Introduction
Bone marrow transplants (BMT) are used to treat
patients with severe aplastic anemia (1), acute lymphoblastic leukemia (2), acute myeloid leukemia (3) and chronic myeloid leukemia (4,5).
However, the clinical application of BMT is limited by
graft-vs.-host disease (GVHD) (6).
Acute GVHD is a common complication which results in severe
morbidity and mortality following BMT and has an occurrence rate of
30-50% (7-9).
Acute GVHD primarily occurs in the skin, intestines and liver
(10). Cytokine dysregulation
resulting from an allogeneic interaction causes tissue injury that
is characteristic of acute GVHD (11,12).
Therefore, identifying a novel therapeutic strategy for GVHD is
required.
Ciclosporin, an immunosuppressant drug, has been
widely used as a treatment strategy for GVHD in pediatric patients
who have undergone BMT (12-14);
however, the therapeutic range is relatively narrow (15) and the drug exhibits wide
inter-individual pharmacokinetic variability (16-18).
Identifying the optimal dose regimen of ciclosporin to achieve and
maintain the target concentration of the drug is crucial (19,20).
Population pharmacokinetics is a tool that can be
used to collect sparse clinical data in order to model and simulate
approaches to assess dosing regimens in specific patients (16,18).
Numerous studies have established population pharmacokinetics of
ciclosporin. For example, Ni et al (21) established population pharmacokinetics
of ciclosporin in Chinese children with aplastic anemia, Fanta
et al (22) and Irtan et
al (23) in patients receiving
pediatric renal transplants and Wilhelm et al (24) in patients receiving hematopoietic
allogeneic stem cell transplants. Therefore, the present study
aimed to optimize the initial dosage of ciclosporin for safety and
effectiveness in pediatric Chinese patients who underwent BMT based
on population pharmacokinetics.
Materials and methods
Patients and data collection
Pediatric patients who underwent BMT between
September 2016 and September 2019 at the Children's Hospital of
Fudan University (Shanghai, China) were retrospectively recruited
to the present study. The criteria for inclusion were as follows:
i) Age, <16 years; ii) treated with cyclosporin; and iii) full
set of therapeutic drug monitoring (TDM) data for cyclosporin.
Patients with other transplant statuses, including liver or kidney
transplants were excluded. Ciclosporin concentrations and clinical
data were gathered via TDM records and from medical records,
respectively. The present study was approved by the Research Ethics
Committee of the Children's Hospital of Fudan University [approval
no. (2019)021]. A total of 18 patients were included in the current
study (male/female ratio, 13/5; mean age, 1.60±1.15 years; age
range, 0.29-6.49 years).
Drug administration and concentration
detection
The initial ciclosporin dosage range was 14-100
mg/day. The dose was later adjusted based on clinical efficacy,
adverse events and the trough concentration based on TDM. TDM was
measured twice per week or more frequently if required, especially
in suspected cases of intolerance or adverse events, using the
Emit® 2000 Cyclosporin Specific assay (cat. no. 6R079UL;
Siemens Healthcare Diagnostics, Inc.) according to the
manufacturer's protocol. Blood samples (≥100 µl) were collected
from the elbow vein immediately before the next drug
administration.
Population pharmacokinetic
modeling
A Nonlinear Mixed-Effects Modeling tool
(NONMEM®; version VII; ICON Development Solutions Ltd.)
was used to analyze the clinical data of pediatric patients who
underwent BMT. The absorption phase was described by a
one-compartment model with first-order elimination, where
pharmacokinetic parameters included apparent oral
clearance/bioavailability (CL/F) and apparent volume of
distribution (V/F). Based on published literature, the absorption
rate constant (Ka) of the model was 0.68 h-1 (16,21,25).
Random effect model
Equation A was used to calculate inter-individual
variability: Pi=T(P) x exp (ηi), where
Pi represented the individual parameter value, T(P) was
the typical individual parameter value and ηi was the
symmetrical distribution, which was a zero-mean chance variable
with variance term. Equation B was used to calculate random
residual variability: Y=F x (1+ε1) + ε2,
where Y was the observation, F was the individual predicted
concentration, and ε1 and ε2 were
symmetrically distributed, zero-mean random variables with variance
terms.
Covariate model
The relationship between weight and pharmacokinetic
parameters was calculated using Equation C:
Pi=Pnorm x
(WTi/WTnorm)POW, where
Pi represented the ith individual
pharmacokinetic parameter, WTi represented the
ith individual weight, WTnorm represented the
standard weight of 70 kg, Pnorm represented the typical
individual parameter whose weight was WTnorm and POW
represented the allometric coefficient (0.75 for CL/F; 1 for V/F)
(26).
The relationship between continuous covariates or
categorical covariates and pharmacokinetic parameters was
calculated by Equations D and E, respectively. Equation D:
Pi=T(P) x
(Covi/Covmedian)θ. Equation E:
Pi=T(P) x (1+θ x Covi). Pi
represented the individual parameter value, T(P) was the typical
individual parameter value, θ was the parameter to be estimated,
Covi was the covariate of the ith individual
and Covmedian was the population median for the
covariate.
To explain the variability of pharmacokinetic
parameters, the correlations between covariates were investigated
and the pharmacokinetic parameters were estimated. The potential
covariates, which were obtained from the medical records, included
sex, age, weight, days post-transplant (POD), albumin, alanine
transaminase, aspartate transaminase, creatinine, urea, total
protein, total bile acid, direct bilirubin, total bilirubin,
hematocrit, hemoglobin, mean corpuscular hemoglobin, mean
corpuscular hemoglobin concentration and co-medications
(glucocorticoids, mycophenolate mofetil, omeprazole, phenobarbital
and tacrolimus).
Statistical analysis
Alterations to the objective function values (OFV)
were generated using covariate inclusions and a decrease in OFV of
>3.84 [χ2; α=0.05; degrees of freedom (df)=1] was
considered sufficient for inclusion of the base model. After
establishing a full regression model, the model was further
assessed by eliminating covariates from each pharmacokinetic
parameter to obtain the final model. An increase in OFV of >6.64
(χ2; α=0.01; df=1) was considered to indicate a
statistically significant difference.
Model validation
The reliability and stability of the final
parameters were assessed by bootstrap, an internal validation
method, which was performed using Wings for NONMEM (version VII;
ICON plc) and repeated 1,000 times using different random draws.
The medians and 2.5-97.5% percentiles of the bootstrap results were
compared with the final pharmacokinetic parameter estimates and the
absolute threshold of bias was set at <15% which was calculated
using the following formula: Bias = (median-estimate)/estimate
x100%. The final model was evaluated using goodness of fit plots
and prediction-corrected visual predictive check plots, which were
used to analyze model precision and predictability,
respectively.
Simulation of dosing regimens
The dosage regimen simulations were performed using
the parameter estimates obtained from the final model. The
probability to achieve the target concentration was investigated
using Monte Carlo simulations (NONMEM®; version VII;
ICON plc), based on the established model. According to previous
studies, the target concentrations were determined as 50-350 ng/ml
(27-29).
A total of 1,000 virtual patients were simulated in each of the
four weight groups (5, 10, 20 and 30 kg) and for seven initial
dosage regimens (2, 3, 4, 5, 6, 7 and 8 mg/kg/day ciclosporin split
into 2 doses). Due to the large differences between individuals,
the simulation results were presented with 70% confidence
interval.
Results
Data collection
Clinical data of 18 pediatric patients who underwent
BMT (13 male and 5 female) were collected for the present study.
Patient characteristics and co-medications are presented in
Table I.
 | Table IDemographic data of patients and
co-medications (n=18). |
Table I
Demographic data of patients and
co-medications (n=18).
| Characteristic | Mean ± SD | Median (range) |
|---|
| Sex,
male/female | 13/5 | n/a |
| Age, years | 1.60±1.15 | 1.22
(0.29-6.49) |
| Weight, kg | 8.40±3.28 | 7.60
(5.20-25.60) |
| POD | 61.16±40.16 | 51.50
(1.00-188.00) |
| Albumin, g/l | 34.07±5.51 | 34.40
(1.20-45.20) |
| Alanine
transaminase, IU/l | 51.26±65.97 | 30.00
(1.00-439.00) |
| Aspartate
transaminase, IU/l | 56.00±57.58 | 36.30
(5.80-392.00) |
| Creatinine,
µmol/l | 17.59±5.23 | 16.00
(8.00-38.00) |
| Urea, mmol/l | 3.62±1.86 | 3.45
(0.60-11.10) |
| Total protein,
g/l | 57.74±8.49 | 58.80
(38.40-77.80) |
| Total bile acid,
µmol/l | 21.62±32.09 | 10.85
(0.40-201.20) |
| Direct bilirubin,
µmol/l | 20.73±45.60 | 4.60
(0.10-301.90) |
| Total bilirubin,
µmol/l | 33.30±64.46 | 11.60
(1.30-384.20) |
| Hematocrit, % | 30.64±6.76 | 30.40
(10.50-44.30) |
| Hemoglobin,
g/l | 101.16±21.86 | 101.00
(35.00-149.00) |
| Mean corpuscular
hemoglobin, pg | 29.78±3.30 | 29.70
(20.40-42.00) |
| Mean corpuscular
hemoglobin concentration, g/l | 331.48±19.62 | 333.00
(273.00-396.00) |
| Comedication,
n | | |
|
Glucocorticoids | 15 | n/a |
|
Mycophenolate
mofetil | 7 | n/a |
|
Omeprazole | 16 | n/a |
|
Phenobarbital | 2 | n/a |
|
Tacrolimus | 2 | n/a |
Modeling
The final covariate models were displayed by
equations F and G: i) Equation F, CL/F=θCL/F x
(WT/70)0.75 x (POD/51.5)θPOD; and ii)
Equation G: V/F=θV/F x (WT/70). θCL/F and
θV/F represented the typical population values of CL/F
and V/F, respectively, whilst θPOD represented the
coefficient of the POD. From Table
II, the value of θCL/F was found to be 29.200,
θV/F was 6550.000 and θPOD was 0.749. Using
these two models, WT and POD were included as the covariates of
CL/F, whilst WT was included as the covariate of V/F. If the
potential influencing factors could be included in the model as
covariates, it indicated that there was an influence on
ciclosporin. Conversely, if not, it indicated that there was no
influence on ciclosporin. In the final models co-medications
(glucocorticoids, mycophenolate mofetil, omeprazole, phenobarbital
and tacrolimus) were could not be included into the final covariate
models, suggesting that there was no significant drug interaction
with ciclosporin.
 | Table IIParameter estimates of the final
model and bootstrap validation. |
Table II
Parameter estimates of the final
model and bootstrap validation.
| Parameter | Estimate | SE (%) | Bootstrap
median | 95% confidence
interval | Bias (%) |
|---|
| CL/F (l/h) | 29.200 | 15.3 | 28.700 | (20.000,
38.300) | -1.71 |
| V/F (l) | 6,550.000 | 29.9 | 6,620.000 | (3,500.000,
11,800.000) | 1.07 |
| Ka
(h-1) | 0.680 (fixed) | - | - | - | - |
|
θPOD | 0.749 | 25.4 | 0.730 | (0.330, 1.120) | -2.54 |
|
ωCL/F | 0.627 | 16.5 | 0.611 | (0.338, 0.826) | -2.55 |
|
ωV/F | 0.998 | 20.1 | 0.955 | (0.522, 1.367) | -4.31 |
| σ1 | 0.447 | 15.6 | 0.442 | (0.296, 0.597) | -1.12 |
| σ2 | 70.071 | 30.2 | 67.749 | (14.765,
104.403) | -3.31 |
Validation
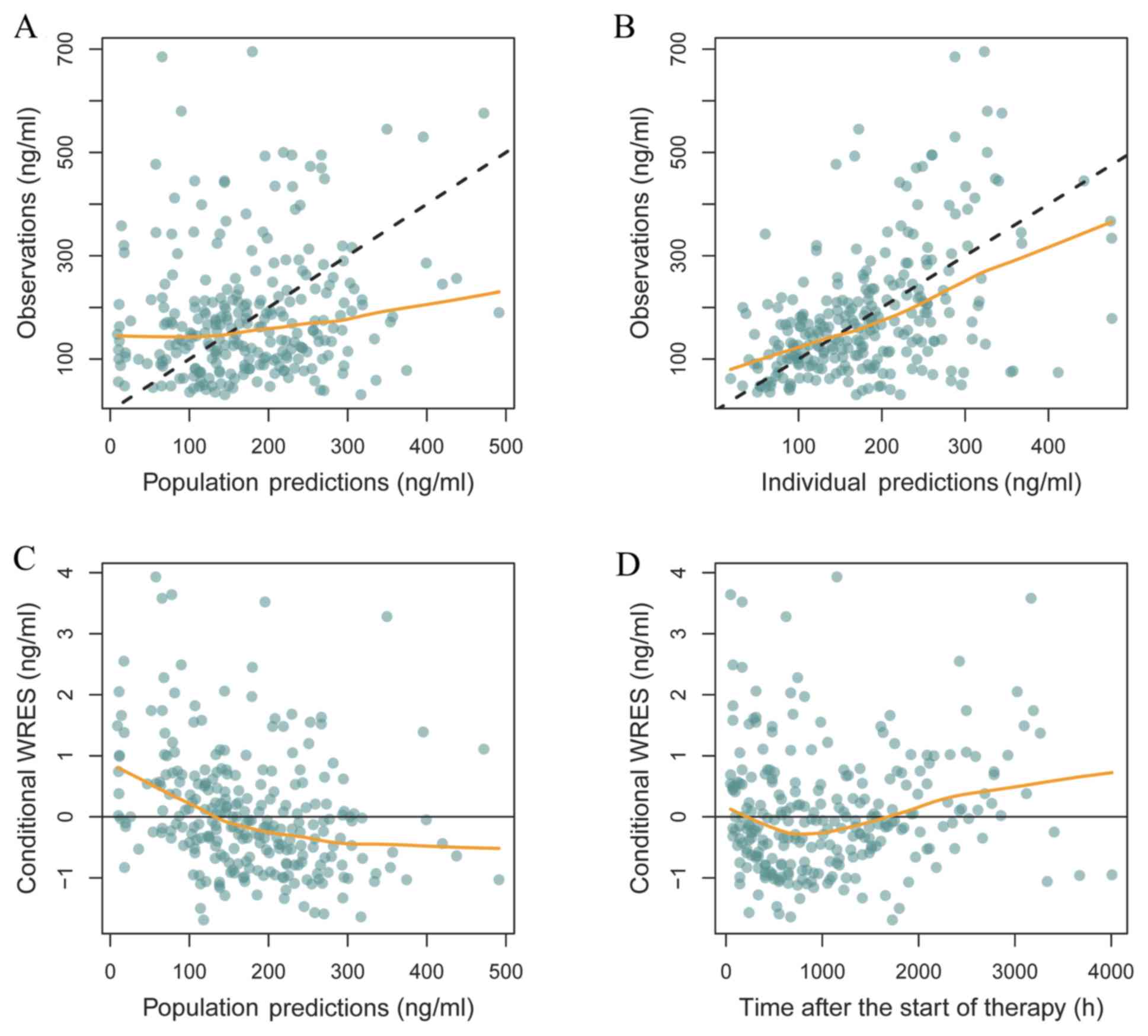
Goodness of fit plots, representing the observed and
predicted drug concentrations in the blood, are presented in
Fig. 1, including observations vs.
population predictions, observations vs. individual predictions,
conditional weighted residuals (WRES) vs. population predictions
and WRES vs. time after the start of therapy. In Fig. 1A and B, the black dashed lines represent the line
of unity, where the predictions matched the observed values and the
smooth yellow line represents the trend of the data. Hence, the
closer the yellow smooth line is to the black dashed line, the more
accurate the predictive the model. In Fig. 1C and D, the yellow smooth line represents the
trend of the data such that the closer the yellow smooth line is to
the line of unity, the more accurate the predictive model.
Therefore, the final model exhibit higher precision and
predictability. The parameter estimates of the final model and
bootstrap validation are presented in Table II, where the median values of the
parameter estimates of bootstraps were close to the respective
values of the final population model. The absolute value of bias
was found to be <5, <15% of the standard, which indicated
that the final population model was accurate and reliable. The
prediction-corrected visual predictive check plots of the final
model are presented in Fig. 2. The
majority of the observations were within the 95% prediction
intervals of the simulation data, which suggested that the
prediction-corrected concentrations were well predicted by the
final model.
Simulation
Weight and POD influenced the clearance of
ciclosporin in pediatric patients who underwent BMT (Fig. 3). Specifically, ciclosporin clearance
was found to be increased as POD increased, whilst ciclosporin
clearance was reduced with increasing weight. Additionally, lighter
weight resulted in higher clearance rates in pediatric patients
with the same POD. The initial dosage of 2-5 and 8 mg/kg/day split
into 2 doses for pediatric patients weighing 5-30 kg exhibited
lower probabilities of achieving the target concentrations, whilst
the initial dosage of 6 or 7 mg/kg/day split into 2 doses for
pediatric patients weighing 5-30 kg displayed higher probabilities
to achieve the target concentrations (Fig. 4). However, the 7 mg/kg/day dose split
into 2 doses exceeded the upper limit of the treatment window (350
ng/ml) in all weight groups (Table
III). Therefore, an initial dose of 6 mg/kg/day ciclosporin
split into 2 doses for pediatric patients weighing 5-30 kg who
underwent BMT was identified as the optimal dose.
 | Table IIIPredicted median (15th
percentile-85th percentile) concentrations (ng/ml) of ciclosporine
in each group. |
Table III
Predicted median (15th
percentile-85th percentile) concentrations (ng/ml) of ciclosporine
in each group.
| Dosea | 5 kg | 10 kg | 20 kg | 30 kg |
|---|
| 2 mg/kg/day | 41.5
(15.05-104.81) | 41.41
(15.07-105.82) | 41.49
(15.09-107.50) | 41.54
(15.11-108.09) |
| 3 mg/kg/day | 61.72
(22.57-157.22) | 62.11
(22.61-158.74) | 62.23
(22.63-161.25) | 62.31
(22.67-162.14) |
| 4 mg/kg/day | 82.30
(30.10-209.65) | 82.82
(30.14-211.65) | 82.98
(30.18-215.00) | 83.08
(30.22-216.20) |
| 5 mg/kg/day | 102.87
(37.63-262.02) | 103.52
(37.68-264.56) | 103.72
(37.72-268.75) | 103.85
(37.77-270.24) |
| 6 mg/kg/day | 123.45
(45.15-314.43) | 124.22
(45.21-317.47) | 124.46
(45.26-322.50) | 124.62
(45.32-324.29) |
| 7 mg/kg/day | 144.02
(52.68-366.84) | 144.93
(52.75-370.39) | 145.20
(52.81-376.25) | 145.39
(52.88-378.34) |
| 8 mg/kg/day | 164.60
(60.20-419.24) | 165.63
(60.28-423.29) | 165.94
(60.35-430.00) | 166.16
(60.43-432.38) |
Discussion
The immunosuppressive drug ciclosporin was initially
approved for use to prevent rejection in organ transplants, such as
in liver (30,31), kidney (32,33),
lung (34) and heart (35,36).
Additionally, ciclosporin has been approved by the US Federal Drug
Association for severe psoriasis and rheumatoid arthritis treatment
(37). It has also been reported
that ciclosporin can be used to treat alopecia (38), chronic autoimmune urticaria (39), pyoderma gangrenosum (40), severe atopic dermatitis (41), systemic lupus erythematosus (42), aplastic anemia (43), Crohn's disease (44) and ulcerative colitis (45). Furthermore, ciclosporin has also been
used for the treatment of GVHD in pediatric patients who underwent
BMT (1,2,13,14).
While ciclosporin has a wide range of clinical
applications, the potential risks in numerous conditions have not
been fully elucidated (46).
Clinically, ciclosporin displays pharmacokinetic challenges which
vary considerably between patients receiving the same dose
(46). Additionally, ciclosporin has
a narrow therapeutic range (47,48). Low
doses are closely associated with the risk of graft rejection or
loss, and overexposure is associated with acute or chronic
toxicity, and irreversible renal damage (48). Therefore, a key challenge for the
clinical use of ciclosporin is maintaining constant drug exposure
in the narrow therapeutic window for each patient (15,49).
While clinical TDM is often used to determine the optimal
ciclosporin concentration and to provide reference for subsequent
dose adjustments, a concentration reference for the initial dose
has not been identified.
Population pharmacokinetics has the potential to aid
individualized therapy by integrating different effects of
variables on drug exposure (50);
therefore, it can be used to determine the initial dose in
different diseases. Population pharmacokinetics has been used for
dosage optimization of tacrolimus in patients with nephritic
syndrome (51,52), oxcarbazepine in pediatric Chinese
patients with epilepsy (53),
azithromycin in children with community-acquired pneumonia
(54), vancomycin in neonates and
young infants (55) and cyclosporin
in pediatric patients with hemophagocytic lymphohistiocytosis
(16). Therefore, the present study
aimed to optimize the initial dosage of ciclosporin in pediatric
patients who underwent BMT based on population pharmacokinetics and
Monte Carlo simulations.
In the present study, the typical value of CL/F from
the final population pharmacokinetic model was 29.200 l/h, which
was similar to the previously reported value for pediatric patients
receiving stem cell or kidney transplants (23.1-29.3 l/h) (23,27).
Weight and POD influenced the clearance of ciclosporin in pediatric
patients who underwent BMT. Ciclosporin clearance associated
positively and negatively with POD and weight, respectively. A
similar previous study demonstrated a non-linear relationship
between drug clearance and body weight in pediatric patients
(26). The association between body
weight and clearance may scale with 0.75 power and a coefficient of
1 for volume (26,56,57).
Therefore, in the present study, the following allometric
coefficient was selected: 0.75 for CL/F and 1 for V/F. For ease of
comparison between studies, body weight is typically standardized
to 70 kg and the standardization of weight is particularly
important in studies investigating children and neonates (21,56,58,59).
Furthermore, in the present study, POD was found to associate
positively with ciclosporin clearance, which may be explained by
the association between patient recovery and an increased ability
to metabolize exogenous drugs (60).
Additionally, lighter weight was associated with higher clearance
rates in pediatric patients with the same POD.
Subsequently, whether there was a significant
difference in initial dose between children with different body
weights was investigated. Monte Carlo simulations were used to
simulate the optimal initial dose, including four weight groups (5,
10, 20 and 30 kg) and seven initial dosing regimens (2, 3, 4, 5, 6,
7 and 8 mg/kg/day split into 2 doses). According to previous
studies, the target concentration range was determined as 50-350
ng/ml (27-29).
The results of the present study suggested that the doses of 6 and
7 mg/kg/day split into 2 doses displayed a similar probability to
achieve the target concentrations. However, the dose of 7 mg/kg/day
resulted in an increased number of cases where the dose exceeded
the upper limit of the therapeutic window. Therefore, an initial
dose of 6 mg/kg/day ciclosporin split into 2 doses for pediatric
patients weighing 5-30 kg who underwent BMT was identified as the
optimal dose. Additionally, the present study also considered
combination drugs, including glucocorticoid, mycophenolate mofetil,
omeprazole, phenobarbital and tacrolimus; however, no significant
interaction with ciclosporin was identified.
The present study had an important limitation.
Ciclosporin is primarily eliminated via biotransformation by
cytochrome P450. Therefore, whether the inclusion of genotyping in
the model generated in the present study explains the variability
of ciclosporin in pediatric Chinese patients who underwent BMT
requires further investigation.
In conclusion, the present study indicated that
weight and POD influenced the clearance of ciclosporin in pediatric
patients who underwent BMT. Furthermore, the results indicated that
the optimal initial dose of ciclosporin was 6 mg/kg/day split into
2 doses for pediatric patients weighing 5-30 kg who underwent
BMT.
Acknowledgements
Not applicable.
Funding
The present study was supported by Important Weak
Subject Construction Project of Shanghai (grant no. 2016ZB0305),
Clinical Pharmacy Key Specialty Construction Project of Shanghai
(grant no. 2017/26), Scientific Research Project of Science and
Technology Commission of Shanghai Municipality (grant no.
18DZ1910604/19DZ1910703) and Shanghai Science and Technology
Commission (grant no. 19XD1400900).
Availability of data and materials
The dataset used and/or analyzed during the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
ZL and HX conceived and designed the study. XC, XY
and DW collected and analyzed the data. XC drafted the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Children's Hospital of Fudan University.
Patient consent was waived by the Ethics Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McCann S, Passweg J, Bacigalupo A,
Locasciulli A, Locatelli F, Ryan J, Schrezenmeier H and Lawler M:
The influence of cyclosporin alone, or cyclosporin and
methotrexate, on the incidence of mixed haematopoietic chimaerism
following allogeneic sibling bone marrow transplantation for severe
aplastic anaemia. Bone Marrow Transplant. 39:109–114.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Teuffel O, Schrauder A, Sykora KW,
Zimmermann M, Reiter A, Welte K and Schrappe M: The impact of
cyclosporin A on acute graft-versus-host disease after allogeneic
bone marrow transplantation in children and adolescents with acute
lymphoblastic leukemia. Bone Marrow Transplant. 36:145–150.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bacigalupo A, Vitale V, Corvò R, Barra S,
Lamparelli T, Gualandi F, Mordini N, Berisso G, Bregante S, Raiola
AM, et al: The combined effect of total body irradiation (TBI) and
cyclosporin A (CyA) on the risk of relapse in patients with acute
myeloid leukaemia undergoing allogeneic bone marrow
transplantation. Br J Haematol. 108:99–104. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brandenburg U, Gottlieb D and Bradstock K:
Antileukemic effects of rapid cyclosporin withdrawal in patients
with relapsed chronic myeloid leukemia after allogeneic bone marrow
transplantation. Leuk Lymphoma. 31:545–550. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Goldman JM, Apperley JF, Jones L, Marcus
R, Goolden AW, Batchelor R, Hale G, Waldmann H, Reid CD, Hows J, et
al: Bone marrow transplantation for patients with chronic myeloid
leukemia. N Engl J Med. 314:202–207. 1986.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kohno A, Morishita Y, Iida H, Sakamaki H,
Yokozawa T, Kitaori K, Ozeki K, Matsuo K and Sao H: Low-dose
cyclosporin A with short-term methotrexate for graft-versus-host
disease prophylaxis in allogeneic bone marrow transplantation from
human leukocyte antigen-identical siblings: A prospective phase II
study in Japanese patients. Int J Hematol. 84:83–89.
2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rowlings PA, Przepiorka D, Klein JP, Gale
RP, Passweg JR, Henslee-Downey PJ, Cahn JY, Calderwood S, Gratwohl
A, Socie G, et al: IBMTR Severity Index for grading acute
graft-versus-host disease: Retrospective comparison with Glucksberg
grade. Br J Haematol. 97:855–864. 1997.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Martin PJ, Schoch G, Fisher L, Byers V,
Anasetti C, Appelbaum FR, Beatty PG, Doney K, McDonald GB, Sanders
JE, et al: A retrospective analysis of therapy for acute
graft-versus-host disease: Initial treatment. Blood. 76:1464–1472.
1990.PubMed/NCBI
|
|
9
|
Martin PJ, Schoch G, Fisher L, Byers V,
Appelbaum FR, McDonald GB, Storb R and Hansen JA: A retrospective
analysis of therapy for acute graft-versus-host disease: Secondary
treatment. Blood. 77:1821–1828. 1991.PubMed/NCBI
|
|
10
|
Ferrara JL, Levine JE, Reddy P and Holler
E: Graft-versus-host disease. Lancet. 373:1550–1561.
2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hill GR and Ferrara JL: The primacy of the
gastrointestinal tract as a target organ of acute graft-versus-host
disease: Rationale for the use of cytokine shields in allogeneic
bone marrow transplantation. Blood. 95:2754–2759. 2000.PubMed/NCBI
|
|
12
|
Fowler DH, Foley J, Whit-Shan Hou J, Odom
J, Castro K, Steinberg SM, Gea-Banacloche J, Kasten-Sportes C,
Gress RE and Bishop MR: Clinical ‘cytokine storm’ as revealed by
monocyte intracellular flow cytometry: Correlation of tumor
necrosis factor alpha with severe gut graft-versus-host disease.
Clin Gastroenterol Hepatol. 2:237–245. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gérard C, Bleyzac N, Girard P, Freyer G,
Bertrand Y and Tod M: Links between cyclosporin exposure in tissues
and graft-versus-host disease in pediatric bone marrow
transplantation: Analysis by a PBPK model. Pharm Res. 28:531–539.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kobayashi R, Yabe H, Hara J, Morimoto A,
Tsuchida M, Mugishima H, Ohara A, Tsukimoto I, Kato K, Kigasawa H,
et al: Preceding immunosuppressive therapy with antithymocyte
globulin and ciclosporin increases the incidence of graft rejection
in children with aplastic anaemia who underwent allogeneic bone
marrow transplantation from HLA-identical siblings. Br J Haematol.
135:693–696. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bowers LD: Therapeutic monitoring for
cyclosporine: Difficulties in establishing a therapeutic window.
Clin Biochem. 24:81–87. 1991.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang DD, Ye QF, Chen X, Xu H and Li ZP:
Population pharmacokinetics and initial dosing regimen optimization
of cyclosporin in pediatric hemophagocytic lymphohistiocytosis
patients. Xenobiotica. 50:435–441. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li TF, Hu L, Ma XL, Huang L, Liu XM, Luo
XX, Feng WY and Wu CF: Population pharmacokinetics of cyclosporine
in Chinese children receiving hematopoietic stem cell
transplantation. Acta Pharmacol Sin. 40:1603–1610. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu YO, Jia B, Chen CY, Zhou Y, Cui YM and
Zhou FD: Population pharmacokinetics of cyclosporine A in Chinese
patients with nephrotic syndrome in individualized drug
administration. Int J Clin Pharmacol Ther. 58:1–9. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Martin P, Bleyzac N, Souillet G, Galambrun
C, Bertrand Y, Maire PH, Jelliffe RW and Aulagner G: Relationship
between CsA trough blood concentration and severity of acute
graft-versus-host disease after paediatric stem cell
transplantation from matched-sibling or unrelated donors. Bone
Marrow Transplant. 32:777–784. 2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Carlens S, Aschan J, Remberger M, Dilber M
and Ringden O: Low-dose cyclosporine of short duration increases
the risk of mild and moderate GVHD and reduces the risk of relapse
in HLA-identical sibling marrow transplant recipients with
leukaemia. Bone Marrow Transplant. 24:629–635. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ni SQ, Zhao W, Wang J, Zeng S, Chen SQ,
Jacqz-Aigrain E and Zhao ZY: Population pharmacokinetics of
ciclosporin in Chinese children with aplastic anemia: Effects of
weight, renal function and stanozolol administration. Acta
Pharmacol Sin. 34:969–975. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fanta S, Jonsson S, Backman JT, Karlsson
MO and Hoppu K: Developmental pharmacokinetics of ciclosporin-a
population pharmacokinetic study in paediatric renal transplant
candidates. Br J Clin Pharmacol. 64:772–784. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Irtan S, Saint-Marcoux F, Rousseau A,
Zhang D, Leroy V, Marquet P and Jacqz-Aigrain E: Population
pharmacokinetics and bayesian estimator of cyclosporine in
pediatric renal transplant patients. Ther Drug Monit. 29:96–102.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wilhelm AJ, de Graaf P, Veldkamp AI,
Janssen JJ, Huijgens PC and Swart EL: Population pharmacokinetics
of ciclosporin in haematopoietic allogeneic stem cell
transplantation with emphasis on limited sampling strategy. Br J
Clin Pharmacol. 73:553–563. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang D, Chen X and Li Z: Cyclosporin
population pharmacokinetics in pediatric refractory nephrotic
syndrome based on real-world studies: Effects of body weight and
spirolactone administration. Exp Ther Med. 17:3015–3020.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Anderson BJ and Holford NH:
Mechanism-based concepts of size and maturity in pharmacokinetics.
Annu Rev Pharmacol Toxicol. 48:303–332. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Willemze AJ, Cremers SC, Schoemaker RC,
Lankester AC, den Hartigh J, Burggraaf J and Vossen JM: Ciclosporin
kinetics in children after stem cell transplantation. Br J Clin
Pharmacol. 66:539–545. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Weiss M, Steinbach D, Zintl F, Beck J and
Gruhn B: Superior outcome using cyclosporin A alone versus
cyclosporin A plus methotrexate for post-transplant
immunosuppression in children with acute leukemia undergoing
sibling hematopoietic stem cell transplantation. J Cancer Res Clin
Oncol. 141:1089–1094. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Choi JS, Lee SH, Chung SJ, Yoo KH, Sung KW
and Koo HH: Assessment of converting from intravenous to oral
administration of cyclosporin A in pediatric allogeneic
hematopoietic stem cell transplant recipients. Bone Marrow
Transplant. 38:29–35. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Muduma G, Saunders R, Odeyemi I and
Pollock RF: Systematic review and meta-analysis of tacrolimus
versus ciclosporin as primary immunosuppression after liver
transplant. PLoS One. 11(e0160421)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Muduma G, Odeyemi I and Pollock RF: A
cost-utility analysis of prolonged-release tacrolimus relative to
immediate-release tacrolimus and ciclosporin in liver transplant
recipients in the UK. J Med Econ. 19:995–1002. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Riegersperger M, Plischke M,
Jallitsch-Halper A, Steinhauser C, Födinger M, Winkelmayer WC,
Dunkler D and Sunder-Plassmann G: A non-randomized trial of
conversion from ciclosporin and tacrolimus to tacrolimus MR4 in
stable long-term kidney transplant recipients: Graft function and
influences of ABCB1 genotypes. PLoS One.
14(e0218709)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gathogo E, Harber M, Bhagani S, Levy J,
Jones R, Hilton R, Davies G and Post FA: UK HIV Kidney
Transplantation Study Group. Impact of tacrolimus compared with
cyclosporin on the incidence of acute allograft rejection in human
immunodeficiency virus-positive kidney transplant recipients.
Transplantation. 100:871–878. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Penninga L, Penninga EI, Møller CH,
Iversen M, Steinbrüchel DA and Gluud C: Tacrolimus versus
cyclosporin as primary immunosuppression for lung transplant
recipients. Cochrane Database Syst Rev: CD008817, 2013.
|
|
35
|
Jia Y, Meng X, Li Y, Xu C, Zeng W, Jiao Y
and Han W: Optimal sampling time-point for cyclosporin A
concentration monitoring in heart transplant recipients. Exp Ther
Med. 16:4265–4270. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Robertsen I, Falck P, Andreassen AK, Naess
NK, Lunder N, Christensen H, Gullestad L and Asberg A:
Endomyocardial, intralymphocyte, and whole blood concentrations of
ciclosporin A in heart transplant recipients. Transplant Res.
2(5)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rosmarin DM, Lebwohl M, Elewski BE and
Gottlieb AB: National Psoriasis Foundation. Cyclosporine and
psoriasis: 2008 National Psoriasis Foundation Consensus Conference.
J Am Acad Dermatol. 62:838–853. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gupta AK, Ellis CN, Cooper KD, Nickoloff
BJ, Ho VC, Chan LS, Hamilton TA, Tellner DC, Griffiths CE and
Voorhees JJ: Oral cyclosporine for the treatment of alopecia
areata. A clinical and immunohistochemical analysis. J Am Acad
Dermatol. 22:242–250. 1990.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Boubouka CD, Charissi C, Kouimintzis D,
Kalogeromitros D, Stavropoulos PG and Katsarou A: Treatment of
autoimmune urticaria with low-dose cyclosporin A: A one-year
follow-up. Acta Derm Venereol. 91:50–54. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ahronowitz I, Harp J and Shinkai K:
Etiology and management of pyoderma gangrenosum: A comprehensive
review. Am J Clin Dermatol. 13:191–211. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Berth-Jones J, Finlay AY, Zaki I, Tan B,
Goodyear H, Lewis-Jones S, Cork MJ, Bleehen SS, Salek MS, Allen BR,
et al: Cyclosporine in severe childhood atopic dermatitis: A
multicenter study. J Am Acad Dermatol. 34:1016–1021.
1996.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zavada J, Pesickova S, Rysava R, Olejarova
M, Horák P, Hrncír Z, Rychlík I, Havrda M, Vítova J, Lukác J, et
al: Cyclosporine A or intravenous cyclophosphamide for lupus
nephritis: The Cyclofa-Lune study. Lupus. 19:1281–1289.
2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zheng Y, Liu Y and Chu Y:
Immunosuppressive therapy for acquired severe aplastic anemia
(SAA): A prospective comparison of four different regimens. Exp
Hematol. 34:826–831. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
McDonald JW, Feagan BG, Jewell D, Brynskov
J, Stange EF and Macdonald JK: Cyclosporine for induction of
remission in Crohn's disease. Cochrane Database Syst Rev: CD000297,
2005.
|
|
45
|
Meier J and Sturm A: Current treatment of
ulcerative colitis. World J Gastroenterol. 17:3204–3212.
2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Paziana K, Del Monaco M, Cardonick E,
Moritz M, Keller M, Smith B, Coscia L and Armenti V: Ciclosporin
use during pregnancy. Drug Saf. 36:279–294. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Barr WH: Cyclosporine: The case for
expanding bioequivalence criteria to include measures of individual
bioequivalence in relevant population subsets. Transplant Proc.
31:25S–30S. 1999.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kahan BD, Welsh M and Rutzky LP:
Challenges in cyclosporine therapy: The role of therapeutic
monitoring by area under the curve monitoring. Ther Drug Monit.
17:621–624. 1995.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Colaizzi JL and Lowenthal DT: Critical
therapeutic categories: A contraindication to generic substitution?
Clin Ther. 8:370–379. 1986.PubMed/NCBI
|
|
50
|
Zheng QS and Li LJ: Pharmacometrics: A
quantitative tool of pharmacological research. Acta Pharmacol Sin.
33:1337–1338. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lu T, Zhu X, Xu S, Zhao M, Huang X, Wang Z
and Zhao L: Dosage optimization based on population pharmacokinetic
analysis of tacrolimus in Chinese patients with nephrotic syndrome.
Pharm Res. 36(45)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wang X, Han Y, Chen C, Ma L, Xiao H, Zhou
Y, Cui Y, Wang F, Su B, Yao Y, et al: Population pharmacokinetics
and dosage optimization of tacrolimus in pediatric patients with
nephrotic syndrome. Int J Clin Pharmacol Ther. 57:125–134.
2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Chen CY, Zhou Y, Cui YM, Yang T, Zhao X
and Wu Y: Population pharmacokinetics and dose simulation of
oxcarbazepine in Chinese paediatric patients with epilepsy. J Clin
Pharm Ther. 44:300–311. 2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zheng Y, Liu SP, Xu BP, Shi ZR, Wang K,
Yang JB, Huang X, Tang BH, Chen XK, Shi HY, et al: Population
pharmacokinetics and dosing optimization of azithromycin in
children with community-acquired pneumonia. Antimicrob Agents
Chemother. 62:e00686–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen Y, Wu D, Dong M, Zhu Y, Lu J, Li X,
Chen C and Li Z: Population pharmacokinetics of vancomycin and
AUC-guided dosing in Chinese neonates and young infants. Eur J Clin
Pharmacol. 74:921–930. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Anderson BJ and Holford NH: Tips and traps
analyzing pediatric PK data. Paediatr Anaesth. 21:222–237.
2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Holford NH: A size standard for
pharmacokinetics. Clin Pharmacokinet. 30:329–332. 1996.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hao GX, Huang X, Zhang DF, Zheng Y, Shi
HY, Li Y, Jacqz-Aigrain E and Zhao W: Population pharmacokinetics
of tacrolimus in children with nephrotic syndrome. Br J Clin
Pharmacol. 84:1748–1756. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wang DD, Chen X and Li ZP: Wuzhi capsule
and haemoglobin influence tacrolimus elimination in paediatric
kidney transplantation patients in a population pharmacokinetics
analysis: A retrospective study. J Clin Pharm Ther. 44:611–617.
2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang DD, Chen X, Fu M, Zheng QS, Xu H and
Li ZP: Model extrapolation to a real-world dataset: Evaluation of
tacrolimus population pharmacokinetics and drug interaction in
pediatric liver transplantation patients. Xenobiotica. 50:371–379.
2020.PubMed/NCBI View Article : Google Scholar
|