the
contraction response to 60 mM KCl or 1 µM U46619 was observed to be
sustained. Relaxations were expressed as the percentage of the
contraction induced by 60 mM KCl or 1 µM U46619, respectively,
prior to treatment with the vasodilators.
Electrophysiological measurements
For single RCASMC isolation, RCAs were dissected in
0.1 mM CaCl2 HEPES solution containing 0.5 mg/ml papain,
1 mg/ml dithiothreitol and 1 mg/ml albumin, which was then
incubated for 25 min at 37˚C. Subsequently, the arteries were
transferred to the same solution deprived of Ca2+ and
incubated for 10 min at 37˚C. Single cells were dispersed
mechanically using a Pasteur pipette. Isolated RCASMCs were used
immediately for electrophysiological recording or stored in
Ca2+-free 4˚C HEPES solution for later use within 8 h of
isolation.
Single RCASMCs were plated onto the glass bottom
dishes and visualized using an inverted microscope (magnification
x400; ECLIPSE TE2000-S; Nikon Corporation). Cells were allowed to
settle for 30 min and were gently washed using HEPES solution to
remove debris. Electrophysiological responses were only recorded in
cells that are firmly adhered to the bottom surface of the chamber
and morphologically characteristic of arterial smooth muscle cells
(ASMCs) with clearly defined and visible cell membranes. All
electrophysiological experiments were performed in an
air-conditioned room at ~25˚C. Patch electrodes were pulled from
borosilicate glass capillary tubing (outside diameter, 1.5 mm;
inside diameter, 0.84 mm; Vitalsense Scientific Instruments Co.,
Ltd.) using a micropipette puller (PP-830; Narishige Group) and
polished on a microforge (MF-830; Narishige Group). The electrodes
had resistances of 3-5 MΩ when filled with a pipette solution as
undermentioned. Whole-cell voltage clamp was performed using an
Axopatch 200B amplifier (Axon Instruments), a 32-bit data
acquisition system (Digidata1440A, Molecular Devices, LLC.) and the
pClampex software 10.4 (Molecular Devices, LLC.). Pipette offset,
whole-cell capacitance and series resistance (to 80%, bandwidth
>5 kHz) were electronically compensated. The average access
resistance was 3-5 MΩ and the cell capacitance was 7-15 pF. Current
traces were filtered at 1 kHz with a low-pass 4-pole Bessel filter
in the clamp amplifier, subjected to P/4 leak subtraction and
digitized at 5 kHz before being stored on the computer hard drive
for subsequent analysis using pClampex 10.4.
Cells were perfused with a Ca2+-free
HEPES bath solution. The pipette was filled with a
Ca2+-free HEPES solution consisting of the following: i)
KCl, 110 mM; ii) MgCl2, 1.2 mM; iii) Na2ATP,
5 mM; iv) EGTA, 10 mM; and v) HEPES, 10 mM; with the pH adjusted to
7.3 using KOH. Under these conditions, ATP-sensitive K+
channel (KATP) currents and Ca2+-activated
K+ channel (KCa) currents were minimized by
the inclusion of high concentrations of ATP and EGTA in the pipette
solution (31). The remainder of the
K+ currents were markedly attenuated by the Kv channel
blocker 4-aminopyridine (4-AP; 3 mM), which were considered as
K+ currents mediated by Kv channels (31). Cells were held at holding potentials
of -60 mV and subsequently subjected to step depolarizations to +80
mV in 10 mV increments at 500 msec each.
RT-qPCR
RCAs freshly isolated from diabetic or non-diabetic
rats were used for RT-qPCR analysis. RT-qPCR was used to detect
transcript levels as previously described (32-34).
SV Total RNA Isolation System (Promega Corporation) was used to
isolate total RNA from the RCAs in accordance to the manufacturer's
protocol. A microplate reader (BioTek Instruments, Inc.) was used
to measure the concentration and purity of the RNA samples. A total
of 1 µg RNA was added into the reaction system (PrimeScript™ RT
reagent kit with gDNA Eraser; Takara Bio, Inc.) comprising of 2 µl
5X gDNA Eraser Buffer, 1 µl gDNA Eraser and appropriate volumes
DEPC-treated water to remove genomic DNA. The mixture was then
incubated for 2 min at 42˚C. Total RNA was converted into cDNA in a
20 µl PCR reaction system with 10 µl reaction system from the
previous step, 1 µl PrimeScript RT Enzyme Mix I, 1 µl RT Primer
Mix, 4 µl 5X Primer Script Buffer 2, 4 µl RNase Free
dH2O (PrimeScript™ RT reagent kit with gDNA
Eraser; Takara Bio, Inc.). C1000 Touch™ Thermal cyclers
(Bio-Rad Laboratories, Inc.) were used to perform RT with the
following heat cycle: 37˚C for 15 min and 85˚C for 5 sec. A total
of 2 µl cDNA was amplified in a 23 µl reaction system containing
12.5 µl SYBR® Premix Ex Taq™ (Takara Bio,
Inc.), 8.5 µl DEPC-treated water, 1 µl forward primer (10 µM) and 1
µl reverse primer (10 µM). The following thermocycling conditions
were used for the PCR: Initial denaturation at 95˚C for 30 sec;
followed by 40 cycles of 95˚C for 5 sec and 60˚C for 30 sec. The
melting curve included an increase from 65˚C to 95˚C for 5 sec. The
following primer pairs were used for the qPCR: Kv1.2 forward,
5'-TGTCTATCACCCAGGAACATGGAG-3' and reverse,
5'-GAGCTTGGGTCTGAAGCCTTTG-3'; Kv1.5 forward,
5'-CCTCCGACGTCTGGACTCAATAA-3' and reverse,
5'-CCTCATCCTCAGCAGATAGCCTTC-3' and GAPDH forward,
5'-GGCACAGTCAAGGCTGAGAATG-3' and reverse,
5'-ATGGTGGTGAAGACGCCAGTA-3' (Takara Bio, Inc.). Relative gene
expression was calculated using the 2-ΔΔCq method
(35).
Western blotting
Total protein was extracted from RCA samples using
RIPA buffer mixed with PMSF (Beijing SolarBio Science &
Technology Co., Ltd.), protease inhibitor cocktail (Beyotime
Institute of Biotechnology) and phosphatase inhibitor (Boster
Biological Technology) at 4˚C for 30 min. The homogenate was
cleared of debris by centrifugation at 13,000 x g at 4˚C for 20
min. Protein concentration was determined using a Bio-Rad protein
assay (Bio-Rad Laboratories, Inc.) using BSA as a standard. Total
protein (24 µg) was separated in a 10% Tris-HCl polyacrylamide gel
and transferred onto nitrocellulose blotting membranes. The
membranes were blocked in 5% non-fat milk containing TBS-0.02%
Tween-20 (TBS-T) at room temperature for 2 h. The membranes were
then incubated with polyclonal antibodies against Kv1.2 (1:400;
cat. no. APC-004; Alomone Labs), Kv1.5 (1:400; cat. no. APC-010;
Alomone Labs) or β-actin (1:4,000; cat. no. D110001-0100; Sangon
Biotech Co., Ltd.) overnight at 4˚C. Following incubation with
primary antibodies, the membranes were washed three times with
TBS-T for 10 min and incubated with horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat. no.
ZB2301; Origene technologies, Inc.) for 1 h at room temperature.
After the membrane was washed three times at 10 min intervals in
TBS-T, the bound antibodies were detected with an ECL reagent using
a ChemiDoc XRS chemiluminescence detection system (Bio-Rad
Laboratories, Inc.). The levels of β-actin were used as internal
controls for sample loading. Protein levels were quantified using
Image Lab software (5.2.1, Bio-Rad Laboratories, Inc.).
Acute incubation experiment
As previously described (36), RCAs isolated from normal rats were
incubated with various concentrations of glucose for 8 h in the
presence or the absence of HSP prior to RCASMC isolation for patch
clamping analysis, RT-qPCR and western blot assays. The incubation
was performed in DMEM with 10% FBS (Sangon Biotech Co., Ltd.), 100
U/ml penicillin G and 100 mg/ml streptomycin for 24 h at 37˚C. DMEM
was supplemented with either 5.5 mM D-glucose [normal glucose (NG);
Tianjin Damao Chemical Reagent Factory], 44 mM D-glucose (high
glucose, HG) or 5.5 mM D-glucose + 38.5 mM L-glucose (HLG). HLG
served as an osmotic control as L-glucose cannot be metabolized.
All media were filtered (0.2-mm filter) before use.
Statistical analysis
All data are expressed as the mean ± SD. Statistical
analysis was performed using ANOVA followed by Tukey's multiple
comparisons test using SPSS version 13 (SPSS, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
‘n’ represents the number of vascular rings or cells
isolated from separate rats. The maximal contraction
(Emax), the values of EC50 (vasoconstrictor
concentration producing 50% of the maximal contraction) and
RC50 (vasodilator concentration inducing 50% relaxation
on the precontraction) were calculated by non-linear regression
using GraphPad Prism version 6.0 (GraphPad Software, Inc.).
Results
Chronic HSP attenuates
diabetes-induced losses in body weight and elevations in plasma
glucose levels
At the end of 8 weeks after STZ injection, the body
weight of vehicle-treated diabetic rats was found to be
significantly lower (219.19±10.32 g vs. 328.55±9.91 g; P<0.05)
whilst the plasma glucose concentration was significantly higher
(29.77±2.73 mM vs. 5.94±0.60 mM; P<0.05) compared with those in
non-diabetic rats (Table I).
Compared with vehicle-treated diabetic rats, those treated with HSP
exhibited significantly higher body weights (264.08±9.40 g vs.
219.19±10.32; P<0.05) and lower plasma glucose levels
(20.54±2.34 mM vs. 29.77±2.73 mM; P<0.05; Table I).
 | Table IEffects of chronic HSP treatment on
body weight and plasma levels of glucose. |
Table I
Effects of chronic HSP treatment on
body weight and plasma levels of glucose.
| Groups | n | Body weight
(g) | Plasma glucose
(mM) |
|---|
| Non-diabetic | 16 | 328.55±9.91 | 5.94±0.60 |
| Diabetic | 13 |
219.19±10.32a |
29.77±2.73a |
| Diabetic+HSP | 14 |
264.08±9.40b |
20.54±2.34b |
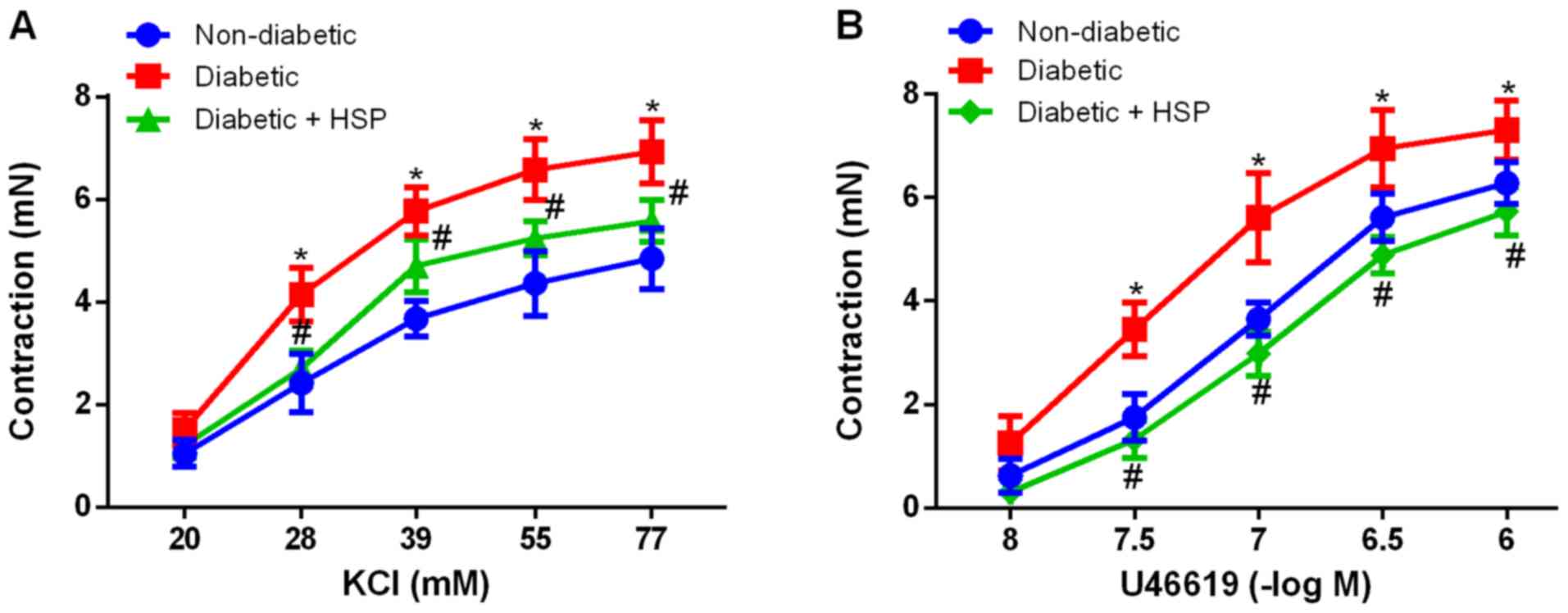
Chronic HSP treatment reverses
contractile hypersensitivity in diabetic RCAs
Both KCl (20-77 mM) and U46619
(10-8-10-6 M) application resulted in RCA
contraction in a dose-dependent manner in all three experimental
groups (Fig. 1). In the non-diabetic
group, the Emax values for KCl and U46619 were
calculated to be 4.85±0.59 and 6.28±0.41 mN, respectively (Fig. 1). By contrast, diabetic rats
exhibited significantly higher Emax values, 6.93±0.61 mN
for KCl (P<0.05) and 7.31±0.57 mN for U46619 (P<0.05).
Compared with the vehicle-treated diabetic group, HSP treatment
significantly decreased Emax for KCl to 5.58±0.34 mN
(P<0.05; Fig 1A) and
Emax for U46619 to 5.73±0.47 mN (P<0.05; Fig 1B).
Chronic HSP treatment restores
relaxant responsiveness in diabetic RCAs
Ach (Fig. 2A) and
forskolin (Fig. 2B) induced
dose-dependent relaxations in all RCA rings pre-contracted with 1
µM U46619. The degree of relaxation as a result of Ach or forskolin
treatment was observed to be significantly lower in the
vehicle-treated diabetic group compared with that in the
non-diabetic group (P<0.05). By contrast, Ach and forskolin
treatment resulted in significantly higher degrees of relaxation in
the HSP-treated diabetic group compared with those in the
vehicle-treated diabetic group (P<0.05).
Chronic HSP treatment increases Kv
currents in diabetic RCASMCs
Under the present experimental conditions, outward
K+ currents mediated through KATP and
KCa channels were minimized by the inclusion of high
concentrations of ATP and EGTA in the pipette solution. The
remaining outward K+ currents were found to be markedly
attenuated by the Kv channel blocker 4-AP (3 mM), which were
considered to be currents mediated by Kv channels (31). At a testing potential of +80 mV, the
Kv current densities were significantly reduced (26.96±4.33 pA/pF
vs. 52.51±4.86 pA/pF, P<0.05) in the vehicle-treated diabetic
group, compared with those in the non-diabetic group (Fig. 3). By contrast, the Kv current
densities were found to be significantly increased (42.27±5.02
pA/pF vs. 26.96±4.33 pA/pF, P<0.05) in the HSP-treated diabetic
group compared with those in the vehicle-treated diabetic
group.
Chronic HSP treatment increases the
expression of Kv1.2 channels in diabetic RCASMCs
To explore the possible involvement of HSP treatment
on the expression of Kv1.2 and Kv1.5 channel subtypes, both of
which are important for coronary vasomotor function (37,38),
Kv1.2 and Kv1.5 mRNA and protein expression were measured in
RCASMCs by RT-qPCR and western blot analysis. The expression of
Kv1.2, but not Kv1.5 channels, was significantly lower in diabetic
rats compared with that in non-diabetic rats (Fig. 4). Diabetic rats treated with HSP
exhibited significantly increased Kv1.2 expression on both mRNA and
protein levels compared with those of vehicle-treated diabetic rats
(Fig. 4).
Acute application of HSP attenuates
the suppression of Kv currents by high glucose in RCASMCs
Incubation of RCASMCs with 44 mM glucose (HG) for 8
h suppressed the Kv current density at a test potential of +80 mV
compared with that in the normal control (NG) (36.48±4.62 pA/pF vs.
56.17±3.85, P<0.05), but not osmotic elevation with L-glucose
(HLG, 56.83±5.01 pA/pF). Co-incubation with 30 µM HSP significantly
attenuated high glucose-induced suppression of the Kv current
(47.47±3.68 pA/pF, P<0.05), whilst 100 µM HSP treatment
significantly reversed the suppression (61.25±5.51 pA/pF,
P<0.05; Fig. 5).
Acute application of HSP attenuates
high-glucose-induced downregulation of Kv1.2 expression in
RCASMCs
Incubation of RCASMCs for 8 h with 44 mM glucose
(HG) reduced Kv1.2 protein expression by 41.93% compared with NG,
which was significantly reversed by co-incubation with 100 µM HSP
(Fig. 6). Treatment with 30 µM HSP
also increased Kv1.2 protein expression, but no statistical
significance was observed compared with that in HG (Fig. 6).
Discussion
The present study investigated the effects of
intragastric HSP administration on coronary arterial vasomotor
function in rats with STZ-induced diabetes. The main findings of
the present study were as follows: i) Chronic HSP administration
improved contraction-relaxation responsiveness of diabetic RCAs;
ii) chronic HSP administration augmented Kv channel currents and
upregulated the expression of Kv1.2 channels in diabetic RCASMCs;
and iii) acute incubation with HSP counteracted both the reduction
of Kv channel currents and downregulation of Kv1.2 expression
induced by high glucose in RCASMCs.
The STZ-induced diabetic rat model is a widely
accepted animal model of diabetes as the rats typically exhibit
characteristic symptoms of diabetes, including hyperglycemia,
glucose intolerance, body weight loss and vascular dysfunction
(39). In the present study, the
diabetic rats exhibited significant decreases in body weight and
higher glucose levels compared with non-diabetic rats. Chronic
administration of HSP (100 mg/kg per day for 8 weeks) attenuated
the diabetes-induced reductions in body weight loss and
hyperglycemia. These effects could be due to the stimulation of
glucose uptake by peripheral tissues, antioxidation, renoprotection
and protective effects on pancreatic islet β-cells, based on
previous reports (40,41). Compared with RCAs from non-diabetic
rats, RCAs from diabetic rats exhibited increased sensitivity to
vasoconstrictors KCl and U46619 whilst exhibiting opposite effects
to vasodilators Ach and forskolin. Ach is an endothelium-dependent
vasodilator whereas forskolin is an endothelium-independent
vasodilator, which induces vasodilation through adenylyl cyclase
activation (42). These data suggest
that both endothelium-dependent and endothelium-independent
vasodilation pathways were impaired in diabetic rats, consistent
with previous findings (43-47).
The present study demonstrated that chronic HSP not
only attenuated diabetes-induced RCA hypersensitivity to KCl and
U46619, but also restored the sensitivity of RCAs to vasodilators.
These results implicate at the extension of the vasospasmolytic
effects of HSP to diabetic RCAs. The observation that HSP restored
responsiveness to both endothelium-dependent and -independent
vasodilators suggested that in addition to possible effects on the
endothelium, HSP also may exert direct effects on RCASMCs. This is
consistent with a previous finding that HSP inhibited the myocyte
voltage-dependent Ca2+ channels and increased the
myocyte Kv channel activity (26).
K+ channels are pivotal in the
maintenance of cell membrane potential and regulation of vascular
tone in coronary arteries (48). The
activation of K+ channels leads to K+ efflux,
membrane hyperpolarization, reducing Ca2+ influx into
the cell and resistance to vascular myocyte contraction (49). Among a number of K+
channel families, Kv channels are significant in the regulation of
coronary arterial resistance (50-53). Both diabetes
(1) and high glucose (5) have been previously demonstrated to
reduce Kv channel currents and downregulated the expression of Kv
1.2 channels in RCASMCs. Therefore, it was hypothesized in the
present study that improvement of Kv channel activity may be
involved in the beneficial effects of HSP on RCAs of diabetic
rats.
Although it was suggested that a number of
mechanisms are involved in the vasorelaxant effects of HSP
(22-25),
none could adequately explain the vascular effects of HSP. A recent
in vitro study demonstrated that HSP relaxed coronary
arteries isolated from normal rats by increasing Kv currents and
inhibiting Ca2+ influx in ASMCs (26). To explore the mechanism underlying
the protective effects of HSP on RCAs from diabetic rats, Kv
channel function was evaluated using the patch clamp technique. Kv
currents were found to be increased in chronically HSP-treated
diabetic rats compared with those in vehicle-treated diabetic rats.
These results suggest that recovery of Kv function in RCASMCs may
underlie the improvements in diabetic coronary arteries in response
to HSP. To substantiate this, Kv channel expression was measured in
RCAs from diabetic rats and in RCAs pre-incubated with high
glucose. Diabetic induction was found to reduce the expression of
Kv1.2 channels, but not Kv1.5. Chronic HSP administration increased
the expression of Kv1.2, but not Kv1.5, in diabetic rats. Since
elevated glucose is the main cause of diabetes-induced damage to
the vasculature (54), the present
study also investigated the in vitro effects of high glucose
on the physiology of Kv channels. It was found that high glucose
reduced Kv currents and downregulated Kv1.2 protein expression,
both of which were reversed by co-incubation with HSP.
HSP depressed depolarization-induced contractions in
both non-diabetic and diabetic RCAs, suggesting that apart from its
potential effects on Kv channels, other mechanisms may be involved
in its protective effects (22-26).
The molecular signaling mechanisms underlying vascular
contraction-relaxation and the regulation of Kv channel function
are complex (55-57). The effects of
HSP on the expression profiles of the Kv1 channel subunits and
associated signaling pathways remain to be clarified. To the best
of our knowledge, the present study was the first to report the
beneficial effects of HSP on diabetic coronary arteries via the
upregulation of myocyte Kv channels.
In conclusion, the present study demonstrated that
HSP restored the contraction-relaxation responsiveness of diabetic
RCAs, augmented Kv currents and upregulated the expression of Kv1.2
channels in RCASMCs of diabetic rats or in RCASMCs exposed to high
glucose. These results suggested that HSP may be a promising
therapeutic agent for the treatment of coronary arterial
dysfunction as a result of diabetes.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. NSFC81603111 and
NSFC 81773738), The Fund for Shanxi Key Subjects Construction and
The Fund for Shanxi 1331 Project Key Subjects Construction (grant
no. XK200708).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and MZ designed the experiments, analyzed the
data, prepared the figures and wrote the manuscript. YL, LD, QS,
PG, YW and ZC performed the chronic experiments and myograph study.
LZ, LD and PG performed the patch clamp technique; QS and YW
performed RT-qPCR and western blot analysis. All authors
contributed to drafting the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The authors declare that all protocols and
procedures described in this animal study were approved by the
Animal Ethics Committee of Shanxi Medical University (approval no.
2018LL348; Taiyuan, China). No human body materials and data were
used in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chai Q, Liu Z and Chen L: Effects of
streptozotocin-induced diabetes on Kv channels in rat small
coronary smooth muscle cells. Chin J Physiol. 48:57–63.
2005.PubMed/NCBI
|
|
2
|
Bubolz AH, Li H, Wu Q and Liu Y: Enhanced
oxidative stress impairs cAMP-mediated dilation by reducing Kv
channel function in small coronary arteries of diabetic rats. Am J
Physiol Heart Circ Physiol. 289:H1873–H1880. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chai Q, Xu X, Jia Q, Dong Q, Liu Z, Zhang
W and Chen L: Molecular basis of dysfunctional Kv channels in small
coronary artery smooth muscle cells of streptozotocin-induced
diabetic rats. Chin J Physiol. 50:171–177. 2007.PubMed/NCBI
|
|
4
|
Jackson R, Brennan S, Fielding P, Sims MW,
Challiss RA, Adlam D, Squire IB and Rainbow RD: Distinct and
complementary roles for alpha and beta isoenzymes of PKC in
mediating vasoconstrictor responses to acutely elevated glucose. Br
J Pharmacol. 173:870–887. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu Y, Terata K, Rusch NJ and Gutterman
DD: High glucose impairs voltage-gated K(+) channel current in rat
small coronary arteries. Circ Res. 89:146–152. 2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Garg A, Garg S, Zaneveld LJ and Singla AK:
Chemistry and pharmacology of the citrus bioflavonoid hesperidin.
Phytother Res. 15:655–669. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Knekt P, Kumpulainen J, Järvinen R,
Rissanen H, Heliövaara M, Reunanen A, Hakulinen T and Aromaa A:
Flavonoid intake and risk of chronic diseases. Am J Clin Nutr.
76:560–568. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morand C, Dubray C, Milenkovic D, Lioger
D, Martin JF, Scalbert A and Mazur A: Hesperidin contributes to the
vascular protective effects of orange juice: A randomized crossover
study in healthy volunteers. Am J Clin Nutr. 93:73–80.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rendeiro C, Dong H, Saunders C, Harkness
L, Blaze M, Hou Y, Belanger RL, Corona G, Lovegrove JA and Spencer
JPE: Flavanone-rich citrus beverages counteract the transient
decline in postprandial endothelial function in humans: A
randomised, controlled, double-masked, cross-over intervention
study. Br J Nutr. 116:1999–2010. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choi EJ: Antioxidative effects of
hesperetin against 7,12-dimethylbenz(a)anthracene-induced oxidative
stress in mice. Life Sci. 82:1059–1064. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim JY, Jung KJ, Choi JS and Chung HY:
Hesperetin: A potent antioxidant against peroxynitrite. Free Radic
Res. 38:761–769. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pollard SE, Whiteman M and Spencer JP:
Modulation of peroxynitrite-induced fibroblast injury by hesperet
in: A role for intracellular scavenging and modulation of ERK
signalling. Biochem Biophy Res Commun. 347:916–923. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Morin B, Nichols LA, Zalasky KM, Davis JW,
Manthey JA and Holland LJ: The citrus flavonoids hesperetin and
nobiletin differentially regulate low density lipoprotein receptor
gene transcription in HepG2 liver cells. J Nutr. 138:1274–1281.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hirata A, Murakami Y, Shoji M, Kadoma Y
and Fujisawa S: Kinetics of radical-scavenging activity of
hesperetin and hesperidin and their inhibitory activity on COX-2
expression. Anticancer Res. 25:3367–3374. 2005.PubMed/NCBI
|
|
15
|
Huang SM, Tsai SY, Lin JA, Wu CH and Yen
GC: Cytoprotective effects of hesperetin and hesperidin against
amyloid beta-induced impairment of glucose transport through
downregulation of neuronal autophagy. Mol Nutr Food Res.
56:601–609. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Trivedi PP, Kushwaha S, Tripathi DN and
Jena GB: Cardioprotective effects of hesperetin against
doxorubicin-induced oxidative stress and DNA damage in rat.
Cardiovasc Toxicol. 11:215–225. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamamoto M, Suzuki A and Hase T:
Short-Term effects of glucosyl hesperidin and hesperetin on blood
pressure and vascular endothelial function in spontaneously
hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 54:95–98.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jin YR, Han XH, Zhang YH, Lee JJ, Lim Y,
Chung JH and Yun YP: Antiplatelet activity of hesperetin, a
bioflavonoid, is mainly mediated by inhibition of PLC-gamma2
phosphorylation and cyclooxygenase-1 activity. Atherosclerosis.
194:144–152. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shih CH, Lin LH, Hsu HT, Wang KH, Lai CY,
Chen CM and Ko WC: Hesperetin, a selective phosphodiesterase 4
inhibitor, effectively suppresses ovalbumin-induced airway
hyperresponsiveness without influencing xylazine/ketamine-induced
anesthesia. Evid Based Complement Alternat Med.
2012(472897)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kumar B, Gupta SK, Srinivasan BP, Nag TC,
Srivastava S and Saxena R: Hesperetin ameliorates hyperglycemia
induced retinal vasculopathy via anti-angiogenic effects in
experimental diabetic rats. Vascul Pharmacol. 57:201–207.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jin YR, Han XH, Zhang YH, Lee JJ, Lim Y,
Kim TJ, Yoo HS and Yun YP: Hesperetin, a bioflavonoid, inhibits rat
aortic vascular smooth muscle cells proliferation by arresting cell
cycle. J Cell Biochem. 104:1–14. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Orallo F, Alvarez E, Basaran H and Lugnier
C: Comparative study of the vasorelaxant activity,
superoxide-scavenging ability and cyclic nucleotide
phosphodiesterase-inhibitory effects of hesperetin and hesperidin.
Naunyn Schmiedebergs Arch Pharmacol. 370:452–463. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu L, Xu DM and Cheng YY: Distinct
effects of naringenin and hesperetin on nitric oxide production
from endothelial cells. J Agric Food Chem. 56:824–829.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Takumi H, Nakamura H, Simizu T, Harada R,
Kometani T, Nadamoto T, Mukai R, Murota K, Kawai Y and Terao J:
Bioavailability of orally administered water-dispersible hesperetin
and its effect on peripheral vasodilatation in human subjects:
Implication of endothelial functions of plasma conjugated
metabolites. Food Funct. 3:389–398. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Calderone V, Chericoni S, Martinelli C,
Testai L, Nardi A, Morelli I, Breschi MC and Martinotti E:
Vasorelaxing effects of flavonoids: Investigation on the possible
involvement of potassium channels. Naunyn Schmiedebergs Arch
Pharmacol. 370:290–298. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu Y, Niu L, Cui L, Hou X, Li J, Zhang X
and Zhang M: Hesperetin inhibits rat coronary constriction by
inhibiting Ca(2+) influx and enhancing voltage-gated K(+) channel
currents of the myocytes. Eur J Pharmacol. 735:193–201.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
McGrath JC and Lilley E: Implementing
guidelines on reporting research using animals (ARRIVE etc.): New
requirements for publication in BJP. Br J Pharmacol. 172:3189–3193.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Scholey JW and Meyer TW: Control of
glomerular hypertension by insulin administration in diabetic rats.
J Clin Invest. 83:1384–1389. 1989.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kanaze FI, Bounartzi MI, Georgarakis M and
Niopas I: Pharmacokinetics of the citrus flavanone aglycones
hesperetin and naringenin after single oral administration in human
subjects. Eur J Clin Nutr. 61:472–477. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kumar B, Gupta SK, Srinivasan BP, Nag TC,
Srivastava S, Saxena R and Jha KA: Hesperetin rescues retinal
oxidative stress, neuroinflammation and apoptosis in diabetic rats.
Microvasc Res. 87:65–74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cogolludo A, Moreno L, Bosca L, Tamargo J
and Perez-Vizcaino F: Thromboxane A2-induced inhibition of
voltage-gated K+ channels and pulmonary vasoconstriction: Role of
protein kinase Czeta. Circ Res. 93:656–663. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gradel AKJ, Salomonsson M, Sorensen CM,
Holstein-Rathlou NH and Jensen LJ: Long-term diet-induced
hypertension in rats is associated with reduced expression and
function of small artery SKCa, IKCa, and Kir2.1 channels. Clin Sci
(Lond). 132:461–474. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Albarwani S, Nemetz LT, Madden JA, Tobin
AA, England SK, Pratt PF and Rusch N: Voltage-Gated K+ channels in
rat small cerebral arteries: Molecular identity of the functional
channels. J Physiol. 551:751–763. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shen X, Li H, Li W, Wu X and Ding X:
Pioglitazone prevents hyperglycemia induced decrease of AdipoR1 and
AdipoR2 in coronary arteries and coronary VSMCs. Mol Cell
Endocrinol. 363:27–35. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li H, Chai Q, Gutterman DD and Liu Y:
Elevated glucose impairs cAMP-mediated dilation by reducing Kv
channel activity in rat small coronary smooth muscle cells. Am J
Physiol Heart Circ Physiol. 285:H1213–H1219. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dick GM, Bratz IN, Borbouse L, Payne GA,
Dincer UD, Knudson JD, Rogers PA and Tune JD: Voltage-Dependent K+
channels regulate the duration of reactive hyperemia in the canine
coronary circulation. Am J Physiol Heart Circ Physiol.
294:H2371–H2381. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ohanyan V, Yin L, Bardakjian R, Kolz C,
Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, et al:
Requisite role of kv1.5 channels in coronary metabolic dilation.
Circ Res. 117:612–621. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gheibi S, Kashfi K and Ghasemi A: A
practical guide for induction of type-2 diabetes in rat:
Incorporating a high-fat diet and streptozotocin. Biomed
Pharmacother. 95:605–613. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fang XK, Gao J and Zhu DN: Kaempferol and
quercetin isolated from Euonymus alatus improve glucose uptake of
3T3-L1 cells without adipogenesis activity. Life Sci. 82:615–622.
2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang DK and Kang HS: Anti-Diabetic effect
of cotreatment with quercetin and resveratrol in
streptozotocin-induced diabetic rats. Biomol Ther (Seoul).
26:130–138. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wright IK, Amirchetty-Rao S and Kendall
DA: Potentiation by forskolin of both SNP- and ANP-stimulated
cyclic GMP accumulation in porcine isolated palmar lateral vein. Br
J Pharmacol. 112:1146–1150. 1994.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Elms SC, Toque HA, Rojas M, Xu Z, Caldwell
RW and Caldwell RB: The role of arginase I in diabetes-induced
retinal vascular dysfunction in mouse and rat models of diabetes.
Diabetologia. 56:654–662. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mokhtar SS, Vanhoutte PM, Leung SW,
Suppian R, Yusof MI and Rasool AH: Reduced nitric oxide-mediated
relaxation and endothelial nitric oxide synthase expression in the
tail arteries of streptozotocin-induced diabetic rats. Eur J
Pharmacol. 773:78–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Malakul W, Thirawarapan S, Ingkaninan K
and Sawasdee P: Effects of kaempferia parviflora wall. Ex baker on
endothelial dysfunction in streptozotocin-induced diabetic rats. J
Ethnopharmacol. 133:371–377. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Goulopoulou S, Hannan JL, Matsumoto T,
Ogbi S, Ergul A and Webb RC: Reduced vascular responses to soluble
guanylyl cyclase but increased sensitivity to sildenafil in female
rats with type 2 diabetes. Am J Physiol Heart Circ Physiol.
309:H297–H304. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Radovi T, Bömicke T, Kökény G, Arif R,
Loganathan S, Kécsán K, Korkmaz S, Barnucz E, Sandner P, Karck M
and Szabó G: The phosphodiesterase-5 inhibitor vardenafil improves
cardiovascular dysfunction in experimental diabetes mellitus. Br J
Pharmacol. 156:909–919. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wu GB, Zhou EX, Qing DX and Li J: Role of
potassium channels in regulation of rat coronary arteriole tone.
Eur J Pharmacol. 620:57–62. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Salomonsson M, Brasen JC and Sorensen CM:
Role of renal vascular potassium channels in physiology and
pathophysiology. Acta Physiol (Oxf). 221:14–31. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dick GM and Tune JD: Role of potassium
channels in coronary vasodilation. Exp Biol Med (Maywood).
235:10–22. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Berwick ZC, Moberly SP, Kohr MC, Morrical
EB, Kurian MM, Dick GM and Tune JD: Contribution of
voltage-dependent K+ and Ca2+ channels to coronary pressure-flow
autoregulation. Basic Res Cardiol. 107(264)2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nishijima Y, Cao S, Chabowski DS,
Korishettar A, Ge A, Zheng X, Sparapani R, Gutterman DD and Zhang
DX: Contribution of KV1.5 channel to hydrogen peroxide-induced
human arteriolar dilation and its modulation by coronary artery
disease. Circ Res. 120:658–669. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ohanyan V, Yin L, Bardakjian R, Kolz C,
Enrick M, Hakobyan T, Kmetz J, Bratz I, Luli J, Nagane M, et al:
Requisite role of kv1.5 channels in coronary metabolic dilation.
Circul Res. 117:612–621. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dong Y, Fernandes C, Liu Y, Wu Y, Wu H,
Brophy ML, Deng L, Song K, Wen A and Wong S: Role of endoplasmic
reticulum stress signalling in diabetic endothelial dysfunction and
atherosclerosis. Diab Vasc Dis Res. 14:14–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kerr PM, Clement-Chomienne O, Thorneloe
KS, Chen TT, Ishii K, Sontag DP, Walsh MP and Cole WC:
Heteromultimeric Kv1.2-Kv1.5 channels underlie
4-aminopyridine-sensitive delayed rectifier K(+) current of rabbit
vascular myocytes. Circ Res. 89:1038–1044. 2001.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Thorneloe KS, Chen TT, Kerr PM, Grier EF,
Horowitz B, Cole WC and Walsh MP: Molecular composition of
4-aminopyridine-sensitive voltage-gated K(+) channels of vascular
smooth muscle. Circ Res. 89:1030–1037. 2001.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Nieves-Cintrón M, Syed AU, Nystoriak MA
and Navedo MF: Regulation of voltage-gated potassium channels in
vascular smooth muscle during hypertension and metabolic disorders.
Microcirculation. 25:2018.PubMed/NCBI View Article : Google Scholar
|