Introduction
As modern medical technology develops, the number of
patients undergoing cardiac surgery under cardiopulmonary bypass
(CPB) has increased (1). CPB enables
cardiovascular surgery to be safer and more practical, but
complications from surgery can lead to a systemic inflammatory
response (2). Previous studies have
demonstrated that the intestinal tract plays a key role in the
continuous occurrence and development of systemic inflammatory
response syndrome (SIRS), as well as multiple organ dysfunction
syndrome (3,4). The occurrence of gastrointestinal
complications following CPB cardiac surgery is <3%, but the
mortality rate can be as high as 90% (5,6).
Previous studies investigating the damage to and protection of
tissues and organs caused by CPB have focused on the heart and
brain (7,8). However, increased intestinal
permeability and bacterial translocation occur in animal models and
patients during CPB (9,10). Therefore, further study is needed
into the intestinal complications of cardiac surgery. Prevention
and treatment of intestinal damage during peri-intestinal
circulation is crucial for reducing the incidence of complications
and the mortality rate.
The κ-opioid receptor (KOR) serves a key role in
regulating hypoxia and ischemia-induced damage (11,12), by
mechanisms including protection of the microcirculation of skeletal
muscle or activation of the PI3K/Akt signaling pathway (13). The KOR agonist U50448H protects
against retinal ischemia-reperfusion injury in rats by activating
the PI3K/Akt signaling pathway or via the production of tumor
necrosis factor-α (TNF-α) in the retina (14). KOR agonists effectively reduce the
occurrence of ischemia/reperfusion arrhythmia in rats (15,16). KOR
agonists can also inhibit β-adrenergic receptors, thus reducing the
contractile response of blood vessels as well as myocardial oxygen
consumption (17). Zhang et
al (18), has shown that KOR
agonists can reduce pulmonary arterial hypertension caused by
hypoxia by inhibiting pulmonary arterial smooth muscle cell
proliferation and remodeling the pulmonary artery. However, the
specific regulatory mechanism by which KOR agonists attenuate
intestinal barrier damage following CPB remains unknown.
Hypoxia-inducible factor-1 (HIF-1) is a
transcriptionally active nuclear protein that is produced by cells
under hypoxic conditions and plays a key role in the hypoxic
compensatory response of the body (19). HIF-1 is also important in cellular
energy production, the metabolism of iron and catecholamines, as
well as vasoconstriction, neovascularization and apoptosis in tumor
cells under ischemic and hypoxic conditions (20-25).
Previous studies have shown that HIF-1α is a disruptor of the
intestinal mucosal barrier during hypoxia, ischemia-reperfusion and
inflammation (26,27). HIF-1α can be activated not only by
hypoxia, but also by several non-hypoxic stimuli, including various
inflammatory mediators and cytokines (28,29). A
common mechanism of these non-hypoxic stimuli of HIF-1α involves
the upregulation of NF-κB-dependent HIF-1α expression levels
(30).
In the present study, a model of post-operative
intestinal barrier injury in rats undergoing CPB was established to
observe the effects of a KOR agonist on intestinal barrier function
injury, inflammation, oxidative stress response and the expression
of NF-κB signaling pathway-associated proteins in CPB rats. In
addition, the present study investigated the protective effect and
mechanism of KOR agonists in the intestinal barrier of the CPB
model rats. The present results may provide theoretical and
experimental evidence that could facilitate the treatment of
patients who have intestinal barrier function damage following
CPB.
Materials and methods
Experimental animals and
groupings
A total of 50 male Sprague Dawley rats, eight weeks
old, specific pathogen free, 350-450 g were provided by the Animal
Experimental Department of the General Hospital of Northern Theater
Command [rodent use permit: SYXK (Military) 20120007; rodent
production permit: SCXK (Military) 20120006]. All rats were in good
health condition, maintained at 22-26˚C, in a 12 h light/dark cycle
with 40-60% relative humidity in the animal rooms. Rats were
maintained on standard laboratory diet with tap water ad
libitum throughout the experiment, except for an overnight fast
before surgery. The whole animal study has been reviewed and
approved by Animal Ethical and Welfare Committee of The General
Hospital of Northern Theater Command.
Rats were randomly divided into five groups, with 10
rats in each group. The groups were as follows: i) Sham operation
(group Sham); ii) CPB surgery (group CPB); iii) KOR agonist
(U50488H) + CPB (group K); iv) KOR agonist (U50488H) + specific KOR
antagonist (norBNI) + CPB (group NK); and v) KOR agonist (U50488H)
+ NF-κB inhibitor pyrrolidinedithiocarbamic acid (PDTC) + CPB (NF
group). The CPB rat model was established using a CPB bypass for
all rats, with the exception of those in the sham group. In group
K, an intravenous injection of 1.5 mg/kg U50488H (cat. no. 0495/25;
Tocris Bioscience) was administered prior to the bypass. In group
NK, an intravenous injection of 1.5 mg/kg U50488H was given after
rats were catheterized and then 2 mg/kg norBNI (cat. no.
sc-396970A; Santa Cruz Biotechnology, Inc.) was administered
intravenously after 30 min (31,32). In
group NF, 1.5 mg/kg U50488H was given intravenously after rats were
catheterized, and then 50 mg/kg NF-κB inhibitor PDTC (cat. no.
sc-203224A; Santa Cruz Biotechnology, Inc.) was injected
intravenously 30 min later. After 2 h of CPB bypass, rats were
anesthetized and following the intestinal microcirculation test (as
described below), 5 ml of blood was taken from the right internal
vein. The serum was separated by centrifugation 1,000 x g for 20
min and stored at -80˚C for subsequent experimentation. The jejunum
and ileum tissues (section thickness, 2 µm) were stored at -80˚C
for further analysis. Moreover, additional jejunum and ileum tissue
(thickness, 2 cm) were immersed in 4% formalin for subsequent
analysis.
Preparation of CPB model
CPB surgery was performed following the procedure
described in a previous study (33).
Rats received an intraperitoneal injection of 30 mg/kg
pentobarbital sodium (Shanghai Ziyuan Pharmaceutical Co., Ltd.)
every 2 h during surgery. Photopic oral intubation was conducted
using a 16G intravenous catheter, and the rats were mechanically
ventilated using a small animal ventilator (frequency, 60
beats/min; tidal volume, 3 ml/kg; inspiratory to expiratory ratio,
1:1.5), which was connected to a monitor to observe heart rate,
oxygen saturation and rectal temperature.
The puncture site was sterilized using iodophor
(Shandong Lierkang Disinfection Technology Co., Ltd.), which was
followed by exposure and puncture of the femoral vein. Right
femoral vein catheterization (24G) was performed in order to open
the fluid path, which was then transfused with 6% hydroxyethyl
starch (Guangdong Jiabao Pharmaceutical Co., Ltd.) and connected to
a micro-infusion pump. In addition, the left femoral artery was
catheterized (22G) and multi parameter ECG monitor (cat. no.
CMS7000, Contec Medical Systems Co., Ltd.) used to monitor blood
pressure. Both coccygeal artery catheterization (22G) and right
internal jugular vein catheterization (18G) were performed so that
blood could be drained for the CPB. A drainage tube, homemade blood
storage device, constant peristaltic pump (Baoding Longer Precision
Pump Co., Ltd.), silicone tubing (internal diameter, 4 mm) and rat
membrane oxygenator (Guangdong Kewei Medical Instrument Co., Ltd.)
were installed between the two puncture sites to establish the CPB
circuit. Then, 300 IU/kg heparin sodium (Shenyang Haitong
Pharmaceutical Co., Ltd.) was injected into the left femoral
vein.
CPB was performed with a membrane oxygenator to
supply oxygen; the low-flow CPB velocity was 35 ml/kg/min, which
was later increased to 100-120 ml/kg/min at full-flow bypass. In
order to prevent an air embolism, 1-2 ml of blood was retained in
the blood storage device. Heart rate (HR) and mean arterial
pressure (MAP) were monitored on a Gould ES2000 recorder (Gould,
Inc.). Blood and body temperatures were maintained using heating
lamps and controlled by esophageal temperature monitoring. Arterial
blood gases taken from the right carotid artery were analyzed at 0,
30, 60, 90 and 180 min using a blood gas analyzer (GEM Premier
3000; Heal Force Bio-meditech Holdings Ltd. The mean arterial
pressure was maintained at >60 mmHg, partial pressure of
CO2 (PaCO2) 35-45 mmHg, base excess -3-3
mmol/l mmHg, pH 7.35-7.45 and hematocrit >0.25. 2-20 µg/100 g of
epinephrine hydrochloride (Wuhan Grand Pharmaceutical Group Co.,
Ltd.) was infused into fluid during surgery to maintain rats stable
circulation.
Intestinal microcirculation detection
in rats
Once the rats were anesthetized, the abdominal
cavity was incised. The lower part of the ileum was extracted and
fixed securely in a constant temperature perfusion box, which was
maintained at 37˚C with physiological saline. A microscope and
medical image analysis system were connected to the box. The
microvascular diameter of the same mesenteric vessel and the blood
flow state of the rat were recorded using the microscope at 40-fold
magnification. A semi-quantitative flow rate grading method was
used to determine the blood flow state, which was divided into four
levels (34): i) Level 0,
characterized by fast vascular blood, a smooth vessel wall and a
slight or no presence of debris in the blood vessels; ii) level 1,
distinguished by a relatively faster blood flow and an obvious
graininess in the blood vessels; iii) level 2, characterized by
silt vessels and either a slow or variable blood flow; and iv)
level 3, characterized by the stagnation or loss of blood flow. The
rats were euthanized at the end of the study by an overdose of
pentobarbital sodium (200 mg/kg).
Hematoxylin and eosin (H&E)
staining
Jejunum and ileum samples in 4% formalin were
dehydrated using an increasing ethanol gradient (70, 80, 90, 95 and
100%). The samples were then made transparent using xylene, waxed,
embedded into paraffin blocks and then cut into 4-µm sections.
After dewaxing, the sections were stained with hematoxylin for ~5
min and washed with PBS. Then, 1% hydrochloric acid was used for
alcohol differentiation and 0.5% eosin dyeing solution was applied
for 30 sec at room temperature. Gradient alcohol concentrations
(70, 80, 90 and 100%) were used for dehydration, followed by
transparentizing treatment and sealing using a neutral gum.
Pathological changes to the tissues were observed using a light
microscope (x200).
ELISA
ELISA kits were used to detect the inflammatory
factors interleukin (IL)-1β (cat. no. CSB-E08055r; CUSABIO
Technology), IL-6 (cat. no. SEA079Ra; Wuhan USCN Business Co.,
Ltd.), TNF-α (cat. no. SEA133Si; Wuhan USCN Business Co., Ltd.) and
IL-10 (cat. no. SEA056Ra; Wuhan USCN Business Co., Ltd.) in the
serum from the rats. In addition, the oxidative stress indicators
superoxidase dismutase (SOD; cat. no. SES134Hu), malondialdehyde
(MDA; cat. no. CEA597Ge), nitric oxide (NO; cat. no. IS100) and the
intestinal injury markers D-lactic acid in serum (cat. no.
CEV643Ge), diamine oxidase (DAO; cat. no. SEJ298Hu) and intestinal
fatty acid-binding protein (I-FABP; cat. no. SEA559Ra) were
detected in the serum using kits from Wuhan USCN Business Co., Ltd.
The tests were performed according to the manufacturer's
instructions.
Immunohistochemistry
Jejunum and ileum samples were fixed in 4% (v/v)
formalin in distilled water at room temperature for 24 h. Samples
were then separately dehydrated using an increasing concentration
ethanol (70, 80, 90, 95 and 100%) for 30 min each at room
temperature. The samples were then made transparent using xylene,
waxed, embedded into paraffin blocks and cut into 4-µm sections.
Subsequently, the sections were then incubated with 3%
H2O2 for 10 min at room temperature and
washed with PBS for 5 min. The sections were then incubated with
primary antibodies against NF-κB-p65 (1:100; cat. no. ab207297;
Abcam) and HIF-1α (1:500; cat. no. ab16066; Abcam), and washed
twice with PBS for 5 min. Sections were incubated with HRP-labeled
secondary antibody (1:500; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature and washed three
times with PBS for 5 min. The slices were developed with
3,3'-diaminobenzidine at room temperature for 5 min and washed
thrice with PBS for 5 min, before being counterstained with
hematoxylin for 3 min. Sections were then washed thrice with PBS
for 5 min, dehydrated, and sealed with neutral gum. The expression
of NF-κB-p65 and HIF-1α were observed under light microscope
(x400).
Western blotting
After homogenization of the jejunum and ileum
tissues, pre-cooled RIPA (cat. no. 89900; Thermo Fisher Scientific,
Inc.) buffer was added and lysis was conducted on ice for 30 min.
Once the supernatant had been collected, the concentration of the
collected protein solution was determined using a bicinchoninic
acid protein quantification kit (cat. no. 23225; Thermo Fisher
Scientific, Inc.). A total of 20 µg proteins were loaded and
separated using a 10% SDS-PAGE electrophoresis and transferred to a
PVDF membrane. After blocking with 5% low-fat milk buffer at room
temperature, the PVDF membrane was incubated with zonula
occludens-1 (ZO-1; 1:1,000; cat. no. ab96587; Abcam), claudin-1
(1:1,000; cat. no. ab15098; Abcam,), NF-κB-p65 (1:1,000; cat. no.
ab207297; Abcam), HIF-1α (1:2,000; cat. no. ab16066; Abcam) and
GAPDH (1:1,000; cat. no. ab37168; Abcam) antibodies overnight at
4˚C. After washing the PVDF membrane with PBS, a secondary
antibody, goat anti-rabbit IgG H&L (horse radish peroxidase
conjugated; 1:1,000; cat. no. ab205718; Abcam) was added and the
membrane was incubated for 2 h at room temperature. Then, an ECL
luminescence kit (GE Healthcare) was used to develop the color. A
gel imaging system was used for imaging, and Quantity One (version
4.5.2. Bio-Rad Laboratories, Inc.) software was used for
quantification of protein expression.
Statistical analysis
Statistical analyses were performed using SPSS 19.0
software (IBM Corp.). Multiple comparisons were analyzed using
one-way ANOVA followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Rat hemodynamics
In the CPB model rats, the rectal temperature, pH,
arterial blood PaCO2 and PaO2 were not
significantly different from those in the sham group. However, the
MAP, HR, left ventricular diastolic pressure, highest rate of
change of pressure development and hemoglobin levels were
significantly decreased in the CPB group compared with the sham
group, and in group K these parameters were significantly increased
compared with the CBP group (P<0.05; Fig. 1).
 | Figure 1Altered hemodynamics in a rat CPB
model with and without KOR agonist. The rectal temperature, HR,
MAP, pH, PaO2, PaCO2, HB, LVDP, +dP/dtmax of
rats in each group are presented. *P<0.05 as
indicated. HR, heart rate; MAP, mean arterial pressure;
PaO2, partial pressure of oxygen; PaCO2,
partial pressure of carbon dioxide; HB, hemoglobin; LVDP, left
ventricular diastolic pressure; +dP/dtmax, highest rate of change
of pressure development; KOR, κ-opioid receptor; CPB,
cardiopulmonary bypass; K, KOR agonist (U50488H) + CPD; NS, not
significant. |
KOR agonist alleviates intestinal
damage in CPB model rats
In the CPB model, the blood perfusion of important
organs such as the brain is maintained, while that of the abdominal
organs is suddenly reduced and eventually causes intestinal mucosal
ischemia and hypoxia. The intestinal injury of the CPB model rats
was assessed using H&E staining (Fig. 2). The results suggested that the
intestinal mucosa, villi and brush border were normal in the sham
group. However, the following were observed in the CPB group:
Intestinal mucosal edema, infiltration of neutrophils and
lymphocytes, partial atrophy and shedding of the villus, and
filling of flaky capillaries. Following the addition of the KOR
agonist, the intestinal mucosal injury in group K appeared to be
attenuated compared with that in the CPB group; there was only mild
partial villus edema, the intestinal epithelium and lamina propria
were partially separated, and only minor inflammatory cell
infiltration was observed. In group NK, the intestinal mucosa was
thin, the intestinal villus was atrophied and inflammatory cell
infiltration was observed. These results suggest that the
intestinal mucosal damage in group NK was reduced compared with
that in the CPB group, but not as much as that in group K.
Collectively, the present results suggested that KOR agonists may
alleviate intestinal damage in CPB rats.
KOR agonist inhibits the inflammatory
and oxidative stress response in CPB model rats
The present study investigated changes in the levels
of inflammatory and oxidative stress factors in the serum of rats
using ELISA. In terms of the intestinal damage caused by CPB,
factors associated with inflammation of the intestinal mucosa cells
(Fig. 3A) and the oxidative stress
response (Fig. 3B) were identified.
The results suggest that the serum levels of IL-1β, IL-6 and TNF-α
increased, while the level of IL-10 significantly decreased in
group CPB compared with the sham group (P<0.05). In group K, the
serum levels of IL-1β, IL-6 and TNF-α were decreased, while that of
IL-10 was significantly increased compared with group CPB
(P<0.05). The results for oxidative stress factors suggested
that the levels of serum SOD and NO were decreased, and the level
of MDA was significantly increased in the CPB group compared with
the sham group (P<0.05). In addition, serum SOD and NO levels
were significantly increased, and the level of MDA was
significantly reduced in group K compared with group CPB
(P<0.05). Therefore, the present results suggest that CPB
triggers inflammatory and oxidative stress responses in intestinal
mucosal cells, and that a KOR agonist reverses these responses.
KOR agonist improves intestinal
mucosal function in CPB model rats
CPB-induced dysfunction of intestinal mucosa causes
intestinal epithelial cells to release highly active DAO into the
blood, increases the metabolism of D-lactic acid via
gastrointestinal bacterial fermentation, and increases serum levels
of I-FABP (35). The present results
indicate that serum DAO, D-lactic acid and I-FABP levels were
significantly increased in group CPB compared with the sham group
(P<0.05). Compared with the CPB group, serum DAO, D-lactic acid
and I-FABP levels were decreased in group K (P<0.05). In
addition, DAO, D-lactic acid and I-FABP levels in group NK were
significantly higher compared with those in group K (P<0.05).
These results suggest that CPB-induced damage occurred in the
intestinal mucosa of rats, and KOR agonists have the potential to
ameliorate this damage (Fig.
4A).
Between epithelial and endothelial cells, tight
junction molecules including the transmembrane proteins claudins,
occludins, junctional adhesion molecules, ZOs and other peripheral
proteins are involved in maintaining the internal environment and
barrier function (36). In the
present study, the protein expression levels of claudin-1 and ZO-1
were significantly decreased in the CPB group compared with the
sham group, and were significantly increased in group K compared
with group CPB (P<0.05). In addition, the protein expression
levels of claudin-1 and ZO-1 in group NK were significantly
decreased compared with those in group K (P<0.05; Fig. 4B). These results suggest that CPB may
cause damage to the intestinal barrier in rats, and that KOR
agonists could attenuate this damage.
KOR agonist improves intestinal
microcirculation in CPB model rats
The present study estimated intestinal
microcirculation by calculating the microvessel diameter (Fig. 5A) and blood flow state (Fig. 5B) in rats. In group CPB, the mean
microvessel diameter value of rats was 41.74±5.18 µm, which was
significantly narrower compared with that in the sham group
(54.75±6.21 µm; P<0.05). The mean microvessel diameter value of
rats in group K, which had been treated with KOR agonists was
52.83±5.42 µm, which was significantly increased compared with the
CPB group (P<0.05). In group NK, in which rats were treated with
a KOR agonist and KOR antagonist; the microvessel diameter was
44.11±6.34 µm, which was significantly narrower compared with that
in group K (P<0.05).
When the blood flow state of the rats was assessed
using a semi-quantitative flow rate grading method, the blood flow
state of the 10 rats in the sham group was at level 0. However, in
the CPB group there were two rats at level 1, six rats at level 2
and two rats at level 3. In addition, the blood flow state of the
rats in group K showed some improvement compared with the CPB
group. In group K, there were six rats at level 0 and four rats at
level 1, while for group NK there were three rats at level 1, five
rats at level 2 and one rat at level 3. Collectively, these results
suggested that CPB may lead to microcirculatory disturbance in rats
and that KOR agonists could significantly improve the intestinal
microcirculation disturbance caused by CPB.
Effects of KOR agonist on the
NF-κB/HIF-1α signaling pathway in CPB model rats
HIF-1α plays a role in destroying the intestinal
barrier during hypoxia, ischemia-reperfusion and inflammation
(37-39).
Therefore, the present study further investigated the effects of a
KOR on the intestinal barrier of CPB model rats by examining the
expression levels of NF-κB-p65 and HIF-1α using western blotting
(Fig. 6A). The results suggest that
the protein expression levels of NF-κB-p65 and HIF-1α in intestinal
tissue in the CPB group were significantly increased compared with
those in the sham group (P<0.05). In addition, the expression
levels of NF-κB-p65 and HIF-1α in group K were significantly lower
compared with those in group CPB (P<0.05), and the expression
levels of NF-κB-p65 and HIF-1α in group NK were significantly
higher compared with those in group K (P<0.05). These results
were also confirmed by immunohistochemistry (Fig. 6B). Therefore, the present results
suggested that KOR agonists may attenuate intestinal damage in CPB
model rats by inhibiting the NF-κB/HIF-1α signaling pathway.
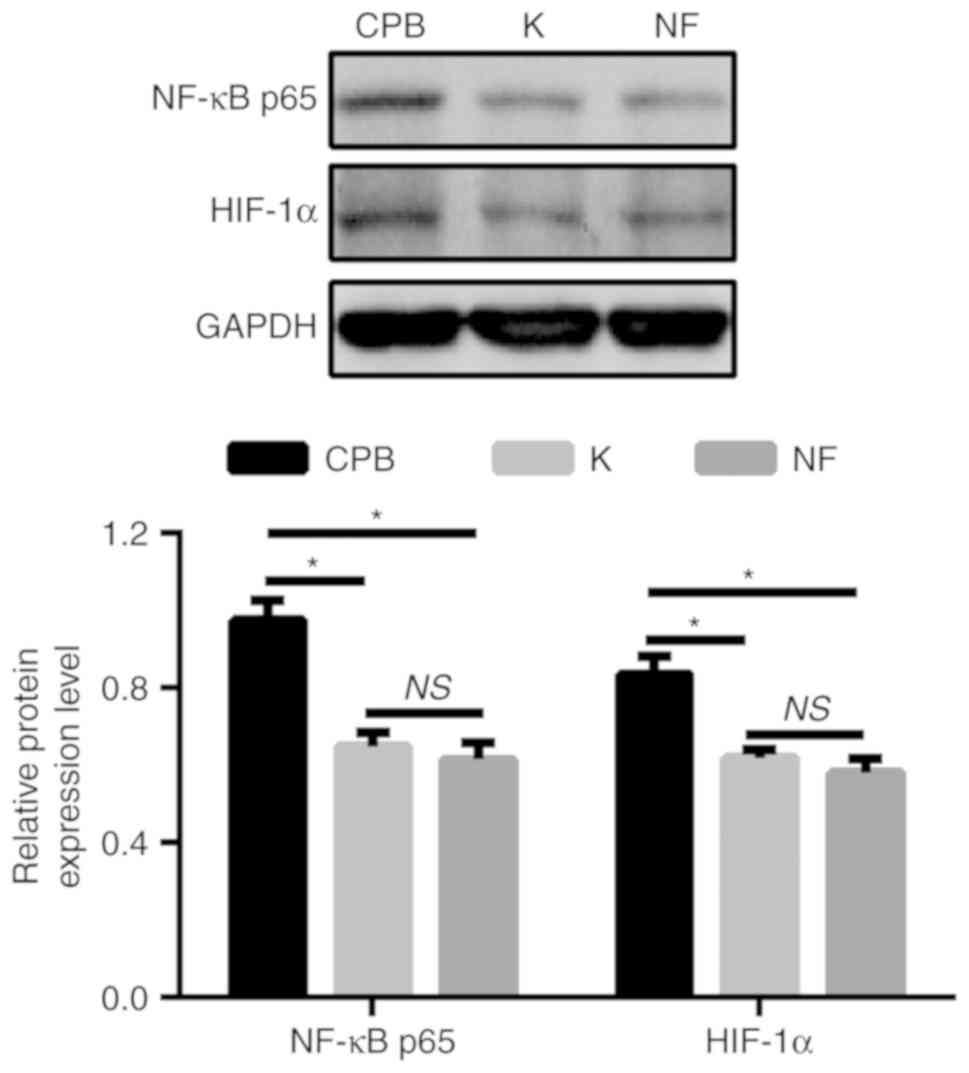
KOR agonist improves intestinal
mucosal function in CPB model rats via the NF-κB/HIF-1α signaling
pathway
To further investigate the relationship between the
KOR agonist, the NF-κB/HIF-1α signaling pathway and intestinal
mucosal function in CPB rats, an NF-κB/HIF-1α signaling pathway
inhibitor was administered to the rats. The results suggested that
protein expression levels of NF-κB-p65 and HIF-1α in intestinal
tissue were significantly decreased in group K compared with the
CPB group (P<0.05). the expression levels of NF-κB-p65 and
HIF-1α in group NF were also significantly decreased compared with
those in the CPB group (P<0.05). However, no significant
difference was found between groups K and NF (Fig. 7). The present results suggest that
KOR agonists may improve intestinal mucosal function in CPB rats
via the NF-κB/HIF-1α signaling pathway.
Discussion
Previous studies have found that pathophysiological
mechanisms of CPB-induced intestinal barrier damage are associated
with SIRS, while intestinal mucosal injury is caused by ischemia
and hypoxia-reperfusion (40,41).
Inflammatory responses caused by CPB include activation of various
systems, including complement in blood serum, platelets and
neutrophils, monocytes and macrophages, as well as the release of
cytokines and leukotrienes in plasma (42). The results of the present study
indicate that the TNF-α and IL-6 levels were significantly
increased in rats after CPB, and were positively associated with
increased intestinal permeability. Furthermore, they suggest that
the inflammatory response caused by CPB may be closely associated
with intestinal mucosal barrier dysfunction. In the present study,
a rat model of CPD-induced intestinal injury was established in
which the intestinal microcirculation was assessed and the
intestinal tissues examined using H&E staining. Oxidative
stress factors, inflammatory factors, intestinal injury markers and
NF-κB/HIF-1α signaling pathway-related proteins were also
investigated. The present study aimed to investigate the role of
KOR agonists in the development and progression of intestinal
barrier dysfunction in CPB model rats.
The expression of KOR mRNA has been detected in the
heart, kidney, adrenal medulla, digestive tract, peripheral blood
vessels, placenta, T cells and macrophages of many animal species,
including humans and the uteri of pregnant mice (43-47).
Therefore, KORs are widely distributed in the body and may be
involved in the regulation of various physiological functions
(43-47).
Previous studies have confirmed that KOR agonists can be used to
treat patients with diseases caused by hypoxia, ischemia or
reperfusion (48,49). When the body is under stress, such as
that caused by shock or ischemia, the endogenous opioid peptide
system is activated and the cardiovascular center of the brain is
regulated via the blood-brain barrier (50).
In addition to the negative inotropic, negative
chronotropic and negative dromotropic effects caused by KORs on the
heart, studies have shown that the activation of KORs can reduce
the area of ischemia/reperfusion myocardial infarction and affect
the occurrence of ischemia/reperfusion arrhythmias (15,16). The
activation of KORs plays a role in cardioprotection (51,52).
When CPB occurs, gastrointestinal tissue is also in an ischemic
state, which leads to damage of the intestinal mucosa (9,10). The
results of the present study reveal that a KOR agonist could
inhibit the inflammatory response of CPB model rats, reduce
oxidative stress, attenuate intestinal damage and relieve
intestinal microcirculation, therefore reducing the occurrence and
development of intestinal barrier dysfunction.
NF-κB is a key transcription factor that regulates
the expression of numerous cytokines and inflammatory mediators,
and plays a central role in the inflammatory response (53). Activation of the NF-κB signaling
pathway promotes the transcription and release of inflammatory
factors such as TNF-α and IL-6 during the inflammatory response
(54). Therefore, inhibiting the
activity of the NF-κB pathway can relieve the inflammatory response
(55). Nicotine can attenuate the
activation of the NF-κB signaling pathway caused by endotoxin
(56,57). In addition, the α7 nicotinic
acetylcholine receptor (α7nAchR) can inhibit the activity of
transcription factor NF-κB, leading to attenuation of inflammatory
cytokines (58). Furthermore, vagus
nerve stimulation prior to α7nAchR antagonist treatment attenuated
the destruction of the intestinal epithelial cells of rats with
endotoxemia, which was mediated by the inhibition of NF-κB-p65 and
transport of myosin light-chain kinase (59). The present results suggest that KOR
agonists may significantly reduce the expression levels of NF-κB
p65 and HIF-1α in intestinal tissues, thereby reducing intestinal
damage in CPB model rats.
In conclusion, the present study revealed that a KOR
agonist can reduce the inflammatory and oxidative stress response
by decreasing intestinal barrier damage in a rat model of CPB, in
which the intestinal barrier plays a key regulatory role.
Collectively, the present results provide theoretical and
experimental evidence on the prevention, occurrence, development
and prognosis of intestinal impairment following cardiopulmonary
bypass.
Acknowledgements
Not applicable.
Funding
This study was supported by The Liaoning Natural
Science Foundation (grant no. 201602790) and The National Natural
Science Foundation of China (grant no. 81471121).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YS and YD designed the research; XZ carried out the
experiments. DS performed the data analysis. YS wrote the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study has been reviewed and approved by the
Animal Ethical and Welfare Committee of The General Hospital of
Northern Theater Command.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hirata Y: Cardiopulmonary bypass for
pediatric cardiac surgery. Gen Thorac Cardiovasc Surg. 66:65–70.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Evora PR, Bottura C, Arcêncio L,
Albuquerque AA, Évora PM and Rodrigues AJ: Key Points for Curbing
Cardiopulmonary Bypass Inflammation. Acta Cir Bras. 31 (Suppl
1):45–52. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chawla BK and Teitelbaum DH: Profound
systemic inflammatory response syndrome following non-emergent
intestinal surgery in children. J Pediatr Surg. 48:1936–1940.
2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Osuka A, Kusuki H, Matsuura H, Shimizu K,
Ogura H and Ueyama M: Acute intestinal damage following severe burn
correlates with the development of multiple organ dysfunction
syndrome: A prospective cohort study. Burns. 43:824–829.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Geissler HJ, Fischer UM, Grunert S,
Kuhn-Régnier F, Hoelscher A, Schwinger RH, Mehlhorn U and Hekmat K:
Incidence and outcome of gastrointestinal complications after
cardiopulmonary bypass. Interact Cardiovasc Thorac Surg. 5:239–242.
2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hashemzadeh K and Hashemzadeh S:
Predictors and outcome of gastrointestinal complications after
cardiac surgery. Minerva Chir. 67:327–335. 2012.PubMed/NCBI
|
|
7
|
De Hert S and Moerman A: Myocardial injury
and protection related to cardiopulmonary bypass. Best Pract Res
Clin Anaesthesiol. 29:137–149. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nan Z, Jin Z, Huijuan C, Tiezheng Z and
Keyan C: Effects of TLR3 and TLR9 signaling pathway on brain
protection in rats undergoing sevoflurane pretreatment during
cardiopulmonary bypass. BioMed Res Int.
2017(4286738)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sun YJ, Cao HJ, Jin Q, Diao YG and Zhang
TZ: Effects of penehyclidine hydrochloride on rat intestinal
barrier function during cardiopulmonary bypass. World J
Gastroenterol. 17:2137–2142. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Riddington DW, Venkatesh B, Boivin CM,
Bonser RS, Elliott TS, Marshall T, Mountford PJ and Bion JF:
Intestinal permeability, gastric intramucosal pH, and systemic
endotoxemia in patients undergoing cardiopulmonary bypass. JAMA.
275:1007–1012. 1996.PubMed/NCBI
|
|
11
|
Tu IH, Yen HT, Cheng HW and Chiu JH:
Baicalein protects chicken embryonic cardiomyocyte against
hypoxia-reoxygenation injury via mu- and delta- but not
kappa-opioid receptor signaling. Eur J Pharmacol. 588:251–258.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cheng L, Ma S, Wei LX, Guo HT, Huang LY,
Bi H, Fan R, Li J, Liu YL, Wang YM, et al: Cardioprotective and
antiarrhythmic effect of U50,488H in ischemia/reperfusion rat
heart. Heart Vessels. 22:335–344. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lin JY, Hung LM, Lai LY and Wei FC:
Kappa-opioid receptor agonist protects the microcirculation of
skeletal muscle from ischemia reperfusion injury. Ann Plast Surg.
61:330–336. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Husain S, Liou GI and Crosson CE: Opioid
receptor activation: Suppression of ischemia/reperfusion-induced
production of TNF-α in the retina. Invest Ophthalmol Vis Sci.
52:2577–2583. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lishmanov AY, Maslov LN, Lasukova TV,
Crawford D and Wong TM: Activation of kappa-opioid receptor as a
method for prevention of ischemic and reperfusion arrhythmias: Role
of protein kinase C and K(ATP) channels. Bull Exp Biol Med.
143:187–190. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Valtchanova-Matchouganska A, Missankov A
and Ojewole JA: Evaluation of the antidysrhythmic effects of delta-
and kappa-opioid receptor agonists and antagonists on calcium
chloride-, adrenaline- and ischemia/reperfusion-induced arrhythmias
in rats. Methods Find Exp Clin Pharmacol. 26:31–38. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sun X, Ma S, Zang YM, Lu SY, Guo HT, Bi H,
Wang YM, Ma H, Ma XL and Pei JM: Vasorelaxing effect of U50,488H in
pulmonary artery and underlying mechanism in rats. Life Sci.
78:2516–2522. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang L, Li J, Shi Q, Fan R, Kaye AJ, Wang
Y, Sun X, Rivera FB, Kaye AD and Pei J: Role of κ-opioid receptor
in hypoxic pulmonary artery hypertension and its underlying
mechanism. Am J Ther. 20:329–336. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Koyasu S, Kobayashi M, Goto Y, Hiraoka M
and Harada H: Regulatory mechanisms of hypoxia-inducible factor 1
activity: Two decades of knowledge. Cancer Sci. 109:560–571.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Anju TR and Paulose CS: Amelioration of
hypoxia-induced striatal 5-HT(2A) receptor, 5-HT transporter and
HIF1 alterations by glucose, oxygen and epinephrine in neonatal
rats. Neurosci Lett. 502:129–132. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barben M, Schori C, Samardzija M and Grimm
C: Targeting Hif1a rescues cone degeneration and prevents
subretinal neovascularization in a model of chronic hypoxia. Mol
Neurodegener. 13(12)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Z, Wang C, Yu N, Si L, Zhu L, Zeng A,
Liu Z and Wang X: INF2 regulates oxidative stress-induced apoptosis
in epidermal HaCaT cells by modulating the HIF1 signaling pathway.
Biomed Pharmacother. 111:151–161. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kappler M, Taubert H, Schubert J,
Vordermark D and Eckert AW: The real face of HIF1α in the tumor
process. Cell Cycle. 11:3932–3936. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Requejo-Aguilar R, Lopez-Fabuel I,
Fernandez E, Martins LM, Almeida A and Bolaños JP: PINK1 deficiency
sustains cell proliferation by reprogramming glucose metabolism
through HIF1. Nat Commun. 5(4514)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Urrutia AA and Aragonés J: HIF oxygen
sensing pathways in lung biology. Biomedicines.
6(E68)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cao M, Wang P, Sun C, He W and Wang F:
Amelioration of IFN-γ and TNF-α-induced intestinal epithelial
barrier dysfunction by berberine via suppression of MLCK-MLC
phosphorylation signaling pathway. PLoS One.
8(e61944)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wu C, Wang X, Jiang T, Li C, Zhang L, Gao
X, Tian F, Li N and Li J: Partial enteral nutrition mitigated
ischemia/reperfusion-induced damage of rat small intestinal
barrier. Nutrients. 8(E502)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kuschel A, Simon P and Tug S: Functional
regulation of HIF-1α under normoxia--is there more than
post-translational regulation? J Cell Physiol. 227:514–524.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nechemia-Arbely Y, Khamaisi M, Rosenberger
C, Koesters R, Shina A, Geva C, Shriki A, Klaus S, Rosen S,
Rose-John S, et al: In vivo evidence suggesting reciprocal renal
hypoxia-inducible factor-1 upregulation and signal transducer and
activator of transcription 3 activation in response to hypoxic and
non-hypoxic stimuli. Clin Exp Pharmacol Physiol. 40:262–272.
2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mi Z, Rapisarda A, Taylor L, Brooks A,
Creighton-Gutteridge M, Melillo G and Varesio L: Synergystic
induction of HIF-1alpha transcriptional activity by hypoxia and
lipopolysaccharide in macrophages. Cell Cycle. 7:232–241.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Collins D, Powell G and Davies I: Cerebral
activity prior to motion task performance: An
electroencephalographic study. J Sports Sci. 9:313–324.
1991.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Szeto HH, Soong Y, Wu D and Fasolo J:
Resensitization of blood pressure response to mu-opioid peptide
agonists after acute desensitization. Anesth Analg. 93:581–586.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou J, Zhou N, Wu XN, Cao HJ, Sun YJ,
Zhang TZ, Chen KY and Yu DM: Role of the Toll-like receptor 3
signaling pathway in the neuroprotective effect of sevoflurane
pre-conditioning during cardiopulmonary bypass in rats. Mol Med
Rep. 12:7859–7868. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
De Backer D, Hollenberg S, Boerma C,
Goedhart P, Büchele G, Ospina-Tascon G, Dobbe I and Ince C: How to
evaluate the microcirculation: Report of a round Table conference.
Crit Care. 11(R101)2007.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Sun YJ, Chen WM, Zhang TZ, Cao HJ and Zhou
J: Effects of cardiopulmonary bypass on tight junction protein
expressions in intestinal mucosa of rats. World J Gastroenterol.
14:5868–5875. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sawada N, Murata M, Kikuchi K, Osanai M,
Tobioka H, Kojima T and Chiba H: Tight junctions and human
diseases. Med Electron Microsc. 36:147–156. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Oliver KM, Taylor CT and Cummins EP:
Hypoxia. Regulation of NFkappaB signalling during inflammation: The
role of hydroxylases. Arthritis Res Ther. 11(215)2009.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Hart ML, Henn M, Köhler D, Kloor D,
Mittelbronn M, Gorzolla IC, Stahl GL and Eltzschig HK: Role of
extracellular nucleotide phosphohydrolysis in intestinal
ischemia-reperfusion injury. FASEB J. 22:2784–2797. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang S, Yu M, Sun L, Xiao W, Yang X, Sun
L, Zhang C, Ma Y, Yang H, Liu Y, et al: Interferon-γ-induced
intestinal epithelial barrier dysfunction by NF-κB/HIF-1α pathway.
J Interferon Cytokine Res. 34:195–203. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Doguet F, Litzler PY, Tamion F, Richard V,
Hellot MF, Thuillez C, Tabley A, Bouchart F and Bessou JP: Changes
in mesenteric vascular reactivity and inflammatory response after
cardiopulmonary bypass in a rat model. Ann Thorac Surg.
77:2130–2137; author reply 2137. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Adamik B, Kübler A, Gozdzik A and Gozdzik
W: Prolonged cardiopulmonary bypass is a risk factor for intestinal
ischaemic damage and endotoxaemia. Heart Lung Circ. 26:717–723.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bronicki RA and Hall M: Cardiopulmonary
bypass-induced inflammatory response: Pathophysiology and
treatment. Pediatr Crit Care Med. 17 (Suppl 1):S272–S278.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hedner T and Cassuto J: Opioids and opioid
receptors in peripheral tissues. Scand J Gastroenterol Suppl. 130
(sup130):27–46. 1987.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Holzer P: Opioid receptors in the
gastrointestinal tract. Regul Pept. 155:11–17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu LJ, Yu JJ and Xu XL: Kappa-opioid
receptor agonist U50448H protects against renal
ischemia-reperfusion injury in rats via activating the PI3K/Akt
signaling pathway. Acta Pharmacol Sin. 39:97–106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang S, Zhou Y, Zhao L, Tian X, Jia M, Gu
X, Feng N, An R, Yang L, Zheng G, et al: κ-opioid receptor
activation protects against myocardial ischemia-reperfusion injury
via AMPK/Akt/eNOS signaling activation. Eur J Pharmacol.
833:100–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhu Y and Pintar JE: Expression of opioid
receptors and ligands in pregnant mouse uterus and placenta. Biol
Reprod. 59:925–932. 1998.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Tsibulnikov SY, Maslov LN, Mukhomedzyanov
AV, Krylatov AV, Tsibulnikova MR and Lishmanov YB: Prospects of
using of κ-opioid receptor agonists U-50,488 and ICI 199,441 for
improving heart resistance to ischemia/reperfusion. Bull Exp Biol
Med. 159:718–721. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xin J, Zhang Y, He Z and Wang Z: Highly
selective non-opioid kappa opioid receptor (KOR) agonist salvinorin
A protects against forebrain ischemia-induced brain injury in rats.
Brain Res. 1637:168–176. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fraessdorf J, Hollmann MW, Hanschmann I,
Heinen A, Weber NC, Preckel B and Huhn R: Role of Endogenous Opioid
System in Ischemic-Induced Late Preconditioning. PLoS One.
10(e0134283)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Maslov LN, Lishmanov YB, Oeltgen PR,
Barzakh EI, Krylatov AV, Naryzhnaya NV, Pei JM and Brown SA:
Comparative analysis of the cardioprotective properties of opioid
receptor agonists in a rat model of myocardial infarction. Acad
Emerg Med. 17:1239–1246. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rong F, Peng Z, Ye MX, Zhang QY, Zhao Y,
Zhang SM, Guo HT, Hui B, Wang YM, Liang C, et al: Myocardial
apoptosis and infarction after ischemia/reperfusion are attenuated
by kappa-opioid receptor agonist. Arch Med Res. 40:227–234.
2009.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer.
12(86)2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Aziz RS, Siddiqua A, Shahzad M, Shabbir A
and Naseem N: Oxyresveratrol ameliorates ethanol-induced gastric
ulcer via downregulation of IL-6, TNF-α, NF-κB, and COX-2 levels,
and up-regulation of TFF-2 levels. Biomed Pharmacother.
110:554–560. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Piao H, Choi YH, Li H, Wang C, Xian Z,
Ogasawara M, Jiang J, Li L, Yamauchi K and Yan G: Recombinant pyrin
domain protein attenuates allergic inflammation by suppressing
NF-κB pathway in asthmatic mice. Scand J Immunol.
89(e12720)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Café-Mendes CC, Garay-Malpartida HM, Malta
MB, de Sá Lima L, Scavone C, Ferreira ZS, Markus RP and Marcourakis
T: Chronic nicotine treatment decreases LPS signaling through NF-κB
and TLR-4 modulation in the hippocampus. Neurosci Lett.
636:218–224. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liu Y, Yang J, Bao J, Li X, Ye A, Zhang G
and Liu H: Activation of the cholinergic anti-inflammatory pathway
by nicotine ameliorates lipopolysaccharide-induced
preeclampsia-like symptoms in pregnant rats. Placenta. 49:23–32.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chen K, Sun Y, Diao Y, Ji L, Song D and
Zhang T: α7 nicotinic acetylcholine receptor agonist inhibits the
damage of rat hippocampal neurons by TLR4/Myd88/NF-κB signaling
pathway during cardiopulmonary bypass. Mol Med Rep. 16:4770–4776.
2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhao YX, He W, Jing XH, Liu JL, Rong PJ,
Ben H, Liu K and Zhu B: Transcutaneous auricular vagus nerve
stimulation protects endotoxemic rat from
lipopolysaccharide-induced inflammation. Evid Based Complement
Alternat Med. 2012(627023)2012.PubMed/NCBI View Article : Google Scholar
|