Introduction
Ulcerative colitis (UC), also known as non-specific
UC, mainly characterized by erosion and ulcers, is a periodic
lifelong disease. The main manifestations of UC are severe
abdominal pain, weight loss, intestinal inflammation, rectal
bleeding, diarrhea, dehydration and tenesmus. The etiology and
pathogenesis of this disease are complicated and have not been
fully elucidated yet. The morbidity rate of UC has an obvious
increasing trend in China, and the patients have been gradually
younger. The occurrence and development of UC have serious affects
on the human health and quality of life, and therefore UC is listed
by the World Health Organization as one of the refractory diseases
of the modern world (1-3). In
UC and other inflammatory bowel diseases, damage of intestinal
mucosal barrier, immune cell dysfunction and intestinal neuronal
dysfunction occur. Currently, the main drugs used for UC include
glucocorticoids, immunosuppressants and tumor necrosis factor (TNF)
(4), which have certain effects, but
also great side-effects, and the recurrence rate and treatment
expenses are high. Most scholars argue that persistent intestinal
infection, intestinal mucosal barrier defect, intestinal mucosal
immunoregulatory abnormality, genetic and environmental factors are
jointly involved in the occurrence of UC (5,6). UC is a
frequently occurring inflammatory bowel disease and its morbidity
rate has increased significantly in recent years worldwide. Studies
have shown that the morbidity rate of UC remains at a high level in
developed countries, whereas it obviously increases in developing
countries or regions previously having a lower morbidity rate,
making UC a global disease (7,8). The
complicated pathogenesis of UC cannot be clarified by research
based on ‘immune-mediated inflammatory response’ ideas, leading to
limited diagnosis and treatment means. Therefore, studying and
developing new treatment means is imperative.
Matrix metalloproteinase (MMP)-2 and MMP-9 are two
types of MMPs (9-11).
According to previous studies, MMP-2 and MMP-9 are actively
involved in the pathophysiological processes in patients with
inflammatory bowel diseases (12-14).
Studies have shown that MMP-2 and MMP-9 affect the tight junctions
among mucosal cells, increase the intestinal mucosal permeability
and aggravate the impairment of mucosal barrier function. After
three cycles of drug treatment of UC, the levels of MMP-2 and MMP-9
decline repairing the intestinal mucosal damage and reducing the
incidence of inappetence, nausea, vomiting and mucositis (15,16).
Therefore, effectively controlling the content of MMPs is important
for the treatment of UC.
Studies have shown that UC patients have
hypercoagulability, hyperfunction of the fibrinolytic system,
increased prothrombin time (PT) and activated partial
thromboplastin time (APTT), and changes in intestinal mucosal
permeability, which can be used as indexes for evaluating the UC
activity (17,18). In the present study, UC patients and
normal subjects receiving physical examination were enrolled. The
content of early predictors, C-reactive protein (CRP),
follistatin-like protein 1 (FSTL1) and D-dimer in the serum was
detected, the disease activity index (DAI) score was recorded, the
content of MMP-2 and MMP-9 was determined, and the inflammatory
factors interleukin (IL)-1, IL-6 and TNF-α were also detected.
Moreover, the blood coagulation factors, platelet count, PT, APTT
and fibrinogen level were detected, and the mucosal permeability in
terms of the lactulose (L)/mannitol (M) ratio was calculated,
aiming to explore the pathogenesis of UC and provide theoretical
and experimental bases for the treatment and prognosis of UC
patients.
Subjects and methods
General data
A total of 50 active UC patients treated in the
Hospital of Liaoning University of Traditional Chinese Medicine
(Shenyang, China) from January 2016 to December 2018 were selected
as the UC group, whereas another 50 healthy subjects receiving
physical examination were selected as the control group. Inclusion
criteria: Patients diagnosed with UC via intestinal microscopy,
those who voluntarily participated in the study and signed the
informed consent, those who had received no treatment before, and
those with DAI score >2 points. Exclusion criteria: Patients
with bacterial colitis caused by Salmonella, hemorrhagic
necrotic enteritis, secondary infection complicated with severe
renal or hepatic dysfunction, colorectal cancer or liver cancer.
All clinical specimens in this experiment were collected upon the
agreement of the Ethics Committee of the Hospital of Liaoning
University of Traditional Chinese Medicine and the family members.
The study was approved by the Ethics Committee of the hospital and
signed written informed consents were obtained from all
participants or their guardians before the study. The specific
clinical data, including age, sex, body weight and disease severity
of patients were collected at the time of admission (Table I).
 | Table IClinical characteristics of the study
subjects. |
Table I
Clinical characteristics of the study
subjects.
| Characteristics | Control group | UC group |
|---|
| Sample size | 50 | 50 |
| Males | 24 | 25 |
| Mean age (years) | 40±10 | 39±9 |
| Mean weight (kg) | 47±9 | 49±10 |
| BMI
(kg/m2) | 21.5±1.0 | 21.2±0.9 |
| Severe cases | 0 | 25 |
| Moderate cases | 0 | 25 |
DAI score
The DAI scoring includes the evaluation of
hemafecia, diarrhea, mucosal manifestations and disease conditions.
Hemafecia: None (0 point), a little (1 point), obvious (2 points),
and frequent (3 points). Diarrhea: Normal (0 point), once to twice
a day (1 point), 3-4 times a day (2 points), and 5 times or more (3
points). Mucosal manifestations: Normal (0 point), mildly brittle
(1 point), moderately brittle (2 points), and severely brittle with
exudation (3 points). Disease conditions: Normal (0 point), mild (1
point), moderate (2 points), and severe (3 points). The DAI score
is the sum of the scores in all four categories and was recorded in
detail by special personnel for subsequent study and analysis.
Detection of serum CRP, FSTL1 and
D-dimer
In this study, 5 ml of venous blood were drawn from
the arm of the study subjects to detect the content of CRP, FSTL1
and D-dimer, in order to predict the development of the disease in
advance. The blood was inserted into 5-ml Eppendorf (EP) tubes
containing anticoagulant and placed at room temperature for 20 min,
followed by centrifugation at 4˚C, 2,000 x g for 15 min. Next, the
supernatant was collected to detect the changes in the content of
CRP, FSTL1 and D-dimer via enzyme-linked immunosorbent assay
(ELISA), providing an important theoretical reference for early
detection of UC. CRP kit (cat. no. H126), FSTL1 kit (cat. no.
E027-1-3) and D-dimer kit (cat. no. E029-1-1) were all purchased
from Nanjing Jiancheng Bioengineering Institute.
Detection of serum inflammatory
factors via ELISA
A total of 5 ml of venous blood were drawn from the
arm of the study subjects, placed into EP tubes containing
anticoagulant and centrifuged at 2,000 x g at room temperature for
15 min. Next, the supernatant was collected to detect the serum
inflammatory factors IL-6, IL-1 and TNF-α following the
manufacturer's instructions of the ELISA kits (Nanjing Jiancheng
Bioengineering Institute). IL-6 kit (cat. no. H007), IL-1 kit (cat.
no. H002) and TNF-α kit (cat. no. H052) were all purchased from
Nanjing Jiancheng Bioengineering Institute. Finally, the absorbance
in each group was detected using a microplate reader.
Detection of content of plasma MMP-9
and MMP-2
A total of 5 ml of fasting venous blood were drawn
early in the morning from the elbow of the study subjects and
centrifuged at 2,000 x g at room temperature for 15 min. Next, the
supernatant was collected to detect the levels of plasma MMP-9 and
MMP-2 using double-antibody sandwich ELISA according to the
manufacturer's instructions. Finally, the absorbance in each group
was detected using a microplate reader. MMP-9 kit (cat. no. H146-4)
and MMP-2 kit (cat. no. H146-1) were purchased from Nanjing
Jiancheng Bioengineering Institute.
Detection of blood coagulation
function
Fasting peripheral venous blood was drawn early in
the morning from all the study subjects, inserted into
anticoagulant tubes with 0.2 ml of sodium citrate, and placed at
room temperature for 20 min, followed by centrifugation at 4˚C,
2,000 x g for 10 min. Next, the separated plasma was collected to
determine the platelet count, PT, APTT and fibrinogen level within
24 h using a full-automatic biochemical analyzer (BS-220; Shenzhen
Mindray Bio-Medical Electronics Co., Ltd.).
Determination of intestinal mucosal
permeability
The concentration of L and M in the urine was
measured via Waters 515 high-performance liquid chromatography
(Waters Corporation). Alltima-NH2 Column (Alltech Medical Systems,
LLC) was used. The mobile phase was acetonitrile-water (67:33). The
flow rate was 1.0 ml/min and the column temperature was 45˚C. Urine
(1 ml) was collected from all subjects into EP tubes, and
centrifuged at 4˚C, 2,000 x g for 15 min. The supernatant was
aspirated, and added with 100 ml of acetonitrile to precipitate the
protein. After vortex mixing, the mixture was centrifuged at 4˚C,
2,000 x g for 15 min, and the supernatant was aspirated and
deionized, followed by vortex mixing and centrifugation at 4˚C,
2,000 x g for 5 min. Next, the supernatant was taken, and filtered
using the water-based filter membrane. Finally, the content of L
and M was detected and the L/M ratio was calculated (column
temperature, 25˚C; internal heating in differential detector at
32˚C, according to the manufacturer's instructions).
Statistical analysis
All data obtained from the experiments were
statistically analyzed using Statistical Product and Service
Solutions (SPSS) 21.0 software (IBM Corp.). The experimental
results were expressed as the mean ± standard deviation. Student's
t-test (two-tailed) was used for the comparison of variables
between two groups. The L/M ratios between two groups were compared
using Wilcoxon-Mann-Whitney two-tailed test. The bar graphs were
plotted using GraphPad Prism 5.0 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
DAI score
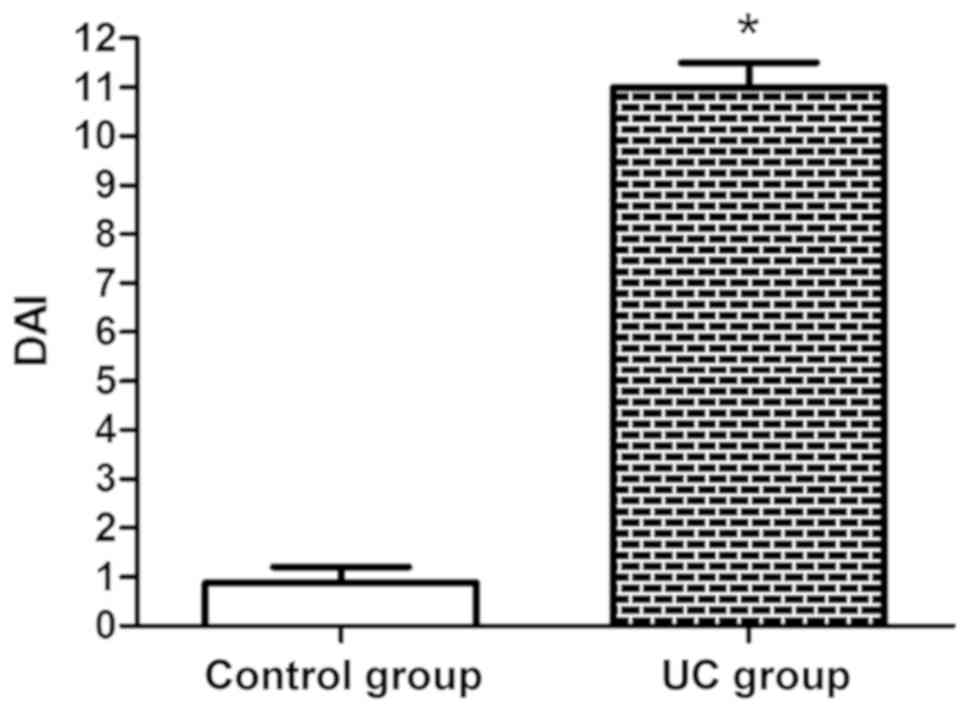
As shown in Fig. 1,
the DAI score was basically close to 1 in the control group,
whereas it was higher in the UC group, and there was a significant
difference (P<0.05).
Serum CRP, FSTL1 and D-dimer
The content of serum CRP, FSTL1 and D-dimer can
predict the occurrence of UC in advance. As shown in Table II, the content of CRP, FSTL1 and
D-dimer in the UC group was markedly higher than that in the
control group (P<0.05), indicating that there are significant
changes in the content of the three indexes when UC occurs.
 | Table IIContent of serum CRP, FSTL1 and
D-dimer. |
Table II
Content of serum CRP, FSTL1 and
D-dimer.
| Groups | CRP (mg/l) | FSTL1 (µg/l) | D-dimer (mg/l) |
|---|
| Control group | 2.2±0.2 | 8±0.8 | 0.4±0.3 |
| UC group | 10.1±0.1a | 29±0.6a | 2.5±0.4a |
Serum inflammatory factors detected
via ELISA
The levels of IL-1, IL-6 and TNF-α were obviously
increased in the UC group (P<0.05), whereas they were normal in
the control group (Table III).
 | Table IIILevels of serum IL-1, IL-6 and
TNF-α. |
Table III
Levels of serum IL-1, IL-6 and
TNF-α.
| Groups | IL-1 (mg/l) | TNF-α (fmol/ml) | IL-6 (mg/l) |
|---|
| Control group | 20. 5±1.9 | 14.5±1.2 | 24.4±1.1 |
| UC group | 43.2±2.0a | 34.7±1.1a | 40.1±1.0a |
Content of plasma MMP-9 and MMP-2
The content of MMP-2 and MMP-9 in the UC group was
raised markedly compared with that in the control group (P<0.05)
(Fig. 2), which further promotes the
development of UC.
Blood coagulation function
The subjects in the UC group had evidently increased
platelet count, PT, APTT and fibrinogen level compared with those
in the control group (P<0.05) (Table
IV), which indicates that the blood coagulation function is
changed during UC, further promoting the development of UC.
 | Table IVBlood coagulation function. |
Table IV
Blood coagulation function.
| Groups | Platelet count
(109/l) | PT (S) | APTT (S) | Fibrinogen level
(g/l) |
|---|
| Control group | 220±5 | 11±0.8 | 20±1.2 | 3±0.5 |
| UC group | 350±6a | 14±0.6a | 31±0.8a | 5±0.7a |
Intestinal mucosal permeability
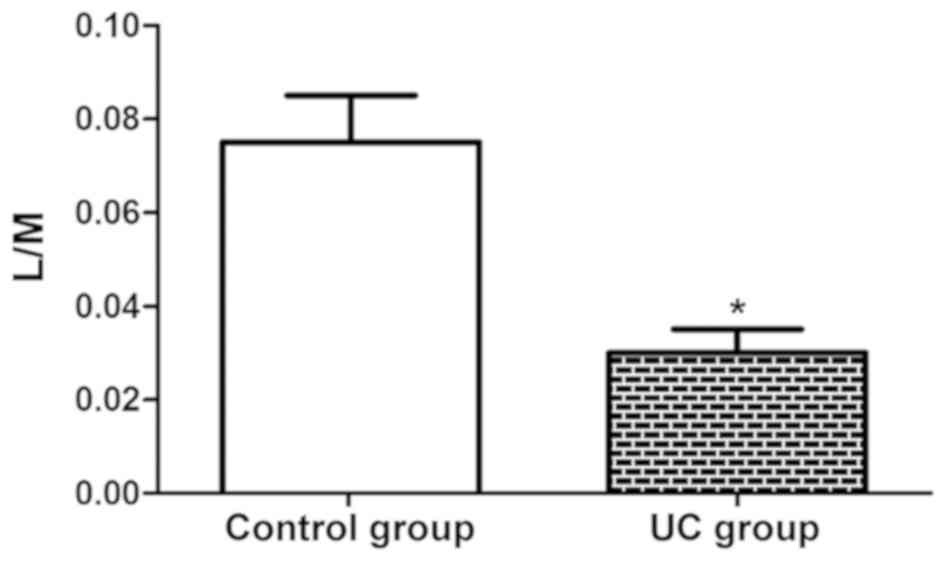
As shown in Fig. 3,
the L/M ratio in the UC group (0.03) was notably lower than that in
the control group (0.07) (P<0.05), which suggests that the
intestinal mucosal permeability evidently rises in UC patients,
further facilitating the development of the disease.
Discussion
UC and Crohn's disease are two major inflammatory
bowel diseases that share some common features. They can be
distinguished by differences in genetic susceptibility, risk
factors, and clinical, endoscopic and histological features. The
exact pathogenesis of inflammatory bowel diseases remains unclear.
Genetically susceptible individuals seemingly have the disordered
mucosal immune response, leading to intestinal inflammation. The
inflammation of UC is mainly confined to the mucosal surface. UC
begins in the rectum and usually extends proximally to the entire
colon. However, part of patients with left-sided colitis or
proctitis may suffer from cecal dilation (19,20).
Based on the degree of colon involvement, proctitis, left-sided
colitis and extensive colitis (pancolitis) have different
conditions, and they are also difficult to be cured. In the present
study, UC patients and normal subjects receiving physical
examination were enrolled, and the serum early predictors,
including MMP-2, MMP-9, IL-6, TNF-α, blood coagulation factors and
mucosal permeability indexes were detected, aiming to explore the
pathogenesis of UC and provide theoretical and experimental bases
for the treatment and prognosis of UC patients. The content of
serum CRP, FSTL1 and D-dimer can predict the occurrence of UC in
advance. The detection results revealed that the content of CRP,
FSTL1 and D-dimer in the UC group was apparently higher than that
in the control group, indicating that there are remarkable changes
in these three indexes when UC occurs, which provides a diagnostic
basis for the early-onset UC, similar to previous studies (6,18). In
addition, DAI was scored in each group. It was found that the DAI
score was basically close to 1 in control group, whereas it was
generally higher in the UC group, demonstrating that UC patients
have severer hemafecia, diarrhea and mucosal manifestations.
Inflammation plays an important role in UC, and
inflammatory cytokines have attracted widespread attention. TNF-α,
one of the major cytokines that mediate the early response to
intestinal injury, can stimulate the production of IL-6. Under
normal conditions, the concentration of IL-6 in healthy subjects is
low or even undetectable. The changes in the concentration of
inflammatory factors are related to the duration and severity of
UC, and the increases in their levels have been proven to be
associated with the raised morbidity rate of UC (21). In the present study, the levels of
IL-1, IL-6 and TNF-α were obviously elevated in the UC group,
whereas they were normal in the control group. MMPs play important
roles in the degradation of ECM and destruction of proteolytic
enzymes. Proteolytic enzymes are stimulated by pro-inflammatory
cytokines, and fully activated MMPs may aggravate the intestinal
inflammatory injury. In addition, some components, such as IL-1,
TNF and lipopolysaccharide, can specifically induce the
upregulation of MMP-3 and MMP-9, which are important factors for
intestinal injury (22). In this
study, it was observed that the content of MMP-2 and MMP-9 in the
UC group was obviously increased, which further promotes the
development of UC. After intestinal injury in UC, the blood
coagulation disorder is early detected. The ability to form fibrin
clots at the injury site is indispensable for limiting bleeding and
subsequent survival. Therefore, the standard coagulation assay,
including the detection of PT and APTT, can accurately reflect the
blood coagulation function of UC patients (23). In the present study, the patients in
the UC group had evidently increased platelet count, PT, APTT and
fibrinogen level, which indicates that the blood coagulation
function is altered during UC, further promoting the development of
UC. The increased L/M ratio corresponds to the enhancement of
intestinal mucosal permeability, and the reason is that the damage
of intestinal mucosal cells causes atrophy of intestinal mucosa,
thus resulting in the increase in intercellular space. Research has
shown that glutamine is one of the essential amino acids to
maintain the intestinal mucosal barrier, and glutamine nutrition
support can reduce intestinal injury. Therefore, inhibiting
intestinal mucosal intercellular space, regulating the expression
of tight junction protein, and protecting the intestinal mucosal
barrier function can gradually lower the L/M ratio (24). In this study, the L/M ratio in the UC
group was remarkably higher than that in the control group, which
suggests that the intestinal mucosal permeability evidently rises
in UC patients, further facilitating the development of the
disease. The aforementioned findings are similar to the research
results of Gao et al (25)
and Li et al (26).
Differently, it was found that the content of MMP-2 and MMP-9 was
increased in patients with active UC rather than in patients with
Crohn's disease or in animal models. The patients included in our
study were all with active UC, whereas the other studies did not
emphasize on this point. In addition, up to our knowledge, it is
the first time that serum early predictors, DAI scores, MMP-2,
MMP-9, inflammatory factors, blood coagulation indexes and the
permeability intestinal mucosa are all included in one study,
suggesting that UC is a complex disease involving multiple
mechanisms. The present study provides a more comprehensive
theoretical basis for the pathogenesis, prevention and treatment of
UC, as well as new ideas for subsequent further research.
In conclusion, in the present study it was confirmed
through a series of experiments that there are changes in the MMPs,
inflammatory factors, blood coagulation function and intestinal
mucosal permeability in active UC patients, further promoting the
development of disease. In the future, such changes can be further
verified by animal experiments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XB, YL and WJ designed the study and performed the
experi-ments. XB, LT, LL and GB were involved in the conception and
design of the study. LT, YL and WJ analyzed the data. XB, YL and WJ
prepared the manuscript. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Hospital of Liaoning University of Traditional Chinese Medicine
(Shenyang, China). Signed written informed consents were obtained
from all participants or their guardians before the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mason A, Malik S, McMillan M, McNeilly JD,
Bishop J, McGrogan P, Russell RK and Ahmed SF: A prospective
longitudinal study of growth and pubertal progress in adolescents
with inflammatory bowel disease. Horm Res Paediatr. 83:45–54.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sun PL and Zhang S: Correlations of
25-hydroxyvitamin D3 level in patients with ulcerative colitis with
inflammation level, immunity and disease activity. Eur Rev Med
Pharmacol Sci. 22:5635–5639. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lamas B, Richard ML, Leducq V, Pham HP,
Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW,
Natividad JM, et al: CARD9 impacts colitis by altering gut
microbiota metabolism of tryptophan into aryl hydrocarbon receptor
ligands. Nat Med. 22:598–605. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Jørgensen KK, Olsen IC, Goll GL, Lorentzen
M, Bolstad N, Haavardsholm EA, Lundin KEA, Mørk C, Jahnsen J, Kvien
TK, et al: NOR-SWITCH study group: Switching from originator
infliximab to biosimilar CT-P13 compared with maintained treatment
with originator infliximab (NOR-SWITCH): A 52-week, randomised,
double-blind, non-inferiority trial. Lancet. 389:2304–2316.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Baumgart DC and Sandborn WJ: Crohn's
disease. Lancet. 380:1590–1605. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ordás I, Eckmann L, Talamini M, Baumgart
DC and Sandborn WJ: Ulcerative colitis. Lancet. 380:1606–1619.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ananthakrishnan AN: Epidemiology and risk
factors for IBD. Nat Rev Gastroenterol Hepatol. 12:205–217.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mbachi C, Attar B, Oyenubi O, Yuchen W,
Efesomwan A, Paintsil I, Madhu M, Ajiboye O, Simons-Linares CR,
Trick WE and Kotwal V: Association between cannabis use and
complications related to ulcerative colitis inhospitalized
patients: A propensity matched retrospective cohortstudy. Medicine
(Baltimore). 98(e16551)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jakubowska K, Pryczynicz A, Iwanowicz P,
Niewiński A, Maciorkowska E, Hapanowicz J, Jagodzińska D, Kemona A
and Guzińska-Ustymowicz K: Expressions of matrix metalloproteinases
(MMP-2, MMP-7, and MMP-9) and their inhibitors (TIMP-1, TIMP-2) in
inflammatory bowel diseases. Gastroenterol Res Pract.
2016(2456179)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Khan S, Shukla S, Sinha S, Lakra AD, Bora
HK and Meeran SM: Centchroman suppresses breast cancer metastasis
by reversing epithelial-mesenchymal transition via downregulation
of HER2/ERK1/2/MMP-9 signaling. Int J Biochem Cell Biol. 58:1–16.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Giannopoulos G, Pavlakis K, Parasi A,
Kavatzas N, Tiniakos D, Karakosta A, Tzanakis N and Peros G: The
expression of matrix metalloproteinases-2 and -9 and their tissue
inhibitor 2 in pancreatic ductal and ampullary carcinoma and their
relation to angiogenesis and clinicopathological parameters.
Anticancer Res. 28B:B1875–B1881. 2008.PubMed/NCBI
|
|
12
|
Jakubowska K, Pryczynicz A, Januszewska J,
Sidorkiewicz I, Kemona A, Niewiński A, Lewczuk Ł, Kędra B and
Guzińska-Ustymowicz K: Expressions of matrix metalloproteinases 2,
7, and 9 in carcinogenesis of pancreatic ductal adenocarcinoma. Dis
Markers. 2016(9895721)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kreijne JE, van der Giessen J, Verhaar AP,
Peppelenbosch MP, de Vries AC, van der Woude CJ and Fuhler GM:
Fecal matrix metalloproteinase-9 measurement for optimizing
detection of disease activity in inflammatory bowel disease. J Clin
Gastroenterol. 53:395–397. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Godefroy E, Gallois A, Idoyaga J, Merad M,
Tung N, Monu N, Saenger Y, Fu Y, Ravindran R, Pulendran B, et al:
Activation of toll-like receptor-2 by endogenous matrix
metalloproteinase-2 modulates dendritic-cell-mediated inflammatory
responses. Cell Rep. 9:1856–1870. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang J, Li Y and Qi Y: Effect of
glutamine-enriched nutritional support on intestinal mucosal
barrier function, MMP-2, MMP-9 and immune function in patients with
advanced gastric cancer during perioperative chemotherapy. Oncol
Lett. 14:3606–3610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Langers AM, Verspaget HW, Hawinkels LJ,
Kubben FJ, van Duijn W, van der Reijden JJ, Hardwick JC, Hommes DW
and Sier CF: MMP-2 and MMP-9 in normal mucosa are independently
associated with outcome of colorectal cancer patients. Br J Cancer.
106:1495–1498. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Park MS, Martini WZ, Dubick MA, Salinas J,
Butenas S, Kheirabadi BS, Pusateri AE, Vos JA, Guymon CH, Wolf SE,
et al: Thromboelastography as a better indicator of hypercoagulable
state after injury than prothrombin time or activated partial
thromboplastin time. J Trauma. 67:266–276. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nguyen NH, Fumery M, Dulai PS, Prokop LJ,
Sandborn WJ, Murad MH and Singh S: Comparative efficacy and
tolerability of pharmacological agents for management of mild to
moderate ulcerative colitis: A systematic review and network
meta-analyses. Lancet Gastroenterol Hepatol. 3:742–753.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abraham C and Cho JH: Inflammatory bowel
disease. N Engl J Med. 361:2066–2078. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Silverberg MS, Satsangi J, Ahmad T, Arnott
ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C,
Geboes K, et al: Toward an integrated clinical, molecular and
serological classification of inflammatory bowel disease: Report of
a Working Party of the 2005 Montreal World Congress of
Gastroenterology. Can J Gastroenterol. 19 (Suppl A):A5–A36.
2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
17.Soop M, Nygren J, Thorell A and
Ljungqvist O: Stress-induced insulin resistance: Recent
developments. Curr Opin Clin Nutr Metab Care. 10:181–186.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Altshuler AE, Penn AH, Yang JA, Kim GR and
Schmid-Schönbein GW: Protease activity increases in plasma,
peritoneal fluid, and vital organs after hemorrhagic shock in rats.
PLoS One. 7(e32672)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Brohi K, Singh J, Heron M and Coats T:
Acute traumatic coagulopathy. J Trauma. 54:1127–1130.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lu H, Liu H, Wang J, Shen J, Weng S, Han
L, Sun T, Qian L, Wu M, Zhu S, et al: The chemokine CXCL9
exacerbates chemotherapy-induced acute intestinal damage through
inhibition of mucosal restitution. J Cancer Res Clin Oncol.
141:983–992. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gao Q, Meijer MJ, Schlüter UG, van
Hogezand RA, van der Zon JM, van den Berg M, van Duijn W, Lamers CB
and Verspaget HW: Infliximab treatment influences the serological
expression of matrix metalloproteinase (MMP)-2 and -9 in Crohn's
disease. Inflamm Bowel Dis. 13:693–702. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li WL, Wu CH, Yang J, Tang M, Chen LJ and
Zhao SL: Local inflammation alters MMP-2 and MMP-9 gelatinase
expression associated with the severity of nifedipine-induced
gingival overgrowth: A rat model study. Inflammation. 38:1517–1528.
2015.PubMed/NCBI View Article : Google Scholar
|