Introduction
Liver disease, which includes acute liver injury and
chronic liver disease, is a major cause of morbidity and mortality
worldwide (1). Chemicals or toxins
typically cause acute liver injury, whereas chronic liver disease
is stimulated by numerous factors, such as viral hepatitis,
alcohol, drugs and metabolic and autoimmune diseases (2). Acute liver injury is a lethal condition
characterized by widespread hepatocyte necrosis, acute
deterioration of liver function and subsequent multiorgan failure.
Chronic liver injury-induced liver fibrosis can lead to the
development of liver cirrhosis and hepatocellular carcinoma at the
end stage (3). Liver transplantation
is currently regarded as one of the most effective treatment
options for acute liver injury and end-stage chronic liver injury;
however, the extreme shortage of organ donors, high cost of
surgery, immunological rejection risk and transplantation
complications severely hamper treatment by liver transplantation
(4). Alternatively, transplantation
of hepatocytes, particularly those isolated from fetal liver, is
considered a promising therapy for the treatment of liver diseases
(5). Similar problems, such as the
shortage of organ donors and the risk of immunological rejection of
allogenic hepatocytes, also exist in the clinical application of
this treatment (2).
Stem cell transplantation, particularly mesenchymal
stem cell (MSC) therapy, has shown potential for the treatment of
liver diseases (3). MSCs are
recognized as promising stem cells for cytotherapy due to their
multipotency and paracrine effects. MSCs have been isolated from
various tissues, including bone marrow, adipose tissue, placenta,
dental pulp, endometrium, perinatal tissues and other mesodermal
tissues (6,7). However, there is no consensus on
surface markers that identify MSCs from various sources, with the
exception that the minimum criteria of MSC markers include positive
expression of CD105, CD73, CD44 and CD90, and negative expression
of CD45, CD34, CD14 and HLA-DR (8).
Studies on animal models have revealed that heterogenic MSCs can
ameliorate liver fibrosis and fulminant hepatic failure through
paracrine and immunoregulatory effects (9). In veterinarian applications, MSCs
rescue animals suffering from acute liver injuries caused by
incidents such as accidental ingestion of poison (9-11).
The therapeutic mechanisms underlying the effects of MSCs include
their multipotent capacity to differentiate into various cell
types, including hepatocyte-like cells (HLCs), under appropriate
conditions (12). The substitution
of hepatocytes with MSCs differentiated into HLCs for liver disease
treatment is expected to overcome the shortage of liver donors
(13). However, studies have
reported that MSCs from various sources or tissues present
different cell characteristics, molecular functions and clinical
therapeutic effects (14-16).
Bone marrow has commonly been regarded as the most
conventional stem cell source in the field of cytotherapy for liver
diseases, due to the ability of bone marrow cells to differentiate
into HLCs in vitro and in vivo (17). However, the collection of bone marrow
is an invasive procedure that can cause severe pain to the donor,
which limits the applicability of bone marrow-derived MSCs
(BM-MSCs) for clinical therapy (18). Conversely, adipose tissue is
ubiquitous; it is easy to obtain, and the collection procedure is
associated with less morbidity and patient discomfort (19). Therefore, the application of adipose
tissue-derived MSCs (AT-MSCs) for cellular therapeutic research is
feasible and has been shown to be both safe and efficacious in
preclinical and clinical studies (19). Although previous studies have
reported that AT-MSCs can differentiate into HLCs in vitro
and in vivo, knowledge about the differentiation potential
of BM-MSCs and AT-MSCs is lacking (20-22).
Only one study has compared the differentiation success rates of
equine BM-MSCs and AT-MSCs into HLCs, and the results revealed that
BM-MSCs could completely differentiate into HLCs, whereas AT-MSCs
failed to fully differentiate (23).
An investigation into the differentiation potential of BM-MSCs and
AT-MSCs into HLCs will therefore be beneficial for the
identification of liver disease stem cell therapies.
Rhesus macaques are one of the most widely used
laboratory animals in biomedical research due to their genetic,
physiological, behavioral and neurological similarities to humans,
and because macaques provide excellent translational validity in
preclinical studies (24). The
present study therefore aimed to investigate the differentiation
potential of rhesus macaque BM-MSCs and AT-MSCs into HLCs in
vitro, and to provide the basis for selection of seed cells
that trans-differentiate into HLCs for cytotherapy of acute or
chronic liver injuries in either clinical or veterinary
medicine.
Materials and methods
Animals
A total of two male rhesus macaques (age, 2 years)
with a body weight of 2-3 kg were used as bone marrow and adipose
tissue donors. The rhesus macaques were individually caged in an
animal room with a 12/12 h light/dark cycle, and provided with
commercial monkey chow, sterile water, fresh fruits and vegetables
ad libitum. The temperature of the animal room was
controlled between 18-26˚C and with humidity from 40 to 70%. Animal
studies were approved by the Institutional Animal Care and Use
Committee of Kunming University of Science and Technology (approval
number: LPBR20170201) and were performed in accordance with the
Guide for the Care and Use of Laboratory Animals (25).
Preparation of rhesus macaque BM-MSCs
and AT-MSCs
BM-MSCs were isolated from the tibias of young
rhesus macaques using the procedures described in detail in a
previous study (9). AT-MSCs were
isolated from the mesenteric adipose tissue of young rhesus
macaques. Briefly, the adipose tissues were washed with 75% alcohol
and PBS, cut into small pieces(~0.5x0.5 cm) and placed into 10-cm
plastic dishes containing Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% (v/v)
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific) and 1%
(v/v) penicillin/streptomycin (Gibco; Thermo Fisher Scientific) in
sterile conditions. The adipose tissues were cultured in an
incubator at 37˚C with a humidified atmosphere of 5%
CO2, which was the same as the BM-MSC culture
conditions. The medium was refreshed every 48 h. After 10 days, the
primary cell culture was passaged at 80% confluence with 0.25%
trypsin (Gibco; Thermo Fisher Scientific, Inc.).
BM-MSCs and AT-MSCs were resuspended in culture
medium at a dilution ratio of 1:3 and expanded on a new plastic
petri dish. The morphology, surface markers and differentiation
potency of the MSCs were determined at passage 3.
Flow cytometry of the immunophenotype
surface markers of MSCs
The procedures were described in detail in our
previous study (9). Briefly,
5x105 MSCs were collected, washed and centrifuged (500 x
g; 5 min; room temperature) in 500 µl PBS containing 3% FBS
(PBSF). MSCs were then resuspended in 100 µl PBSF and incubated
with 5 µl (10 µg/µl) antibody markers (Human MSC Analysis kit; cat.
no. 562245; BD Biosciences) on ice for 30 min, washed and
resuspended in 500 µl PBSF, and examined using flow cytometry (C6;
BD Biosciences) and analyzed using using the built-in software.
Evaluation of the differentiation
potential of MSCs
These procedures were described in detail in our
previous study (9). Briefly, for
adipogenic differentiation, BM-MSCs and AT-MSCs were seeded into
24-well plates (8x104 cells per well), cultured for 12 h
and treated with adipogenic differentiation medium (Gibco; Thermo
Fisher Scientific, Inc.) for 7 days; the medium was refreshed every
3 days. Adherent cells were stained red with 60% Oil Red O
(Sigma-Aldrich; Merck KGaA) for 1 min at room temperature. For
osteogenic differentiation, BM-MSCs and AT-MSCs were seeded into
24-well plates (4x104 cells per well), cultured for 12 h
and treated with osteogenic differentiation medium (Gibco; Thermo
Fisher Scientific, Inc.) for 21 days; the medium was refreshed
every 3 days. Osteogenic differentiation was confirmed by 0.2%
Alizarin Red staining for 5 min at room temperature. For
chondrogenic differentiation, 2x105 MSCs were collected
in 15-ml centrifuge tubes and cultured with chondrogenic
differentiation medium (Gibco; Thermo Fisher Scientific, Inc.) for
21 days; the medium was refreshed every 3 days. The chondroid
pellets were sectioned (8 µm) with a freezing microtome. The slices
were stained with 1% toluidine blue for 3 min at room temperature
and were captured using a light microscope (Olympus Corporation) at
a x50 magnification.
Hepatogenic differentiation
protocol
For hepatogenic differentiation, the commonly used
three-step protocol was applied, which includes serum-free culture,
differentiation and maturation steps (26). Firstly, passage 3 cells were seeded
on a 6-well plastic plate at a density of
2x104/cm2 and cultured in serum-deprived
medium containing 20 ng/ml epidermal growth factor (EGF; PeproTech
EC Ltd.) and 10 ng/ml basic fibroblast growth factor (bFGF;
PeproTech EC Ltd.) for 2 days. Hepatogenic differentiation was
sustained for 7 days, and the cells were cultured in
differentiation medium consisting of DMEM supplemented with 10%
FBS, 10 ng/ml bFGF, 0.6 mg/ml nicotinamide (PeproTech EC Ltd.) and
20 ng/ml hepatocyte growth factor (HGF; PeproTech EC Ltd.). At the
maturation step, the cells were cultured in maturation medium
consisting of DMEM supplemented with 10% FBS, 20 ng/ml oncostatin M
(PeproTech EC Ltd.), 1 µM dexamethasone (Beijing Solarbio Science
& Technology Co., Ltd) and 50 µg/ml
insulin-transferrin-selenium (ITS; PeproTech EC Ltd.) for 2 weeks.
The culture medium was changed every 3 days.
Hepatocyte isolation
Whole liver was obtained from a 2-year-old rhesus
macaque following euthanasia with intravenous sodium pentobarbital
at 100 mg/kg body weight, according to the Guide for the Care and
Use of Laboratory Animals Primary monkey hepatocytes were isolated
using a modified multipoint puncture perfusion technique (27). Briefly, the liver tissue samples were
flushed with 38˚C perfusion buffer (NaCl, 9.3 g/l; KCl, 0.5 g/l;
HEPES, 2.4 g/l; EGTA, 0.95 g/l) via repetitive multipoint puncture
with a 10-ml sterile syringe until the liver changed to an
off-white color and the perfusion buffer turned clear. The tissue
was then continuously perfused with a prewarmed digestion buffer
solution (perfusion buffer with 0.05% collagenase IV). After
sufficient digestion, the liver tissue was mechanically disrupted
using ophthalmic scissors and an operating knife. The chopped
tissue was suspended in DMEM (containing 10% FBS and 1%
penicillin/streptomycin) and repeatedly pipetted up and down prior
to gentle shaking. The hepatocyte suspension was divided into equal
aliquots, which were then filtered through a 500-µm strainer and
centrifuged at 50 x g for 2 min at 4˚C. The suspension was removed,
added to red blood cell lysis buffer (Beyotime Institute of
Biotechnology) and incubated for 5 min at room temperature before
centrifugation at 50 x g for 2 min at 4˚C. Sedimentary cells were
washed with DNaseI solution (Beyotime Institute of Biotechnology)
and filtered through a 250-µm nylon membrane, and the cells were
harvested by low-speed centrifugation at 50 x g for 5 min at 4˚C.
Finally, the hepatocytes were seeded on 6-well plates at a density
of 2x104 cells/cm2 and cultured with
high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C
and 5% CO2. Due to their inability to proliferate,
hepatocytes were cultured for 5 days before testing in the present
study.
Periodic acid-Schiff (PAS) staining
for hepatic glycogen detection
PAS staining is a typical technique used to detect
hepatocyte function (28). The
functional features of HLCs were assessed for glycogen deposition
using a PAS staining kit (Beijing Leagene Biotech Co., Ltd). The
cell culture medium was removed from the plates, and the cells were
rinsed with PBS three times. Subsequently, the cells were fixed in
methyl alcohol (99% purity) for 10 min at room temperature. After
being washed three more times with PBS, the cells were oxidized for
15 min with 1% periodic acid and washed three times with deionized
water, then stained with Schiff's reagent at room temperature for
20 min. After being washed three times with PBS, the cells were
stained with Mayer's hematoxylin (room temperature) for 1 min.
Finally, the dye was washed off with PBS prior to further
evaluation under a light microscope (Olympus Corporation) at a
magnification of x50.
Urea production assay
The urea production test is one of the most widely
used methods to detect the function of hepatocytes (28). The culture media from MSCs and HLCs
were changed every 3 days normally and collected 3-day culture
before the timepoints (0, 9, 16 and 23 days), and the culture media
from hepatocytes were changed at 2 days and collected after a 3-day
culture. All culture media were assessed for urea production using
a urease method kit (Beijing Leagene Biotech Co., Ltd.; cat.no:
TC1165), according to the manufacturer's protocol. Briefly, urease
working solution, phenol coloring solution and urea test solution
were mixed with standard urea and with the samples, which were
measured at 540 nm with a 96-well microplate reader following
incubation at 37˚C for 30 min. Subsequently, the concentrations of
the samples were calculated. Three independent urea samples were
assessed at each time point and each assay was repeated three
times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from monkey hepatocytes and
HLCs differentiated from monkey BM-MSCs and AT-MSCs using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The precipitated RNA was solubilized in sterile
diethylpyrocarbonate-treated water (Sangon Biotech), and cDNA was
then synthesized using a Prime-Script RT reagent kit (Takara
Biotechnology Co., Ltd) at 37˚C for 5 min and 85˚C for 5 sec.
Quantification of specific genes was performed using
SYBR® Premix Ex TaqTM II kit (Takara
Biotechnology Co., Ltd) and CXF real-time PCR system (Bio-Rad
Laboratories, Inc.) at particular condition (Stage 1: 95˚C for 30
sec; stage 2: 95˚C for 3 sec; 60˚C for 30 sec and repeat for 40
cycles). All experiments were performed in triplicate, and the data
were analyzed using the 2-∆∆Cq method (29). The gene-specific primers were
commercially synthesized (Sangon Biotech), and sequence information
is shown in Table I.
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
sequences (5'-3') | Reverse primer
sequences (5'-3') |
|---|
| GAPDH |
ACGGATTTGGTCGTATTGG |
GCTCCTGGAAGATGGTGAT |
| CK-18 |
GCCCGCTATGCCCTACAGAT |
TTCACTGACACCATTCTTTCG |
| HNF-4α |
CCACGGGCAAACACTACGG |
TGGACGGCTTCCTTCTTCAT |
| ALB |
AAGGCTTGGTGCTGGTT |
GTTCGGGTTGTCATCTTTGT |
| CYP3A4 |
AAAAGAAAGTCGCCTCAAAGA |
GAAGGAAAGAACACTGCTGGT |
| CYP7A1 |
TTTCCAGTGCCTCCCTCAAC |
GGTAGTCTTTGTCTTCCCGTTTT |
Data analysis
All data are presented as the mean ± SD. Statistical
significance among groups was analyzed using repeated measures
ANOVA with a post-hoc paired t-test with Bonferroni's correction.
Statistical significance within groups at time-point was analyzed
using Student's t-test. P<0.05 was considered to indicate a
statistically significant difference. Statistically analyze and
histograms were generated using GraphPad Prism 5 (GraphPad
Software, Inc.).
Results
Characterization of BM-MSCs and
AT-MSCs
BM-MSCs and AT-MSCs were obtained from bone marrow
and adipose tissue. To evaluate whether the expanded cells were
genuine MSCs, the characterization of BM-MSCs and AT-MSCs at
passage 3 was performed. Both cell types were compared according to
morphology, immunophenotyping profiles and trilineage
differentiation potential. During primary culture, BM-MSCs and
AT-MSCs adhered to the plastic dishes in a scattered manner and
exhibited similar morphology to each other. Cells appeared
fibroblast-like, elongated and spindle-shaped with a single nucleus
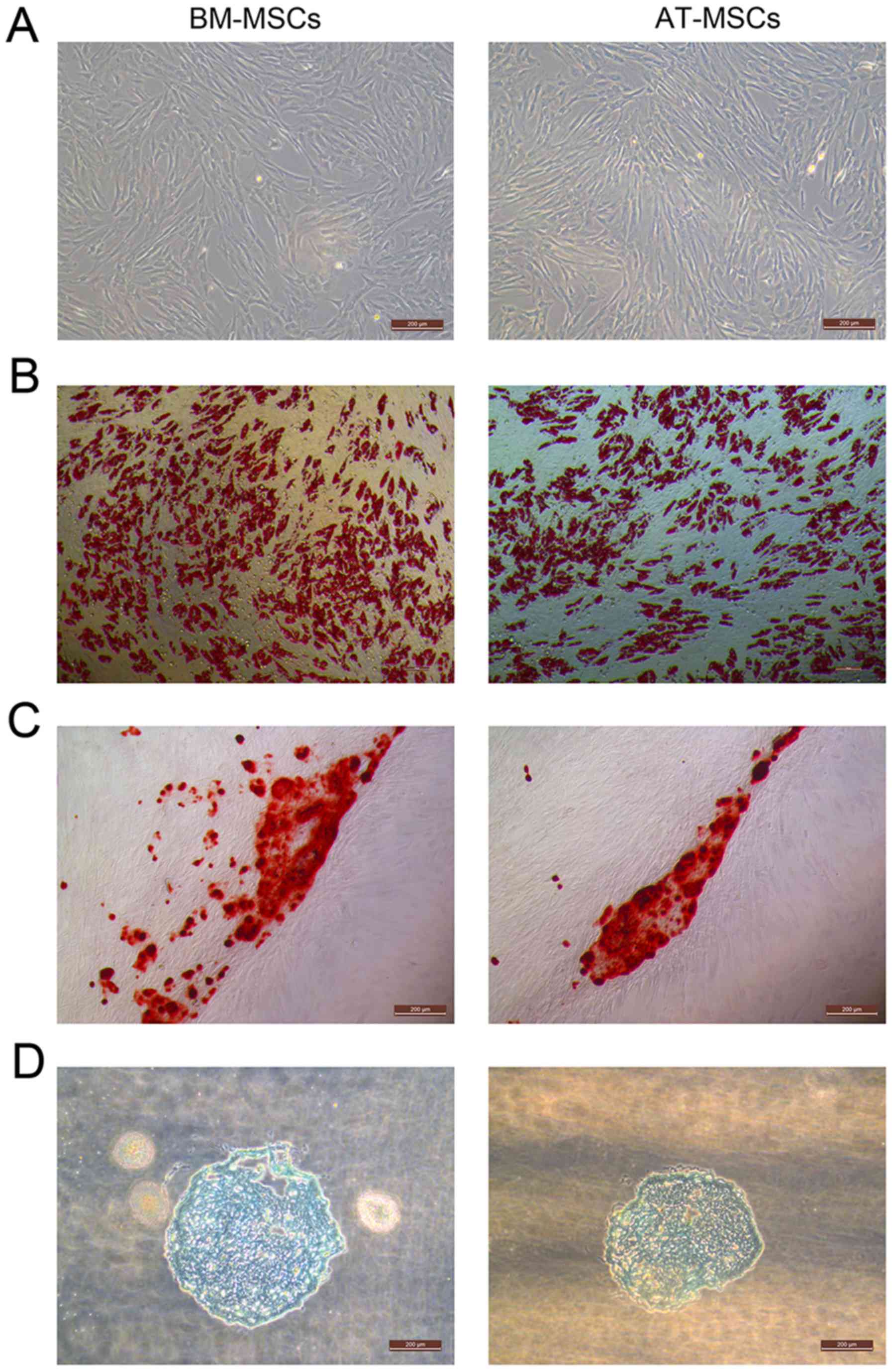
(Fig. 1A).
The BM-MSCs and AT-MSCs could differentiate into
adipocytes (Fig. 1B), osteocytes
(Fig. 1C) and chondrocytes in
vitro (Fig. 1D). After the
induction of adipogenic differentiation, numerous neutral lipid
droplets stained with Oil Red O were observed in the cytoplasm of
BM-MSCs and AT-MSCs. After the induction of osteogenic
differentiation, the cells presented an aggregation of micronodules
or calcium deposits that were stained by Alizarin Red. The
chondrogenic differentiation of both types of MSCs was observed
using an Alcian Blue stain.
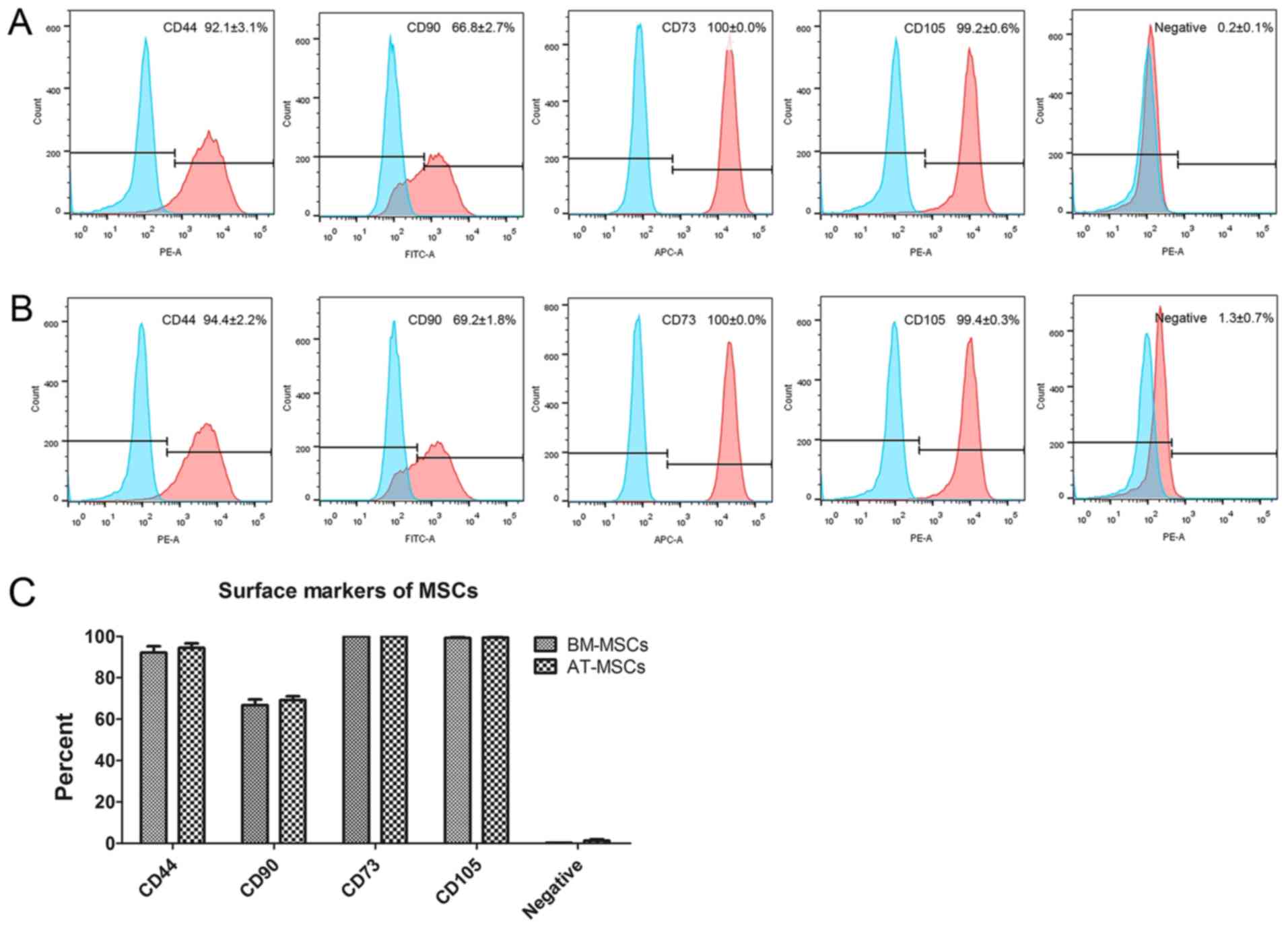
The immunophenotyping profiles of BM-MSCs and
AT-MSCs were analyzed by flow cytometry. The results revealed that
both BM-MSCs and AT-MSCs expressed high levels of the positive
markers CD44, CD90, CD73 and CD105, but did not express the
negative markers CD45, CD34, CD11b, CD19 and human leukocyte
antigen-DR (HLA-DR; Fig. 2A and
B). No differences were observed
between BM-MSCs and AT-MSCs using a t-test (Fig. 2C).
Morpholog yand glycogen deposition of
BM-MSCs and AT-MSCs during differentiation into HLCs
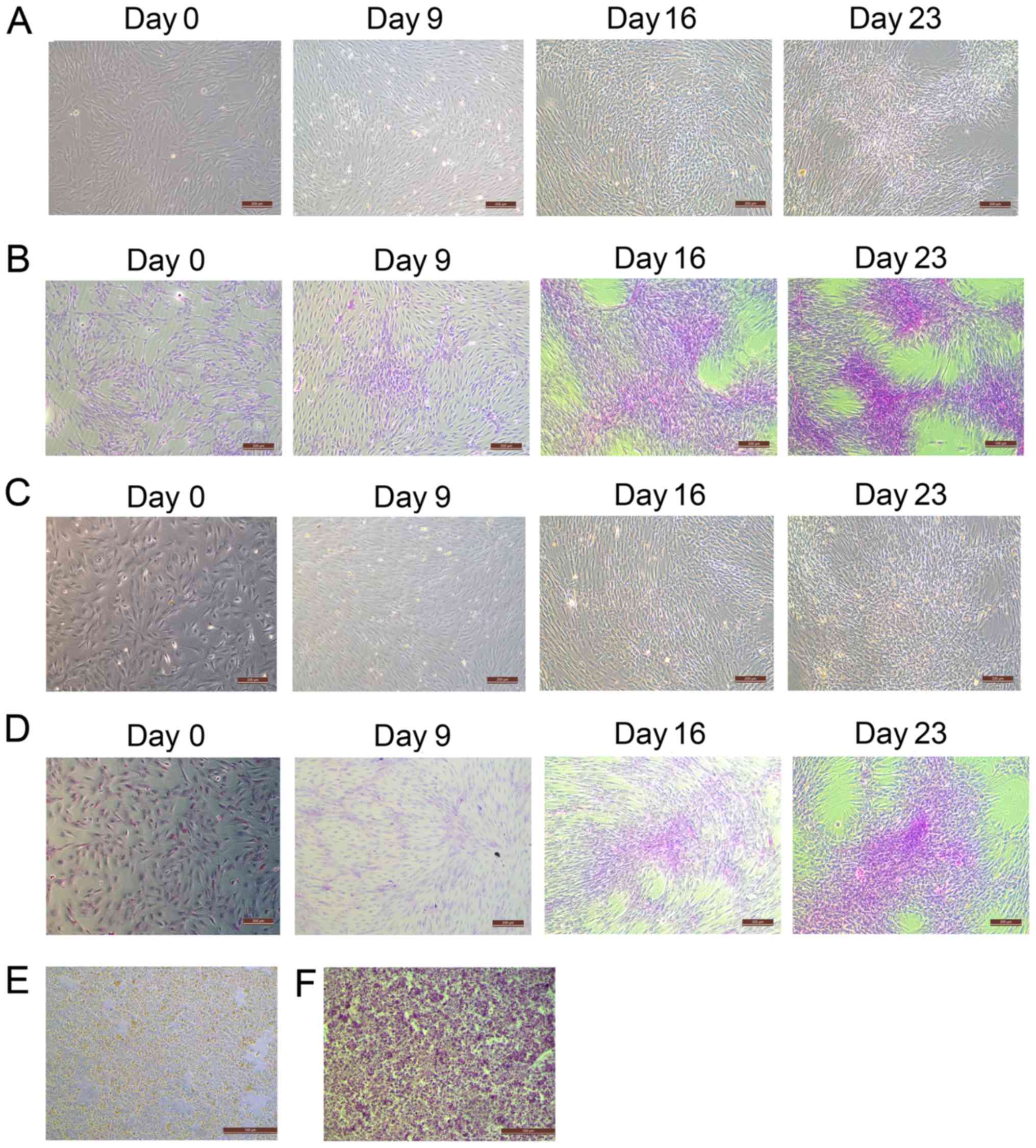
During the differentiation of BM-MSCs and AT-MSCs
into HLCs, BM-MSCs (Fig. 3A)
gradually changed from spindle and fibroblast-like cells to round
or polygonal epithelioid cells. These changes were also observed in
AT-MSCs (Fig. 3C). At day 0, before
hepatogenic differentiation induction, BM-MSCs and AT-MSCs
exhibited similar fibroblast-like morphology. However, a greater
number of flattened and polygonal cells were observed in the BM-MSC
group than in the AT-MSC group on day 9. On days 16 and 23, the
induced MSCs showed epithelioid and cuboidal shapes, which were
similar to the morphology of the primary hepatocytes of the control
group (Fig. 3E). In addition, the
presence of deposited glycogen was determined by PAS staining, to
further characterize the glycogen deposition function of HLCs
differentiated from BM-MSCs and AT-MSCs. After 23 days of
hepatogenic differentiation induction, magenta-stained glycogen was
detected in the differentiated cells but not in the
undifferentiated cells. The PAS intensity of HLCs differentiated
from BM-MSCs (Fig. 3B) was higher
than that of HLCs differentiated from AT-MSCs (Fig. 3D) on days 16 and 23. The level of
staining was similar to the PAS staining of the hepatocytes derived
from liver tissue that had been cultured for 5 days (Fig. 3F). These results suggested that the
morphology of HLCs differentiated from BM-MSCs and AT-MSCs was
similar to primary hepatocytes. Moreover, the HLCs differentiated
from both BM-MSCs and AT-MSCs exhibited the hepatic function of
glycogen deposition and could be stained with PAS.
Urea secretion in HLCs differentiated
from BM-MSCs and AT-MSCs
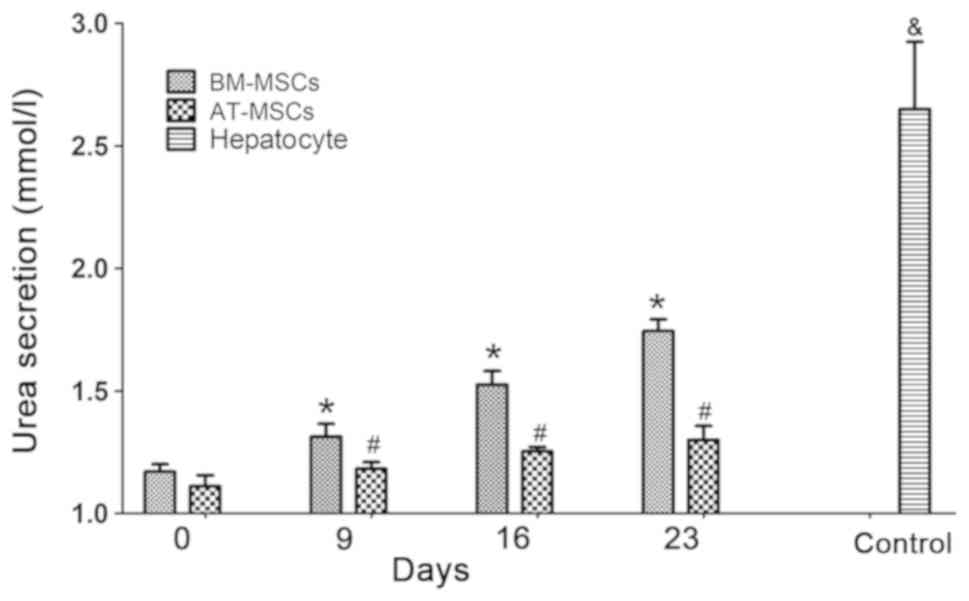
Urea assays detected the urea secretion function of
HLCs differentiated from BM-MSCs and AT-MSCs at various time points
(days 0, 9, 16 and 23), while urea secretion in the culture medium
of hepatocytes derived from liver tissue cultured for 3 days was
tested as a control. The urea production of the BM-MSCs and AT-MSCs
gradually increased over the culture time (days 9, 16 and 23)
during the HLC differentiation process compared to the
undifferentiated BM-MSCs and AT-MSCs (Fig. 4), and the urea secretion function of
differentiated hepatocytes from BM-MSCs was superior to that of
AT-MSCs (P<0.05). These results showed that HLCs differentiated
from rhesus macaque BM-MSCs and AT-MSCs possessed the hepatic
function of urea secretion.
RT-qPCR analysis of hepatocyte marker
expression
To further investigate the differentiation potential
and hepatocyte function of BM-MSCs and AT-MSCs differentiated into
HLCs, the mRNA expression levels of the hepatocyte markers, albumin
(ALB; Fig. 5A), keratin 18 (CK-18;
Fig. 5B), hepatocyte nuclear
factor-4α (HNF-4α; Fig. 5C),
cytochrome P450 family 7 subfamily A member 1 (CYP7A1; Fig. 5D) and cytochrome P450 family 3
subfamily A member 4 (CYP3A4; Fig.
5F), in BM-MSCs and AT-MSCs during the HLC differentiation
process were evaluated by RT-qPCR analysis. In these assays,
hepatocytes derived from liver tissue cultured for 5 days were used
as a positive control. During the HLC differentiation process, the
expression of these differentiated hepatocyte makers in BM-MSCs and
AT-MSCs increased from day 0 to day 23. In the process of BM-MSCs
differentiation into HLCs, the expression of ALB, CYP7A1 and CYP3A4
was significantly different on days 9-23 compared with expression
at day 0. CK-18 and HNF-4α expression was significantly different
on days 16 and 23 compared with at day 0. In the process of AT-MSC
differentiation, the expression of ALB was significantly different
on days 9-23 compared with that on day 0; HNF-4α, CYP7A1 and CYP3A4
expression was significantly different on days 16 and 23, and CK-18
expression was significantly different on day 23 (Fig. 5).
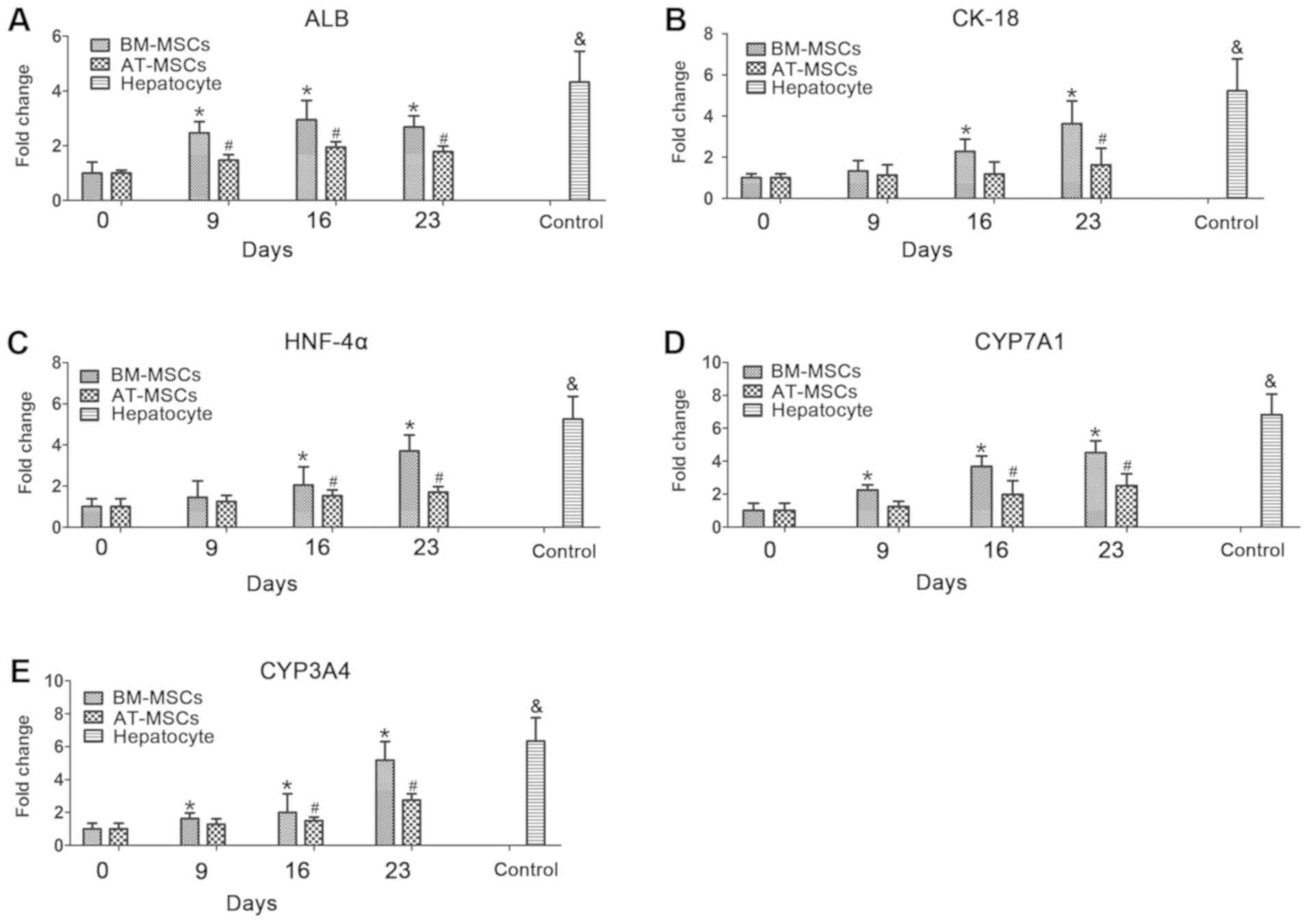 | Figure 5Hepatocyte-specific gene expression
was detected in BM-MSCs and AT-MSCs during the HLC differentiation
process. The mRNA expression of the hepatocyte-specific genes ALB
(A), CK-18 (B), HNF-4α (C), CYP7A1 (D) and CYP3A4 (E) was analyzed
on days 0, 9, 16 and 23 by reverse transcription-quantitative
polymerase chain reaction and normalized to GAPDH expression.
Control, collected hepatocytes after 5 days (n=3).
*P<0.05 BM-MSCs vs. day 0, #P<0.05
AT-MSCs vs. day 0. &denotes hepatocyte samples vs.
BM-MSCs and AT-MSCs. ALB, albumin; AT-MSCs, adipose tissue-derived
mesenchymal stem cells; BM-MSCs, bone marrow-derived mesenchymal
stem cells; CK-18, keratin 18; CYP3A4, cytochrome P450 family 3
subfamily A member 4; CYP7A1, cytochrome P450 family 7 subfamily A
member 1; HLCs, hepatocyte-like cells; HNF-4α, hepatocyte nuclear
factor-4α. |
Discussion
The extreme shortage of liver donors hampers
clinical therapy with orthotopic liver or hepatocyte
transplantation for patients with end-stage liver diseases. MSCs
will likely continue to be used in future clinical applications;
therefore, the differentiation of MSCs into HLCs as an alternative
source of seed cells, such as hepatocytes, shows considerable
promise to overcome the problem of organ donation shortage in liver
disease therapy. Previous studies have reported that MSCs derived
from various tissues exhibit different cell characteristics,
molecular function and clinical therapeutic effects (1,15). Our
previous study investigated whether MSCs could improve liver
fibrosis. The results indicated that transplanted MSCs could
migrate to the liver and that the paracrine effects of MSCs may
play an important role in vivo (9). To understand the therapeutic effect of
MSCs on liver disease therapy, it is essential to determine whether
MSCs can be successfully differentiated into HLCs. Currently, human
MSCs isolated from various tissue sources, including bone marrow
(30), adipose tissue (31), umbilical cord blood (32) and menstrual blood (33), have been proven to have the potential
to differentiate into HLCs under suitable induction conditions. A
previous study reported that equine MSCs derived from peripheral
blood, adipose tissue and bone marrow had different hepatogenic
differentiation efficiency (23).
The tissue-specific differentiation potency of human MSCs derived
from perinatal tissues, including the amnion, chorion and umbilical
cord, has been revealed through adipogenic, osteogenic and
chondrogenic differentiation, and the discovery of the innate
tissue-specific differentiation potency of various types of MSCs
will be helpful in choosing the appropriate cell sources for better
outcomes in specific diseases (34).
Although rhesus macaques have genetic and
physiological similarities with humans, and are widely used as a
laboratory animal (24), the
differentiation potency of BM-MSCs and AT-MSCs remains unclear in
this important species. Therefore, the differentiation potencies of
rhesus BM-MSCs and AT-MSCs into HLCs were analyzed in the present
study; this comparison may help to identify an optimal seed cell
for liver disease therapy either in preclinical or veterinary
applications.
In the present study, BM-MSCs and AT-MSCs were
isolated from age-matched rhesus macaques and cultured based on
conventional plastic adherence. According to the International
Society for Cellular Therapy definition of MSCs (35), both rhesus BM-MSCs and AT-MSCs
exhibited the characteristics of homogeneous fibroblast-like
adherent cells, positively expressed MSC markers and negatively
expressed hematopoietic markers, and exhibited trilineage
differentiation potential into osteoblasts, adipocytes and
chondroblasts. Using a cytokine induction cocktail in vitro
is one strategy for the differentiation of stem cells into HLCs.
Culture medium containing various combinations of cytokines and
growth factors, such as HGF, EGF, bFGF, oncostatin M (OSM), ITS,
nicotinamide and hexadecadrol have been shown to augment the
differentiation of MSCs into functional HLCs (26,33,36,37). Mou
et al (33) successfully
induced menstrual blood-derived MSC differentiation into HLCs using
an HGF, FGF-4, OSM, dexamethasone and ITS premix. Ayatollahi et
al (38) used a combination of
insulin growth factor-1 and liver-specific factors, including HGF,
OSM and dexamethasone, to differentiate human BM-MSCs into HLCs.
Shi et al (37) induced hair
follicle MSCs differentiation into hepatocytes by treating the
cells with L-glutamine and activin A and then culturing the cells
with bone morphogenetic protein-4, FGF-4, HGF, OSM and
dexamethasone for maturation. In the present study, a modified
three-step protocol (serum-free culture for 2 days, differentiation
for 7 days and maturation for 14 days) was used to induce rhesus
MSC differentiation into HLCs. The results confirmed that BM-MSCs
and AT-MSCs of rhesus macaques have the potential to differentiate
into HLCs. Compared with the isolated hepatocytes from rhesus
macaque liver tissue, the HLCs differentiated from BM-MSCs and
AT-MSCs not only displayed hepatocyte morphology but also exhibited
mature hepatocyte-specific functions, including glycogen
deposition, urea production and hepatocyte-related gene expression.
Xu et al (22) compared mouse
AT-MSCs and BM-MSCs in vitro by using a one-step culture
protocol (continuously culturing in the same medium for 10 days)
with a cocktail containing HGF, FGF4, OSM, EGF, acidic FGF, bFGF,
dexamethasone, ITS, vitamin C and nicotinamide, and did not observe
differences between the two types of mouse MSCs. Pennington et
al (23) detected differences in
hepatogenic differentiation efficiency by comparing various
tissue-derived equine MSCs; the results indicated that the
difference between BM-MSC and AT-MSC differentiation into HLCs may
be protocol-, duration-, and species-dependent.
The typical functional assays for hepatocyte
identification are glycogen deposition, urea production, albumin
secretion and low-density lipoprotein uptake assays. In addition,
hepatocyte-related gene expression is widely used in the detection
of MSCs differentiating into HLCs (39-41).
In the present study, HLCs differentiated from both BM-MSCs and
AT-MSCs acquired the functions of glycogen deposition, urea
secretion and expression of hepatocyte-related genes (including
CK-18, HNF-4α, ALB, CYP3A4 and CYP7A1) in the differentiation
process. The differentiation of rhesus macaque BM-MSCs into HLCs
occurred prior to that of AT-MSCs in the culture timeline. The HLCs
morphology appeared first at 9 days in BM-MSCs, which showed
clustered and globular cells, which were similar to primary
hepatocytes from liver tissue. The function of differentiated
hepatocytes from BM-MSCs was superior to that of AT-MSCs, due to
the differences shown in the glycogen deposition and urea secretion
assays. This superiority of BM-MSCs was also indicated in
hepatocyte-related gene expression after 23 days. The ALB, CK-18,
and HNF-4α genes are regarded as mature hepatocyte markers, and the
major cytochrome P450 forms, including CYP3A4 and CYP7A1, are
highly expressed during hepatocyte differentiation (26,42-44).
In the present study, the expression levels of ALB, CK-18, HNF-4α,
CYP3A4 and CYP7A1 in BM-MSCs were higher than those in AT-MSCs
after 23 days of differentiation into HLCs. These results indicated
that the differentiation potential of BM-MSCs into HLCs is better
than that of AT-MSCs in rhesus macaques. Although AT-MSCs are
easier to harvest from donors than BM-MSCs and the harvest
procedure causes much less pain for donors, the differentiation
potential of AT-MSCs into HLCs is inferior to that of BM-MSCs in
rhesus macaques according to glycogen deposition, urea secretion
assays and gene expression detection. Previous studies have
reported that the HLC differentiation potential of cells derived
from various tissues may be dependent on the species and methods
(23,26). The present results are consistent
with these previous studies and indicated that different species
require specific methods for MSC differentiation into HLCs, which
will be necessary to study further in the future.
In conclusion, rhesus macaque BM-MSCs and AT-MSCs
have the potential to differentiate into HLCs, which was confirmed
by morphology, glycogen deposition, urea secretion and
hepatocyte-related gene expression using a modified three-step
protocol of culture for 23 days. To the best of our knowledge, this
study is the first to report the potential of BM-MSCs and AT-MSCs
differentiating into HLCs in rhesus macaques and indicates that the
potential of various tissue-derived MSCs differentiating into HLCs
may be species- and method-dependent. These results will be
beneficial to improve hepatocyte differentiation protocols for MSCs
to ensure high efficiency for preclinical cytotherapy of liver
diseases as well as veterinary medicine.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 31872973 and
81960270), the Major Project of Yunnan Science and Technology
Program (grant no. 2018ZF007-05), Yunnan Medical Scientific
Research Foundation (grant no. 2017NS248), Ningxia Natural Science
Foundation (grant no. 2018AAC03210) and Ningxia Higher Education
Scientific Research Project (grant no. NGY2018-70).
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request.
Author's contributions
JW, XF and YY carried out the experiments, SL, YD
and BMI performed sampling and analysed the data, XF, WS and BZ
designed the experiments and wrote the manuscript. BMI and BZ
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Animal studies were approved by the Institutional
Animal Care and Use Committee of Kunming University of Science and
Technology (approval number: LPBR20170201).
Patient consent for publication
Not applicable.
Competing interests
Thecauthors declare that they have no competing
interests.
References
|
1
|
Alfaifi M, Eom YW, Newsome PN and Baik SK:
Mesenchymal stromal cell therapy for liver diseases. J Hepatol.
68:1272–1285. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Michalopoulos GK: Hepatostat: Liver
regeneration and normal liver tissue maintenance. Hepatology.
65:1384–1392. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jalan R, Yurdaydin C, Bajaj JS, Acharya
SK, Arroyo V, Lin HC, Gines P, Kim WR and Kamath PS: World
Gastroenterology Organization Working Party. Toward an improved
definition of acute-on-chronic liver failure. Gastroenterology.
147:4–10. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nasralla D, Coussios CC, Mergental H,
Akhtar MZ, Butler AJ, Ceresa CDL, Chiocchia V, Dutton SJ,
García-Valdecasas JC, Heaton N, et al: A randomized trial of
normothermic preservation in liver transplantation. Nature.
557:50–56. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yu Y, Fisher JE, Lillegard JB, Rodysill B,
Amiot B and Nyberg SL: Cell therapies for liver diseases. Liver
Transpl. 18:9–21. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Zhao L, Chen S, Shi X, Cao H and Li L: A
pooled analysis of mesenchymal stem cell-based therapy for liver
disease. Stem Cell Res Ther. 9(72)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen Y, Shao JZ, Xiang LX, Dong XJ and
Zhang GR: Mesenchymal stem cells: A promising candidate in
regenerative medicine. Int J Biochem Cell Biol. 40:815–820.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: The surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1019. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fu X, Jiang B, Zheng B, Yan Y, Wang J,
Duan Y, Li S, Yan L, Wang H, Chen B, et al: Heterogenic
transplantation of bone marrow-derived rhesus macaque mesenchymal
stem cells ameliorates liver fibrosis induced by carbon
tetrachloride in mouse. Peer J. 6(e4336)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Marx C, Silveira MD and Beyer Nardi N:
Adipose-derived stem cells in veterinary medicine: Characterization
and therapeutic applications. Stem Cells Dev. 24:803–813.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shi D, Zhang J, Zhou Q, Xin J, Jiang J,
Jiang L, Wu T, Li J, Ding W, Li J, et al: Quantitative evaluation
of human bone mesenchymal stem cells rescuing fulminant hepatic
failure in pigs. Gut. 66:955–964. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vezzani B, Pierantozzi E and Sorrentino V:
Mesenchymal stem cells: From the perivascular environment to
clinical applications. Histol Histopathol. 7:1235–1246.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nicolas CT, Hickey RD, Chen HS, Mao SA,
Lopera Higuita M, Wang Y and Nyberg SL: Concise review: Liver
regenerative medicine: From hepatocyte transplantation to
bioartificial livers and bioengineered grafts. Stem Cells.
25:42–50. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Barlow S, Brooke G, Chatterjee K, Price G,
Pelekanos R, Rossetti T, Doody M, Venter D, Pain S, Gilshenan K and
Atkinson K: Comparison of human placenta- and bone marrow-derived
multipotent mesenchymal stem cells. Stem Cells Dev. 17:1095–1107.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Heo JS, Choi Y, Kim HS and Kim HO:
Comparison of molecular profiles of human mesenchymal stem cells
derived from bone marrow, umbilical cord blood, placenta and
adipose tissue. Int J Mol Med. 37:115–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Strioga M, Viswanathan S, Darinskas A,
Slaby O and Michalek J: Same or not the same? Comparison of adipose
tissue-derived versus bone marrow-derived mesenchymal stem and
stromal cells. Stem Cells Dev. 21:2724–2752. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sgodda M, Aurich H, Kleist S, Aurich I,
König S, Dollinger MM, Fleig WE and Christ B: Hepatocyte
differentiation of mesenchymal stem cells from rat peritoneal
adipose tissue in vitro and in vivo. Exp Cell Res. 313:2875–2886.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Oliver K, Awan T and Bayes M:
Single-versus multiple-site harvesting techniques for bone marrow
concentrate: Evaluation of aspirate quality and pain. Orthop J
Sports Med. 5(232596711772439)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mizuno H, Tobita M and Uysal AC: Concise
review: Adipose-derived stem cells as a novel tool for future
regenerative medicine. Stem cells. 30:804–810. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Aurich H, Sgodda M, Kaltwasser P, Vetter
M, Weise A, Liehr T, Brulport M, Hengstler JG, Dollinger MM, Fleig
WE and Christ B: Hepatocyte differentiation of mesenchymal stem
cells from human adipose tissue in vitro promotes hepatic
integration in vivo. Gut. 58:570–581. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun J, Yuan Y, Qin H, Ying C, Liu W, Zhang
J, He Y and Liu Z: Serum from hepatectomized rats induces the
differentiation of adipose tissue mesenchymal stem cells into
hepatocyte-like cells and upregulates the expression of hepatocyte
growth factor and interleukin-6 in vitro. Int J Mol Med.
31:667–675. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu LJ, Wang SF, Wang DQ, Ma LJ, Chen Z,
Chen QQ, Wang J and Yan L: Adipose-derived stromal cells resemble
bone marrow stromal cells in hepatocyte differentiation potential
in vitro and in vivo. World J Gastroenterol. 23:6973–6982.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Pennington MR, Curtis TM, Divers TJ,
Wagner B, Ness SL, Tennant BC and Van de Walle GR: Equine
mesenchymal stromal cells from different sources efficiently
differentiate into hepatocyte-like cells. Tissue Eng Part C
Methods. 22:596–607. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fu X, Yan Y, Li S, Wang J, Jiang B, Wang
H, Duan Y, Tan T, Gao F, Gong D, et al: Vitrification of rhesus
macaque mesenchymal stem cells and the effects on global gene
expression. Stem Cells Int. 2017(3893691)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jiang B, Fu X, Yan L, Li S, Zhao D, Wang
X, Duan Y, Yan Y, Li E, Wu K, et al: Transplantation of human
ESC-derived mesenchymal stem cell spheroids ameliorates spontaneous
osteoarthritis in rhesus macaques. Theranostics. 9:6587–6600.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khanjani S, Khanmohammadi M, Zarnani AH,
Akhondi MM, Ahani A, Ghaempanah Z, Naderi MM, Eghtesad S and
Kazemnejad S: Comparative evaluation of differentiation potential
of menstrual blood- versus bone marrow-derived stem cells into
hepatocyte-like cells. PLoS One. 9(e86075)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li Y, Wang Y, Wu Q, Li L, Shi Y, Bu H and
Bao J: Comparison of methods for isolating primary hepatocytes from
mini pigs. Xenotransplantation. 23:414–420. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sarvandi SS, Joghataei MT, Parivar K,
Khosravi M, Sarveazad A and Sanadgol N: In vitro differentiation of
rat mesenchymal stem cells to hepatocyte lineage. Iran J Basic Med
Sci. 18:89–97. 2015.PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pournasr B, Mohamadnejad M, Bagheri M,
Aghdami N, Shahsavani M, Malekzadeh R and Baharvand H: In vitro
differentiation of human bone marrow mesenchymal stem cells into
hepatocyte-like cells. Arch Iran Med. 14:244–249. 2011.PubMed/NCBI
|
|
31
|
Al Battah F, De Kock J, Vanhaecke T and
Rogiers V: Current status of human adipose-derived stem cells:
Differentiation into hepatocyte-like cells. ScientificWorldJournal.
11:1568–1581. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu J, Cao H, Yang J, Pan Q, Ma J, Li J, Li
Y, Li J, Wang Y and Li L: In vivo hepatic differentiation of
mesenchymal stem cells from human umbilical cord blood after
transplantation into mice with liver injury. Biochem Biophys Res
Commun. 422:539–545. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mou XZ, Lin J, Chen JY, Li YF, Wu XX,
Xiang BY, Li CY, Ma JM and Xiang C: Menstrual blood-derived
mesenchymal stem cells differentiate into functional
hepatocyte-like cells. J Zhejiang Univ Sci B. 14:961–972.
2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kwon A, Kim Y, Kim M, Kim J, Choi H,
Jekarl DW, Lee S, Kim JM, Shin JC and Park IY: Tissue-specific
differentiation potency of mesenchymal stromal cells from perinatal
tissues. Sci Rep. 6(23544)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hu C and Li L: In vitro culture of
isolated primary hepatocytes and stem cell-derived hepatocyte-like
cells for liver regeneration. Protein Cell. 6:562–574.
2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shi X, Lv S, He X, Liu X, Sun M, Li M, Chi
G and Li Y: Differentiation of hepatocytes from induced pluripotent
stem cells derived from human hair follicle mesenchymal stem cells.
Cell Tissue Res. 366:1–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ayatollahi M, Soleimani M, Tabei SZ and
Kabir Salmani M: Hepatogenic differentiation of mesenchymal stem
cells induced by insulin like growth factor-1. World J Stem Cells.
3:113–121. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Borhani-Haghighi M, Talaei-Khozani T,
Ayatollahi M and Vojdani Z: Wharton's jelly-derived mesenchymal
stem cells can differentiate into hepatocyte-like cells by hepg2
cell line extract. Iran J Med Sci. 40:143–151. 2015.PubMed/NCBI
|
|
40
|
Wang B, Li W, Dean D, Mishra MK and Wekesa
KS: Enhanced hepatogenic differentiation of bone marrow derived
mesenchymal stem cells on liver ECM hydrogel. J Biomed Mater Res A.
106:829–838. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang YN, Lie PC and Wei X:
Differentiation of mesenchymal stromal cells derived from umbilical
cord Wharton's jelly into hepatocyte-like cells. Cytotherapy.
11:548–558. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Khanjani S, Khanmohammadi M, Zarnani AH,
Talebi S, Edalatkhah H, Eghtesad S, Nikokar I and Kazemnejad S:
Efficient generation of functional hepatocyte-like cells from
menstrual blood-derived stem cells. J Tissue Eng Regen Med.
9:E124–E134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang XF, Ren LW, Yang L, Deng CY and Li
FR: In vivo direct reprogramming of liver cells to insulin
producing cells by virus-free overexpression of defined factors.
Endocr J. 64:291–302. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sa-ngiamsuntorn K, Wongkajornsilp A,
Kasetsinsombat K, Duangsa-ard S, Nuntakarn L, Borwornpinyo S,
Akarasereenont P, Limsrichamrern S and Hongeng S: Upregulation of
CYP 450s expression of immortalized hepatocyte-like cells derived
from mesenchymal stem cells by enzyme inducers. BMC Biotechnol.
11(89)2011.PubMed/NCBI View Article : Google Scholar
|