Introduction
Primary extranodal lymphomas (PELs) are
non-Hodgkin's lymphomas that arise in non-lymphatic tissues
(1,2). PEL is frequently observed in the
gastrointestinal tract and also in the skin, but is seldom seen in
the central nervous system (CNS). The tissue of origin of a PEL
determines its pathological and molecular features, as well as
patient prognosis and therapeutic strategies (3).
Primary central nervous system lymphoma (PCNSL)
represents 4% of intracranial tumors and accounts for 4-6% of all
reported PELs (4). Patients with
CNS-derived PELs usually have a poor prognosis (5,6). Diffuse
large B-cell lymphoma (DLBCL) is a common PCNSL sub-type and is
usually observed in the brain, eyes, meninges and spinal cord
without systemic spread (7).
Differential diagnosis of PCNSL is usually achieved by examining a
stereotactic brain biopsy or cerebrospinal fluid (CSF) and vitreous
fluid (VRF) cytology (if malignancy involves those tissues)
(8). However, CSF and VRF cytology
is not always feasible, owing to the low yield of tumor cells in
the fluid samples (9,10). Therefore other techniques, including
immunocytochemistry, determination of cytokine levels, flow
cytometry, immunoglobulin gene rearrangement analysis and mutation
analysis, are also applied to examine the CSF or VRF (11-13).
Nevertheless, the amount of tumor cells in the fluid sampled
remains a key determining factor affecting accurate diagnosis
(13).
MYD88 (MYD88 innate immune signal transduction
adaptor)L265P mutation is reported in up to 75% of PCNSL
cases and is regarded as a molecular marker for PCNSL (14). In addition, MYD88L265P
mutation is associated with poor prognosis, especially in elderly
patients (15). Therefore,
MYD88L265P mutation detection in the CSF and/or VRF may
be instrumental for the early diagnosis of PCNSL alongside
additional diagnostic tools (10,16).
To date, reverse transcription-quantitative PCR
(RT-qPCR) and panel next generation sequencing (NGS) are the most
commonly used techniques for the detection of MYD88 mutations
(13,16). However, samples containing a low
concentration of tumor DNA may not reach the threshold (% of the
sample containing DNA) required for either technique (NGS, 2-5%;
RT-qPCR, ~0.5%) (17). Droplet
digital PCR (ddPCR) is a relatively new PCR technique with a
superior sensitivity for trace mutation identification compared to
conventional PCR techniques (18).
In the present study, the feasibility of ddPCR in the diagnostic
detection of MYD88L265P mutation in lymphomas was
examined using both CSF and VRF samples and additional tumor tissue
samples.
Materials and methods
Patients
The data from 72 patients that had presented with
DLBCL were retrospectively analyzed in the present study. All
patients were examined in the Department of Hematology, Huashan
Hospital North between January 2013 and December 2016. A total of
44 cases of PCNSL, 15 cases of DLBCS not otherwise specified
(DLBCL-NOS), and 13 cases of other PELs (2 cases in breast, 3 cases
in testis, 3 cases in bone, 4 cases in the gastrointestinal tract
and 1 case in the mediastinum) were analyzed. A total of 55
formalin-fixed paraffin-embedded (FFPE) brain, lymphatic or
malignancy involved tissues were obtained following surgical
resection (PCNSL=29, DLBCL-NOS=15 and PEL=11). CSF samples were
collected by lumbar puncture from 26 PCNSL and 2 testis-PEL
patients. Among them, 18 samples were collected as paired biopsies
of the malignant tissues (16 PCNSL and 2 testis-PEL). A total of 25
VRF samples were collected after either single-side (n=9) or double
side (n=8) vitrectomy, and among them 5 cases were collected as
paired biopsy with the malignant tissues. Further details of
patient characteristics can be found in Table I. The protocol of this study was
approved by the Huashan Hospital Institution Review Board (HIRB)
and informed written consent was obtained from all enrolled
patients. The diagnoses of all enrolled patients were reviewed and
confirmed according to the diagnostic criteria and classification
of the World Health Organization (7).
 | Table IClinical parameters of the enrolled
patients. |
Table I
Clinical parameters of the enrolled
patients.
| Clinical
feature | PCNSL | DLBCL-NOS | Other PEL |
|---|
| Number of
patients | 44 | 15 | 13 |
| Sex |
|
Male | 22 | 8 | 11 |
|
Female | 22 | 7 | 2 |
| Age, years
(range) | 59 (53.5-64.5) | 60 (54.3-72.5) | 52 (46.8-64.0) |
|
<60 | 22 | 7 | 8 |
|
≥60 | 22 | 8 | 5 |
| Clinical
manifestation |
|
Intracranial
hypertension | 9 | 0 | 0 |
|
Movement
disorders | 26 | 2 | 4 |
|
Sensory
dysfunction | 4 | 0 | 0 |
|
Speech
disorders | 5 | 0 | 0 |
|
Visual
disturbance | 10 | 0 | 2 |
|
Cognitive
disorders | 3 | 0 | 1 |
|
Facioplegia | 5 | 1 | 0 |
|
Convulsion | 1 | 0 | 0 |
| Tumor tissue
(n=55) | 29 | 15 | 11 |
|
Brain tumor
tissue | 29 | 0 | 1 |
|
Lymphatic
tumor tissue | 0 | 15 | 0 |
|
Other tumor
tissue | 0 | 0 | 10 |
| CSF (n=28) | 26 | 0 | 2 |
| VRF (n=25) | 22 | 0 | 3 |
-qPCR and ddPCR
Genomic DNA was extracted from the FFPE tissue
sections using the QIAamp DNA FFPE tissue kit (Qiagen GmBH) and
circulating DNA (ctDNA) was extracted from CSF or VRF samples using
the QIAamp circulating nucleic acid kit (Qiagen GmBH) according to
the manufacturer's instructions. In addition, genomic DNA was
extracted from the bone marrow of a lymphoplasmacytic lymphoma
patient (positive control for MYD88L265P mutation) or
from the VFR DNA obtained from an intraocular infiltrated NK/T-cell
lymphoma patient (negative control), using the QIAamp circulating
nucleic acid kit according to the manufacturer's instructions.
TaqMan probes purchased from Thermo Fisher Scientific, Inc were as
follows: MYD88-L265P-CT-T2 (HEX-GCGACTGATCC-BHQ1), and
MYD88-L265P-CT-C2 (FAM-GCGACCGATCC-BHQ1).
The extracted DNA was amplified by qPCR on a Roche
cobas Z 480 real-time PCR platform (Roche Applied Science) using a
Kapa probe fast universal qPCR Kit (Kapa Biosystems; Roche
Diagnostics) according to the standard protocols. Primer sequences
used for amplification were as follows: MyD88-L265P forward,
5'-CATGGCACCCCTTGGCTT-3' and reverse, 5'-CCTCAGGATGCTGGGGAAC-3'.
qPCR was conducted under the following conditions: Initial
denaturation at 95˚C for 3 min followed by 40 cycles of 95˚C for 30
sec, 60˚C for 30 sec and 72˚C 30 sec, with a final extension at
72˚C for 1 min. qPCR data were quantified using
2-ΔΔCq analysis (19). Alternatively, the extracted DNA was
amplified by ddPCR on a QX200 ddPCR system (Bio-Rad Laboratories,
Inc.) according to manufacturer's instructions and the results were
visualized by a QuantaSoft software (version 1.7.4; Bio-Rad
Laboratories, Inc.). ddPCR was conducted under the following
conditions: Initial denaturation at 95˚C for 10 min, followed by 40
cycles of 95˚C for 30 sec, 58˚C for 30 sec and 72˚C for 1 min, with
a final extension at 98˚C for 10 min. GAPDH (forward:
5'-GGAGCGAGATCCCTCCAAAAT-3', reverse:
5'-GGCTGTTGTCATACTTCTCATGG-3') was used as a loading control. Each
sample was analyzed in duplicate and a positive as well as a
negative control sample was included for quality control and to
determine the fluorescence thresholds. The primer sequences and
fluorescent probes used in the ddPCR procedures were identical to
those of qPCR and they were prepared with 2X master-mix solution
(Bio-Rad Laboratories, Inc.). For each sample, the reaction wells
were clustered into four groups, [wild type (HEX positive), mutant
(FAM positive), heterozygote (double positive), and no-template
(double negative)], in the fluorescence signal intensity 2D plot.
Absolute quantification of each sample was subsequently achieved in
copies/µl (molecules DNA/µl) by Poisson's distribution correction.
Furthermore, QuantaSoft 1.7.4 software (Bio-Rad Laboratories, Inc.)
was used to calculate the fractional abundance (mutation
frequency), irrespective of mutant positive droplets amount.
Therefore, samples negative for MYD88L265P showed a
fractional abundance above 0.0%.
Immunohistochemistry
Following fixation at 4˚C at least
overnight and paraffin embedding, the FFPE tissues were sectioned
at 2 to 4 µm thickness on a microtome. Immunohistochemistry was
performed using a Ki67 primary antibody working solution (1:1,000;
cat. no. MAB-0672; MXB Biotechnologies) or MyD88 primary antibody
solution (1:800; OriGene Technologies, Inc.; cat. no. TA502117) and
a REAL EnVision detection system (Dako; Agilent Technologies, Inc.)
according to the manufacturer's instructions. Next, the sections
were counterstained with hematoxylin and eosin (H&E) at room
temperature for 10 min using an H&E staining kit (Baso
Diagnostics, Inc.). Slides were independently examined by two
experienced pathologists using the microscope Nikon50i (Nikon
Corporation) with x400 magnification. The staining was scored
semi-quantitatively and recorded based on both the cytoplasmic
staining (0=negative, 1=1-25% immunoreactive cells, 2=26-50%
immunoreactive cells, 3=51-75% immunoreactive cells, and 4=76-100%
immunoreactive cells) as well as the staining intensity
(0=negative, 1=weak, 2=moderate and 3=strong). The stainings were
manually calculated by two experienced pathologists.
Statistical analysis
The data were presented as means ± SEM. The
differences among the clinical characteristics were compared using
the χ2-test or the Fisher's exact test, according to the
sample size. All statistical analysis was performed with SPSS
(version 21.0; IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic characteristics and
clinical features of the enrolled patients
Analysis of the demographic characteristics
indicated that there was no significant difference in the gender
(P=0.082) or median age (P=0.236) among the enrolled patients
(Table I). The median age of
patients with PCNSL was 59 years (range, 41-72 years). The median
age of patients with DLBCL-NOS was 60 years (range, 45-86 years)
and for patients with other PELs the median age was 52 years
(range, 27-84), whose ages were not significant difference among
these groups. When compared with the DLBCL-NOS and PELs patients,
the PCNSL patients presented with cranial hypertension and
dyskinesia more often (P<0.05), but no B symptoms (fever, night
sweats, or weight loss) were observed (P<0.01). There were no
obvious neurological symptoms in the DLBCL-NOS and other PELs
patients (P<0.01). However, cranial hypertension, dyscinesia and
visual impairment could occur after the CNS was affected (data not
shown).
The diagnosis of PCNSL patients (n=44) was evaluated
according to the guidelines of the International PCNSL
Collaborative Group Report, including by magnetic resonance imaging
of the brain, ophthalmologic evaluation and CSF evaluation
(13) (Table IV). Imaging results demonstrated
that a total of 24 PCNSL patients presented with multifocal lesions
(24/44; 54.5%), while the other 20 patients had a single lesion
(20/44; 45%). The lesions were mainly located in the front-temporal
lobe (35/44; 79.5%) and deep structures (25/44; 56.8%), while the
eyes (14/44; 31.8%) were less frequently involved. There was no
bone marrow invasion observed among the PCNSL patients; whereas,
bone marrow invasion was detected in 6 DLBCL-NOS patients (6/15;
40%). The serum LDH and β2-microglobin concentration was
significantly different among the three examined groups
(P<0.01). There was no significant difference in the CSF
evaluation parameters (pressure, protein concentration or cytology)
among the examined groups (P>0.05).
 | Table IVClinical imaging performance and
clinicopathological features. |
Table IV
Clinical imaging performance and
clinicopathological features.
| Parameter | PCNSL | DLBCL-NOS | Other PEL | P-value |
|---|
| Number of
patients | 44 | 15 | 13 | |
| Lesion
location |
|
Front-temporal
lobe | 35 | 0 | 1 | <0.001 |
|
Deep
structures | 25 | 0 | 3 | <0.001 |
|
Eyes | 14 | 0 | 2 | 0.030 |
| Lesion Number |
|
Multiple | 24 | 0 | 2 | <0.001 |
|
Single | 20 | 0 | 2 | |
| Involvement of bone
marrow | 0 | 6 | 3 | <0.001 |
| LDH
elevateda | 5 | 7 | 3 | 0.007 |
| Serum β2-M
elevatedb | 11 | 11 | 4 | 0.001 |
| CSF
pressurec | 6 | NA | 0 | 0.281 |
| High CSF protein
leveld | 12 | NA | 1 | 0.176 |
| Abnormal CSF
cytology | 10 | NA | 2 | 0.664 |
Detection of MYD88L265P in
tissue and CSF/VRF using ddPCR
Among the collected lymphoma tissue samples, 28 of
the evaluable tissue samples (28/55, 50.9%) harbored the
MYD88L265P mutation. In the PCNSL patients (n=44),
genomic DNA was extracted from 29 tissue samples, 17 VFR samples
and 26 CSF samples. The sensitivity of MYD88L265P
mutation detection was similar between qPCR and ddPCR in the case
of DNA samples obtained from PCNSL tissues. Using both qPCR and
ddPCR, positive MYD88L265P mutation was identified in
72.4% (21/29) PCNSL tissue samples. Compared to qPCR, the
sensitivity of mutation detection was significantly higher in ddPCR
for CSF/VRF DNA samples (P<0.05). Specifically, conventional
qPCR detected positive MYD88L265P mutation in 15.4%
(4/26) of the PCNSL CSF samples, while ddPCR could identify
MYD88L265P mutation in 57.8% (15/26) of the PCNSL CSF,
including the 4 qPCR positive ones (Table II). Meanwhile, qPCR identified
MYD88L265P mutation in 70.61% (12/17) of the VRF samples
while ddPCR detected an additional positive MYD88L265P
mutation (13/17; 76.5%). Interestingly, double side vitrectomy
significantly increased the sensitivity of ddPCR-based
MYD88L265P mutation detection by 35% (13/17 vs. 7/17 in
the single side sample) (P=0.031; P<0.05; Table III).
 | Table IIImmunophenotypic studies and
mutational status of MYD88 L265P. |
Table II
Immunophenotypic studies and
mutational status of MYD88 L265P.
| Positive MYD88
Protein expression ratio |
MyD88L265P mutation using
ddPCR |
|---|
| Tumor tissue
(%) | VRF | CSF |
|---|
| PCNSL | 18/29 (62.1%) | 21/29 (72.4) | 11/15 (73.3%) | 15/26 (57.7%) |
| DLBCL-NOS | 0/15 (0.0%) | 2/15 (13.3) | NA | NA |
| Other PEL | 5/11 (45.5%) | 5/11 (45.5) | 2/2 (100%) | 1/2 (50%) |
| Total | NA | 28/55 (50.9) | 13/17 (76.5%) | 16/28 (57.1%) |
 | Table IIIMYD88 L265P mutation detection by
ddPCR in CSF and vitreous fluid samples. |
Table III
MYD88 L265P mutation detection by
ddPCR in CSF and vitreous fluid samples.
| Sample |
MYD88L265P |
|---|
| Tissue | CSF | VRF |
|---|
| L | R |
|---|
| 1 | Mut | WT (0.00) | NA | NA |
| 2 | Mut | WT (0.07) | NA | NA |
| 3 | Mut | WT (0.05) | NA | NA |
| 4 | Mut | WT (0.00) | NA | NA |
| 5 | Mut | WT (0.00) | NA | NA |
| 6 | Mut | WT (0.00) | NA | NA |
| 7 | Mut | Mut (1.10) | NA | NA |
| 8 | Mut | Mut (1.80) | NA | NA |
| 9 | Mut | Mut (0.92) | NA | NA |
| 10 | Mut | Mut (3.10) | NA | NA |
| 11 | Mut | Mut (1.20) | NA | NA |
| 12 | Mut | Mut (1.03) | NA | NA |
| 13 | Mut | Mut (1.10) | Mut (5.40) | NA |
| 14 | Mut | Mut (0.90) | Mut (3.80) | NA |
| 15 | Mut | Mut (44.3) | WT (0.08) | NA |
| 16 | Mut | WT (0.00) | Mut (10.70) | NA |
| 17 | Mut | WT (0.00) | Mut (7.70) | Mut (1.90) |
| 18 | Mut | WT (0.00) | Mut (12.30) | Mut (11.30) |
| 19 | NA | WT (0.00) | Mut (0.83) | NA |
| 20 | NA | WT (0.00) | Mut (6.30) | Mut (2.2) |
| 21 | NA | WT (0.00) | Mut (5.00) | Mut (0.99) |
| 22 | NA | WT (0.00) | Mut (0.83) | Mut (5.00) |
| 23 | NA | Mut (2.60) | NA | Mut (13.00) |
| 24 | NA | Mut (0.63) | NA | NA |
| 25 | NA | Mut (0.92) | NA | NA |
| 26 | NA | Mut (0.62) | NA | NA |
| 27 | NA | Mut (1.20) | NA | NA |
| 28 | NA | Mut (0.82) | NA | NA |
Among the 18 CSF samples derived from the PEL paired
samples, positive MYD88L265P mutation was detected in 9
(50%) sample pairs by ddPCR using Fisher's exact test, which
emphasizes the value of paired sampling (Table III). Furthermore, multisite
sampling improved the diagnosis efficiency. For instance, in
patient 15, the left eye VRF sample was negative in both cytology
and mutation analysis, while the CSF sample was identified as
MYD88L265P positive by ddPCR (Fig. 1A).
MYD88L265P mutation is
associated with MYD88 upregulation in PCNSL
Next, the immunophenotypic features of MYD88
L265P mutation among patients in the cohort were
investigated (Tables IV and
V; Fig.
3). FFPE tissues from PCNSL, DLBCL-NOS and PEL patients were
immunostained with anti-MYD88 antibody. There was no positive MYD88
expression in the DLBCL-NOS FFPE tissues (0/15; data not shown). In
the PCNSL and PELs tissues, there was no significant difference in
MYD88 protein expression (18/29, 62.1% positive protein expression
vs. 5/11, 45.5% positive MYD expression, respectively; Table IV; Fig.
3). Interestingly, MYD88L265P mutation was
significantly associated with PCNSL (34/72, 47.2%; P<0.05;
Table II). The ddPCR analysis
demonstrated that 28 of the 55 lymphoma tissue samples (28/55;
50.9%) harbored the MYD88L265P mutation (Table II). Among them, 21 cases were from
PCNSL patients (21/29; 72.4%), 2 cases were from DLBCL-NOS patients
(2/15; 13.3%) and 5 samples were obtained from other-PEL patients
(5/11; 45.5%). Therefore, positive MYD88L265P mutation
was significantly more prevalent in PCNSL samples (P<0.001;
Table II). In the present study
cohort (n=72), the PEL tissue of origin included the brain, eye,
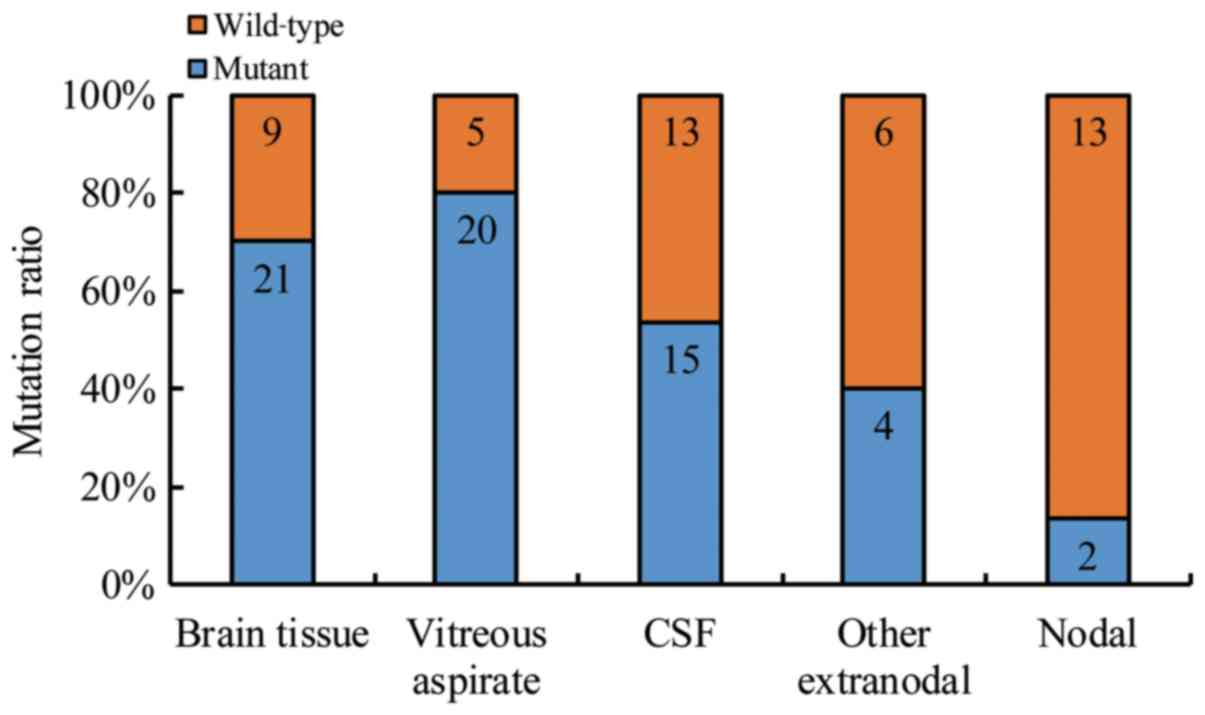
CSF and other extranodal and lymph nodals. However, brain tissue
showed the highest MYD88 L265P mutational rate (21/30,
70%), followed by VRF (20/25; 80.0%), and CSF samples (15/28;
53.6%; Fig. 2). In PCNSL patients,
MYD88L265P mutation in brain tissues was significantly
associated with MYD88 protein upegulation (r=0.421, P=0.038;
Table V). Moreover, the presence of
positive MYD88L265P mutation was observed in up to 40.9%
(9/22) of the CSF samples if the brain tissue was positive for the
same mutation (Fig. 4).
 | Table VRelationship between MYD88 protein
and MYD88L265P mutation in PCNSL. |
Table V
Relationship between MYD88 protein
and MYD88L265P mutation in PCNSL.
| MYD88 Protein |
MYD88L265P | Total |
|---|
| Mut | WT |
|---|
| Negative | 6 | 5 | 11 |
|
Low | 5 | 2 | |
|
Medium | 2 | 0 | |
| Positive | | | 18 |
|
High | 5 | 1 | |
|
Very
high | 3 | 0 | |
| Total | 21 | 8 | 29 |
Discussion
PCNSLs are primary lymphomas of the CNS that include
DLBCL and other rare lymphomas, for example T-cell lymphoma and
Burkitt lymphoma. The incidence of PCNSL increases with age, with
an estimated median age of onset between 55 and 65 years old
(20). The etiology of PCNSL remains
to be elucidated, but Epstein-Barr or human immunodeficiency virus
infection, organ transplantation and immunodeficiency have been
reported to be major contributors to development of the disease
(17,21). Löw et al (22), previously reported that administering
a high methotrexate dose could lead to a high treatment response
rate in PCNSL patients. However, the relapse rate can reach up to
50% with the 5-year survival rate ranging from 22-40% (23,24). In
PCNSL, MYD88L265P is a hot-spot mutation, which alters
interleukin-1 and toll-like receptor signaling and leads to the
hyperactivation of the NF-κB (25)
and JAK/STAT signaling pathways (26-28).
This mutation can be found in extranodal DLBCL in tissues including
the testis, CNS, breast and skin (14,
29-32). In PCNSL, a number of studies have demonstrated that
the rate of MYD88L265P mutation ranges from 73-94.4%
(10,14,16,29-31).
Interestingly, MYD88L265P mutation has not been detected
in other CNS tumors, for example glioblastoma (33). Therefore, accurate identification of
the MYD88L265P mutation may be a critical step for PCNSL
diagnosis.
Identification of circulating tumor cells and
circulating tumor DNA in peripheral fluids has become instrumental
for the micro-invasive diagnosis of tumors (34). Previous studies reported that
MYD88L265P detection in the CSF using NGS or qPCR may be
a powerful tool for disease diagnosis (16,35-37).
In the present study, the diagnostic value of ddPCR in detecting
the MYD88L265P mutation in PCNSL VRF, CSF and FFPE
samples was validated.
In the present study patient cohort, the mutation
rate of MYD88L265P in PCNSL was 77.2% (34/44), which
came in agreement with the reported rates in Caucasians (33.3-38%)
(38,39) and East Asian patients (63.6-85.4%)
(15,30,40). The
MYD88L265P mutation was more frequently observed in the
CNS than in the lymph nodes (70% in brain tissues, 80% in vitreous
bodies and 53.6% in CSF). This phenomenon can be attributed to the
anatomical structure of the immune barrier in the tissue of origin,
such as the CNS, eyes and testicles (29). MYD88L265P mutation
activates the toll-like receptor/MYD88 signal, which can lead to
the selective growth of lymphoma cells in this particular immune
region (41). The results of the
present study indicated an association between
MYD88L265P mutation and increased MYD88 protein
expression in PCNSL tissues, thereby, providing further evidence to
support the abovementioned hypothesis.
To date, NGS and qPCR are the most popular
techniques for the detection of MYD88L265P mutation.
However, the high cost of NGS hinders its wide-scale use for
diagnostic purposes (42). The
results of the present study indicated that the RT-qPCR detection
sensitivity for MYD88L265P mutation in the CSF was only
14.3% (4/28). This could possibly be attributed to a low level of
tumor DNA in the CSF, which hampered the amplification process. On
the other hand, the sensitivity of MYD88L265P mutation
detection was 54.6% (15/28) using ddPCR, which was a significantly
higher rate of MYD88L265P mutation in CSF compared with
that previously reported (31%) (43). The diagnosis of intraocular lymphoma,
when lymphoma cells invade the eye tissues, can sometimes be
challenging (44,45); therefore, vitreous cell pathology
through vitrectomy may be a new gold standard for disease
diagnosis. Using ddPCR, MYD88L265P mutation detection
was successfully achieved in 76% (13/17) of the highVRF samples;
whereas, using qPCR 71% (12/17) of MYD88L265P mutations
were detected. These findings suggested that VRF may be a valuable
micro-invasive sample for the molecular diagnosis of VRL.
Presently, at the early stages of PCNSL, CSF is sufficient for
diagnosis in clinic. With progression of the disease, PCNSL may
affect the eyes in 15-25% patients, which must be confirmed by VRF
analysis (46). VRF analysis may
contribute to improving the sensitivity of vitreoretinal lymphoma
diagnosis. Additionally, MYD88L265P mutation displays
100% specificity for diagnosis in VRF.
PCNSL is a relatively rare intracranial tumor. At
present, its diagnosis is accomplished via intracranial biopsy or
CSF/VRF cytological pathology. CSF/VRF cytology requires the
presence of intact tumor cells in the sample. Consequently, a high
rate of false negative results is usually observed when the number
of tumor cells is low in the CSF/VFR. In addition, treatment with
chemotherapy and steroids may negatively impact the number of
intact tumor cells in the CSF/VRF (47). These shortcomings can be overcome by
the analysis of circulating tumor DNA in CSF/VRF samples.
Therefore, detection of circulating tumor DNA may be a promising
methodology for the diagnosis of CNS lymphoma.
ddPCR has been determined to be the most sensitive
method to detect MYD88L265P in ctDNA of bone marrow or
peripheral blood in cases of Waldenstrom macroglobulinemia
(16,34). In the present study, patient 12 was a
noteworthy case. This 60-year old female was diagnosed with
lymphoplasmacytic lymphoma in December 2016. Her symptoms were
headache, abnormal sensation and dyskinesia. MRI showed that the
left frontal lobe was occupied by lesions. MYD88L265P
mutation was detected in both her bone marrow and her CSF. Her
condition was confirmed to be Bing-Neel syndrome (BNS), a rare
manifestation of Waldenstrom's macroglobulinemia that results from
infiltration of the central nervous system by malignant
lymphoplasmacytic cells (48). It
was puzzling that a large number of tumor cells were found in the
CSF of this patient, which presented with morphology different to
lymphoplasmacytic cells and closer to the morphology of DLBCL
cells. A surgical biopsy of the patient was performed. The
histopathological diagnosis was DLBCL, and MYD88L265P
mutation was also detected. However, the immunohistochemical
staining of the tissue did not indicate evidence of infiltration of
lymphoplasmacytic cells. The immunoglobulin heavy chain (IGH)
rearrangement between brain and bone marrow tissue was then
assessed. According to the results of IGH rearrangement and
histopathological type, it could be concluded that the patient had
two distinct types of tumors. From this case, it can be concluded
that BNS or PCNSL cannot be diagnosed only by the detection of
MYD88L265P mutation in the CSF, which should only be
used as an indicator of auxiliary diagnosis.
In conclusion, MYD88L265P mutation is a
valuable marker for PCNSL diagnosis. Detection of the mutation in
the CSF and VRF samples by ddPCR is a promising micro-invasive tool
to confirm the PCNSL diagnosis or exclude other CNS malignancies.
However, the combination of various molecular techniques with
conventional CSF/VRF cytology should be encouraged to improve
diagnostic specificity and sensitivity.
Acknowledgements
Not applicable.
Funding
This project was supported by the Special Foundation
for Science and Technology of Baoshan District Shanghai (grant no.
17-E-29), the Special Foundation of Fudan University Hua Shan
Hospital North (grant no. 2015106), and the Special clinical
program of Shanghai Health Committee (grant no. 201940004).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request
Authors' contributions
KC, MG and BC designed the study. KC, YM and XZ
performed the experiments. KC and TD analyzed the data. KC and YM
drafted and revised the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The protocol of the current study was approved by
the Huashan Hospital Institution Review Board (HIRB) and informed
written consent was obtained from all enrolled patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oh MY, Oh SB, Seoung HG, et al: Clinical
significance of standardized uptake value and maximum tumor
diameter in patients with primary extranodal diffuse large B cell
lymphoma. The Korean journal of hematology. 47:207–212.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kashyap R, Rai Mittal B, Manohar K, et al:
Extranodal manifestations of lymphoma on [(1)(8)F]FDG-PET/CT: a
pictorial essay. Cancer imaging : the official publication of the
International Cancer Imaging Society. 11:166–174. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Illerhaus G, Schorb E and Kasenda B: Novel
agents for primary central nervous system lymphoma: evidence and
perspectives. Blood. 132:681–688. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ferreri AJ: How I treat primary CNS
lymphoma. Blood. 118:510–522. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shi Y, Han Y, Yang J, et al: Clinical
features and outcomes of diffuse large B-cell lymphoma based on
nodal or extranodal primary sites of origin: Analysis of 1,085 WHO
classified cases in a single institution in China. Chinese journal
of cancer research = Chung-kuo yen cheng yen chiu. 31:152–161.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferreri AJ: Risk of CNS dissemination in
extranodal lymphomas. Lancet Oncol. 15(e159-169)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sabattini E, Bacci F, Sagramoso C and
Pileri SA: WHO classification of tumours of haematopoietic and
lymphoid tissues in 2008: an overview. Pathologica. 102:83–87.
2010.PubMed/NCBI
|
|
8
|
Hoang-Xuan K, Bessell E, Bromberg J, et
al: Diagnosis and treatment of primary CNS lymphoma in
immunocompetent patients: guidelines from the European Association
for Neuro-Oncology. Lancet Oncol. 16(e322-332)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cesana C, Klersy C, Scarpati B, et al:
Flow cytometry and cytomorphology evaluation of hematologic
malignancy in cerebrospinal fluids: comparison with retrospective
clinical outcome. Ann Hematol. 90:827–835. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Raja H, Salomao DR, Viswanatha DS and
Pulido JS: Prevalence Of Myd88 L265p Mutation In Histologically
Proven, Diffuse Large B-Cell Vitreoretinal Lymphoma. Retina.
36:624–628. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Araujo I and Coupland SE: Primary
Vitreoretinal Lymphoma -- A Review. Asia-Pacific journal of
ophthalmology. 6:283–289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shin SY, Lee ST, Kim HJ, et al: Usefulness
of Flow Cytometric Analysis for Detecting Leptomeningeal Diseases
in Non-Hodgkin Lymphoma. Annals of laboratory medicine. 36:209–214.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu L, Cao F, Wang S, Zhou J, Yang G and
Wang C: Detection of malignant B lymphocytes by PCR clonality assay
using direct lysis of cerebrospinal fluid and low volume specimens.
International journal of laboratory hematology. 37:165–173.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Taniguchi K, Takata K, Chuang SS, et al:
Frequent MYD88 L265P and CD79B Mutations in Primary Breast Diffuse
Large B-Cell Lymphoma. Am J Surg Pathol. 40:324–334.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou Y, Liu W, Xu Z, et al: Analysis of
Genomic Alteration in Primary Central Nervous System Lymphoma and
the Expression of Some Related Genes. Neoplasia. 20:1059–1069.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hiemcke-Jiwa LS, Minnema MC, Radersma-van
Loon JH, et al: The use of droplet digital PCR in liquid biopsies:
A highly sensitive technique for MYD88 p.(L265P) detection in
cerebrospinal fluid. Hematol. Oncol. 36:429–435. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Besson C, Goubar A, Gabarre J, et al:
Changes in AIDS-related lymphoma since the era of highly active
antiretroviral therapy. Blood. 98:2339–2344. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Postel M, Roosen A, Laurent-Puig P, Taly V
and Wang-Renault SF: Droplet-based digital PCR and next generation
sequencing for monitoring circulating tumor DNA: a cancer
diagnostic perspective. Expert review of molecular diagnostics.
18:7–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Olson JE, Janney CA, Rao RD, et al: The
continuing increase in the incidence of primary central nervous
system non-Hodgkin lymphoma: a surveillance, epidemiology, and end
results analysis. Cancer. 95:1504–1510. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shiels MS, Pfeiffer RM, Besson C, et al:
Trends in primary central nervous system lymphoma incidence and
survival in the U.S. Br J Haematol. 174:417–424. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Low S, Han CH and Batchelor TT: Primary
central nervous system lymphoma. Therapeutic advances in
neurological disorders. 11(1756286418793562)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yamanaka R, Morii K, Shinbo Y, et al: Late
relapse of primary central nervous system lymphoma. Leuk Lymphoma.
58:475–477. 2017. View Article : Google Scholar
|
|
24
|
Perkins A and Liu G: Primary Brain Tumors
in Adults: Diagnosis and Treatment. Am. Fam. Physician. 93:211–217.
2016.PubMed/NCBI
|
|
25
|
Dubois S, Viailly PJ, Bohers E, et al:
Biological and Clinical Relevance of Associated Genomic Alterations
in MYD88 L265P and non-L265P-Mutated Diffuse Large B-Cell Lymphoma:
Analysis of 361 Cases. Clin Cancer Res. 23:2232–2244.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu Y, Li J, Ouyang J, et al: Prognostic
relevance of protein expression, clinical factors, and MYD88
mutation in primary bone lymphoma. Oncotarget. 8:65609–65619.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ansell SM, Hodge LS, Secreto FJ, et al:
Activation of TAK1 by MYD88 L265P drives malignant B-cell Growth in
non-Hodgkin lymphoma. Blood cancer journal. 4(e183)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wenzl K, Manske MK, Sarangi V, et al: Loss
of TNFAIP3 enhances MYD88L265P-driven signaling in non-Hodgkin
lymphoma. Blood cancer journal. 8(97)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kraan W, Horlings HM, van Keimpema M, et
al: High prevalence of oncogenic MYD88 and CD79B mutations in
diffuse large B-cell lymphomas presenting at immune-privileged
sites. Blood cancer journal. 3(e139)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kraan W, van Keimpema M, Horlings HM, et
al: High prevalence of oncogenic MYD88 and CD79B mutations in
primary testicular diffuse large B-cell lymphoma. Leukemia.
28:719–720. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yamada S, Ishida Y, Matsuno A and Yamazaki
K: Primary diffuse large B-cell lymphomas of central nervous system
exhibit remarkably high prevalence of oncogenic MYD88 and CD79B
mutations. Leuk Lymphoma. 56:2141–2145. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ngo VN, Young RM, Schmitz R, et al:
Oncogenically active MYD88 mutations in human lymphoma. Nature.
470:115–119. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fontanilles M, Marguet F, Bohers E, et al:
Non-invasive detection of somatic mutations using next-generation
sequencing in primary central nervous system lymphoma. Oncotarget.
8:48157–48168. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Drandi D, Genuardi E, Dogliotti I, et al:
Highly sensitive MYD88(L265P) mutation detection by droplet digital
polymerase chain reaction in Waldenstrom macroglobulinemia.
Haematologica. 103:1029–1037. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zorofchian S, Lu G, Zhu JJ, et al:
Detection of the MYD88 p.L265P Mutation in the CSF of a Patient
With Secondary Central Nervous System Lymphoma. Front Oncol.
8(382)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hiemcke-Jiwa LS, Leguit RJ, Snijders TJ,
et al: Molecular analysis in liquid biopsies for diagnostics of
primary central nervous system lymphoma: Review of literature and
future opportunities. Crit Rev Oncol Hematol. 127:56–65.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hattori K, Sakata-Yanagimoto M, Suehara Y,
et al: Clinical significance of disease-specific MYD88 mutations in
circulating DNA in primary central nervous system lymphoma. Cancer
Sci. 109:225–230. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zorofchian S, El-Achi H, Yan Y, Esquenazi
Y and Ballester LY: Characterization of genomic alterations in
primary central nervous system lymphomas. J Neurooncol.
140:509–517. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gonzalez-Aguilar A, Idbaih A, Boisselier
B, et al: Recurrent mutations of MYD88 and TBL1XR1 in primary
central nervous system lymphomas. Clin Cancer Res. 18:5203–5211.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fukumura K, Kawazu M, Kojima S, et al:
Genomic characterization of primary central nervous system
lymphoma. Acta Neuropathol. 131:865–875. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Glass S, Phan A, Williams JN, Flowers CR
and Koff JL: Integrating understanding of epidemiology and genomics
in B-cell non-Hodgkin lymphoma as a pathway to novel management
strategies. Discov Med. 21:181–188. 2016.PubMed/NCBI
|
|
42
|
Ding PN, Becker T, Bray V, et al: Plasma
next generation sequencing and droplet digital PCR-based detection
of epidermal growth factor receptor (EGFR) mutations in patients
with advanced lung cancer treated with subsequent-line osimertinib.
Thoracic cancer. 10:1879–1884. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hiemcke-Jiwa LS, Leguit RJ, Snijders TJ,
et al: MYD88 p.(L265P) detection on cell-free DNA in liquid
biopsies of patients with primary central nervous system lymphoma.
Br J Haematol. 185:974–977. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Takhar JS, Doan TA and Gonzales JA:
Primary vitreoretinal lymphoma: empowering our clinical suspicion.
Curr. Opin. Ophthalmol. 30:491–499. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lai J, Chen K, Shi HM, et al: B-scan
ultrasound and cytology of the vitreous in primary central nervous
system lymphoma with vitreoretinal involvement. Int J Ophthalmol.
12:1001–1007. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chan CC, Rubenstein JL, Coupland SE, et
al: Primary vitreoretinal lymphoma: a report from an International
Primary Central Nervous System Lymphoma Collaborative Group
symposium. Oncologist. 16:1589–1599. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Morell AA, Shah AH, Cavallo C, et al:
Diagnosis of primary central nervous system lymphoma: a systematic
review of the utility of CSF screening and the role of early brain
biopsy. Neuro-oncology practice. 6:415–423. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kopinska AJ, Helbig G, Koclega A and
Kyrcz-Krzemien S: Bing-Neel Syndrome with Detectable MYD88 L265P
Gene Mutation as a Late Relapse Following Autologous Hematopoietic
Stem Cell Transplantation for Waldenstrom's Macroglobulinemia.
Turkish journal of haematology: official journal of Turkish Society
of Haematology. 34:186–187. 2017.PubMed/NCBI View Article : Google Scholar
|