Introduction
Sepsis is a systematic inflammatory response and it
is a major cause of mortality in intensive care units (1). Moreover, sepsis is associated with the
dysfunction of multiple organs (1).
It has been previously documented that 40-50% of patients with
sepsis develop cardiac dysfunction (2). Cardiac dysfunction is a major feature
of sepsis, with the 50% mortality rate increasing in patients with
severe heart failure (3,4). The mortality rate of sepsis is rising
despite the increasing numbers of studies developing antimicrobial
therapies and circulatory support (5). Therefore, the present study aimed to
determine the mechanisms of LPS-induced myocardial injury to
facilitate the development of novel treatment strategies.
Ulinastatin is an endogenous inhibitor of proteases
that is located in the urine and blood, and inhibits various serine
protease (6). Ulinastatin has been
previously used to treat sepsis cardiomyopathy, due to its ability
to function as a protease inhibitor and its anti-inflammatory
effects (7). It was previously
demonstrated that ulinastatin enhanced cardiac function, reduced
the size of the myocardial infarction and decreased the levels of
inflammatory cytokines during hemorrhagic shock and
ischemia-reperfusion (IR) injury (8). A previous study has also suggested that
the protective effects of ulinastatin are based on its
anti-inflammatory, anti-oxidative stress and anti-apoptotic
properties (9). Furthermore,
ulinastatin was indicated to serve an important role in treating
lipopolysaccharide (LPS)-induced myocardial injury (10). However, the specific mechanisms of
the role of ulinastatin in sepsis are not fully understood.
Autophagy is a type of programmed cell death and it
is an adaptation used to respond to a variety of stress stimuli
(11). Previous studies have
identified the presence of activated autophagy in a variety of
heart diseases, such as IR injury, heart failure and sepsis
(12,13). It has been widely demonstrated that
autophagy performs two opposing functions in the heart (14); it serves a protective role during
nutrient deprivation and cellular stress, whereas the excessive
induction of autophagy promotes self-destruction (15). In a model of IR injury, ulinastatin
was revealed to protect cardiomyocytes via the activation of the
mTOR pathway, which is associated with the process of autophagy
(16). A previous study has
identified that ulinastatin exerted a protective effect on sepsis
through its regulation of autophagy in myocardial IR injury models
(16). Therefore, it was
hypothesized that ulinastatin may serve a protective role in
sepsis-induced myocardial injury by regulating autophagy; however,
further research into the role of ulinastatin in sepsis is
required. The present study aimed to investigate whether
ulinastatin protected against sepsis cardiomyopathy and to
determine the possible underlying mechanisms of this
interaction.
Materials and methods
Sepsis model
All procedures were approved by The Animal Ethics
Committees of The Fourth Military Medical University (Xi'an, China)
and were performed in accordance with the guidelines of The China
Council of Animal Care. A total of 84 C57BL/6J mice (age, 8-10
weeks; weight, 25-30 g; sex, male) were purchased from The
Laboratory Animal Center of The Fourth Military Medical University.
Mice were housed at a temperature of 25˚C in ~60% humidity, with a
12 h light/dark cycle. Mice were provided with a standard diet and
free access to water. Endotoxemia was induced via an
intraperitoneal (i.p.) injection of 18 mg/kg lipopolysaccharide
(LPS; cat. no. L2630; Sigma-Aldrich; Merck KGaA) or 10 mg/kg LPS
from the Escherichia coli serotype O111:B4(17). Ulinastatin was obtained from
Guangdong Tianpu Biochemical Pharmaceutical Co., Ltd. To determine
the protective effect of ulinastatin on the survival rate following
lethal endotoxemia, 60 mice were divided into two groups: i) A
total of 30 mice in the LPS group, where mice were treated with the
lethal dose of 18 mg/kg LPS and 0.9% saline; and ii) 30 mice in the
LPS + Ulinastatin group, where the mice received 18 mg/kg LPS and
i.p. injection of 1x105 U/kg Ulinastatin (i.p.) daily
for 4 days, which was determined in a previous study (17).
To investigate the effects of ulinastatin on cardiac
function and the levels of autophagy, 24 mice were randomly divided
into a control group, a LPS group (mice received 10 mg/kg LPS and
0.9% saline) and a LPS + ulinastatin group [mice received 10 mg/kg
LPS and 1x105 U/kg Ulinastatin (i.p.)] (17), with 8 mice in each group. The
administered dose of ulinastatin was selected according to a
previous study, in which ulinastatin was observed to exhibit a
protective effect on sepsis (18).
All mice were anesthetized for echocardiography after treated with
LPS and/or Ulinastatin for 12 h, following which they were
sacrificed for subsequent experiments.
For survival analysis, humane endpoints were
established. In the process of observing the survival of mice, once
they showed labored breathing, they were euthanized immediately by
i.p. injection of 120 mg/kg sodium pentobarbital sodium (20 mg/ml).
Conventional anti-shock therapy was given by an intraperitoneal
injection of 0.9% saline after 4 days of drug treatment. At the end
of the 7 day survival cycle, the rest of the surviving mice in two
groups were euthanized using 120 mg/kg sodium pentobarbital sodium
(20 mg/ml) through the intraperitoneal route. Following cervical
dislocation to ensure death, 600 µl blood samples were collected
from the abdominal aorta and the myocardial tissues of mice were
collected and frozen at 80˚C for further evaluation. To minimize
animal suffering, only qualified personnel were permitted to
perform the experiments.
Echocardiography
After anesthesia with an i.p. injection of 60 mg/kg
sodium pentobarbital sodium (20 mg/ml), conventional
echocardiography of the left ventricle (LV) in each mouse was
performed 12 h after an i.p. injection of LPS using a mouse
echocardiography system (Vevo 2100 Imaging System; VisualSonics,
Inc.) that was equipped with a 30-MHz phased transducer. The
following parameters were measured: LV end diastolic pressure
(LVEDP), LV developed pressure (LVDP), maximal velocity increase of
LV pressure per second (+dP/dtmax) and maximal velocity decrease of
LV pressure per second (-dP/dtmax).
ELISAs
Blood samples were centrifuged at 1,500 x g for 15
min at room temperature to collect the serum. Serum cardiac
troponin-I (cTnI; cat. no. F00503; Shanghai Westang Bio-Tech Co.,
Ltd.), cardiac tumor necrosis factor (TNF)-α (cat. no. T7539;
Sigma-Aldrich; Merck KGaA) and cardiac interleukin (IL)-6 (cat. no.
555240; BD Biosciences) levels were analyzed using their respective
ELISA kits, according to the manufacturer's protocol.
Mitochondrial membrane potential
(MMP)
A mitochondria isolation kit (cat. no. C3606;
Beyotime Institute of Biotechnology) was used to extract the
purified mitochondria from the myocardial tissues, according to the
manufacturer's protocol (19).
Subsequently, a mitochondrial membrane potential assay kit with
JC-1 dye (cat. no. C2006; Beyotime Institute of Biotechnology) and
a fluorescence microplate reader (Chromate 4300; Awareness
Technology, Inc.) were used to measure MMP, according to the
manufacturer's protocol. JC-1 green fluorescence (530 nm) was used
to reflect JC-1 monomers and red fluorescence (590 nm) was used to
determine the formation of J-aggregates. The ratio of J-aggregates
to JC-1 mitochondria were measured using a microplate reader after
JC-1 staining. The ratio of monomeric to aggregated JC-1
fluorescence intensity was used to quantify the changes in MMP.
Data were presented as the normalized percentage of the average
fluorescence intensity and values were normalized to the control
group.
Western blotting
Total proteins were extracted using the RIPA protein
extraction reagent (Beyotime Institute of Biotechnology) from the
myocardial tissues and the protein concentration was determined
using the bicinchoninic acid Protein Assay kit (Beyotime Institute
of Biotechnology). A total of 20 µg protein/lane was separated via
10-15% SDS-PAGE. The separated proteins were subsequently
transferred onto PVDF membranes (EMD Millipore) and blocked with 5%
non-fat milk at room temperature for 1 h. The membranes were then
incubated with the following primary antibodies overnight at 4˚C:
Anti-TNF-α (1:1,000; cat. no. ab6671; Abcam), anti-IL-6 (1:1,000;
cat. no. ab9324; Abcam), anti-microtubule-associated protein light
chain 3 LC3B (1:1,000; cat. no. ab51520; Abcam),
anti-lysosomal-associated membrane protein 1 (LAMP-1; 1:1,000; cat.
no. ab24170; Abcam), anti-sequestosome-1 (SQSTM1)/p62 (1:1,000;
cat. no. ab56416; Abcam) and anti-GAPDH (1:3,000; cat. no.
ab181602; Abcam). Following the primary antibody incubation, the
membranes were washed three times with TBS-0.1% Tween 20 and
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. ZB-2301; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) and horseradish peroxidase-conjugated
secondary antibody (1:5,000; cat. nos. ab6728; Abcam) for 2 h at
room temperature. Protein bands were visualized using an enhanced
chemiluminescence kit (EMD Millipore) and expression levels were
quantified using a densitometric analysis system (Image Lab
software; version 4.0; Bio-Rad Laboratories, Inc.).
Hematoxylin & eosin (H&E)
staining
Following the establishment of the LPS model,
myocardial tissues were excised, washed with ice-cold PBS,
following which 1-mm thick slices were prepared after the hearts
were snap frozen at -80˚C for 5 min. The slices were fixed directly
in 10% neutral formalin for 24 h at room temperature and were
subsequently paraffin-embedded. Sections (3 µm thickness) were then
prepared from the paraffin-embedded tissue blocks by dehydration
through a graded ethanol series and washing with xylene for H&E
staining, for 12 min at room temperature. Morphological changes in
the myocardial tissues were evaluated using an Olympus light
microscope (magnification x400; Olympus Corporation).
Transmission electron microscopy
(TEM)
Myocardial tissues were sliced into 1 mm3
sections and fixed with 2.5% glutaraldehyde overnight at 4˚C. The
tissues were immersed in 1% osmium tetroxide for 2 h at room
temperature, dehydrated in an ascending ethanol series, followed by
dehydration in an 100% acetone for three times at 4˚C and
subsequently embedded in epoxy resin. The tissues were then cut
into ultrathin sections (60-70 nm) using an ultramicrotome.
Sections were stained with 2% uranyl acetate and 2% lead citrate
for 30 min at room temperature. Stained sections were visualized
using a JEM-1010 TE microscope (magnification x15,000 and x30,000;
JEOL, Ltd.) and the amount of autophagosome were quantified using
Gatan Digital Micrograph® software (Gatan, Inc.).
Immunofluorescence staining
Myocardial tissues were excised, washed with
ice-cold PBS, following which 1-mm thick slices were prepared after
the hearts were snap frozen at -80˚C for 5 min. The slices were
sequentially fixed with 4% formaldehyde for 30 min at room
temperature, permeabilized with 0.2% Triton X-100 (HyClone; GE
Healthcare Life Sciences) for 15 min at room temperature and
blocked in 5% bovine serum albumin (Beyotime Institute of
Biotechnology) for 1 h at room temperature. Sections were then
incubated with a rabbit anti-LC3 antibody (1:100; cat. no.
ab128025; Abcam) and a rabbit anti-Beclin 1 antibody (1:100; cat.
no. ab210498; Abcam) at 4˚C overnight. Following the primary
antibody incubation, the sections were incubated with an Alexa
Fluor® 488-conjugated goat anti-rabbit IgG secondary
antibody (1:10; cat. no. ab150077; Abcam) at 37˚C for 1 h. The cell
nucleus was stained with 2 µg/ml DAPI for 30 min at room
temperature and 500 ml/l glycerine was used to seal the sections.
The slides were observed using a confocal laser scanning microscope
(magnification x400) to analyze the expression levels of
autophagy-associated proteins.
Statistical analysis
All experiments were independently performed in
triplicate. Statistical analysis was performed using SPSS version
19 software (IBM Corp.) and data are presented as the mean ± SEM.
Survival rate analysis was analyzed using the Kaplan-Meier method,
with a log-rank test used for comparison (20). Statistical differences between groups
were determined using a one-way ANOVA, followed by a
Student-Newman-Keuls test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Ulinastatin improves the survival rate
of mice with endotoxemia
To determine the protective effect of ulinastatin on
the survival rate following lethal endotoxemia, mice were treated
with a lethal dose of LPS (18 mg/kg), whereas mice in the LPS +
ulinastatin group received 18 mg/kg LPS and ulinastatin
(1x105 U/kg) daily for 4 days daily. After 7 days, it
was revealed that only 12/30 of mice in the LPS + ulinastatin group
survived, thus the survival rate was 40% (Fig. 1). In the LPS group, mice were treated
with an equal amount of saline and it was subsequently demonstrated
that only 3/30 mice had survived 7 days later, thus demonstrating a
survival rate of 10% (Fig. 1).
Therefore, these findings suggested that ulinastatin may reduce the
mortality rate of lethal endotoxemia.
Effect of ulinastatin on the cardiac
function in LPS-stimulated mice
The cardiac function parameters in each experimental
group are presented in Table I.
Following the establishment of the LPS model mice, it was revealed
that the LVEDP was significantly increased compare with that in the
control group. The LVDP, +dP/dtmax and -dP/dtmax were all found to
be significantly decreased following 12 h of treatment in the LPS
group compared with the control group (Table I). Moreover, following ulinastatin
treatment, the LVEDP was significantly decreased by 13.3%, whereas
the LVDP, +dP/dtmax and -dP/dtmax were increased by 30.5, 15.7 and
44.6%, respectively, compared with the LPS group.
 | Table IEffect of ulinastatin on cardiac
function in LPS-induced mice. |
Table I
Effect of ulinastatin on cardiac
function in LPS-induced mice.
| Group | n | Left ventricle end
diastolic pressure (mmHg) | Left ventricle
developed pressure (mmHg) | Maximal velocity
increase of left ventricle pressure per second (mmHg) | Maximal velocity
decrease of left ventricle pressure per second (mmHg) |
|---|
| Control | 8 | 20.7±3.6 | 118.1±11.7 | 8,720.6±135.1 | 6,121.0±212.0 |
| LPS | 8 |
42.1±1.8a |
68.2±7.3a |
6,012.4±312.4a |
3,132.4±254.7a |
| LPS +
ulinastatin | 8 |
36.5±1.3a,b |
89.0±5.6a,b |
6,956.2±216.3a,b |
4,528.1±198.5a,b |
Effects of ulinastatin on the mice
myocardium during endotoxemia
The tissues and serum from individual mice were
obtained following 12 h of LPS induction. The ELISA results
revealed that cTnI levels were significantly increased in the LPS
and LPS + ulinastatin groups compared with the control group
(Table II). Of note, the LPS +
ulinastatin group had significantly decreased cTnI levels compared
with the LPS group (Table II). It
was also indicated that the MMP was significantly decreased in the
LPS and LPS + ulinastatin groups compared with the control group;
however, the LPS + ulinastatin group demonstrated a significantly
increased MMP compared with the LPS group (Table II). Previous studies have reported
that inflammatory cytokines serve an important role in the
progression of sepsis (21). To
investigate the protective effects of ulinastatin on the
LPS-induced inflammatory response, the levels of TNF-α and IL-6 in
the mice myocardial tissue were also investigated. The ELISA
results indicated that the levels of TNF-α and IL-6 were
significantly increased in the LPS and LPS + ulinastatin groups
compared with the control group (Table
II). However, following the treatment with ulinastatin, the
levels of TNF-α and IL-6 were significantly decreased in the
myocardium of mice with endotoxemia compared with the LPS group
(Table II).
 | Table IIEffect of ulinastatin on the mouse
myocardium during endotoxemia. |
Table II
Effect of ulinastatin on the mouse
myocardium during endotoxemia.
| Group | n | Mitochondrial
membrane potential | Serum cardiac
troponin I (ng/ml) | Tumor necrosis
factor-α (pg/ml) | Interleukin-6
(pg/ml) |
|---|
| Control | 8 | 1.00 | 0.136±0.0142 | 39.620±2.140 | 3.820±0.430 |
| LPS | 8 |
0.321±0.067a |
0.919±0.0138a |
268.420±15.920a |
14.560±0.920a |
| LPS +
ulinastatin | 8 |
0.673±0.075a,b |
0.585±0.0129a,b |
172.370±8.560a,b |
9.270±0.610a,b |
Effects of ulinastatin on the
expression levels of inflammatory factors in endotoxemia
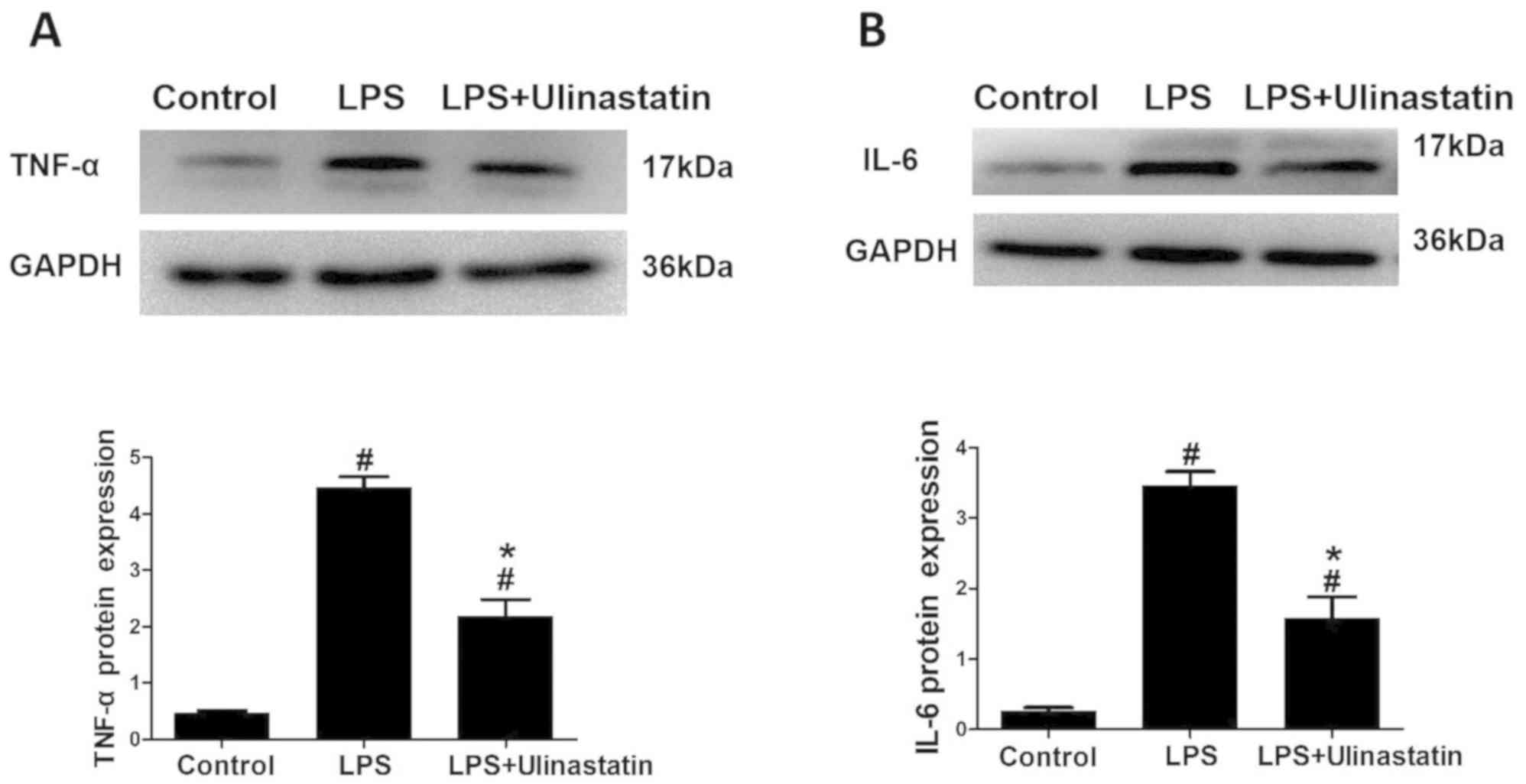
The western blotting data revealed that the protein
expression levels of TNF-α and IL-6 were significantly increased in
the LPS and LPS + ulinastatin groups compared with the control
group (Fig. 2A and B). Notably, following treatment with
ulinastatin, the expression levels of TNF-α and IL-6 were
significantly decreased in the myocardium of mice with endotoxemia
in the LPS + ulinastatin group compared with those in the LPS group
(Fig. 2A and B). Collectively, these findings suggested
that ulinastatin may exert protective effects in the endotoxemia
myocardium and may alleviate the inflammatory response through
decreasing the expression levels of TNF-α and IL-6.
Effects of ulinastatin on the cardiac
ultrastructure and pathological changes
TEM was used to observe the cardiac ultrastructure;
a normal cardiac structure and mitochondrial distribution were
observed in the control group (Fig.
3A), whereas in the LPS group, the myofilaments and
mitochondria were disorganized in the myocardial tissue, alongside
the presence of autophagy (Fig. 3B).
Notably, in the LPS + ulinastatin group, the myocardial tissue
exhibited orderly arranged myofilaments, a normal mitochondrial
distribution and the presence of small magnitudes of autophagy
(Fig. 3C).
H&E staining was subsequently used to assess the
pathological changes in the myocardial tissue. In the LPS group,
myofibril loss, myocardial cell necrosis and structural
abnormalities were all observed, which indicated the occurrence of
severe cardiac injury (Fig. 3E).
However, in the LPS + ulinastatin group, there was a reduction in
the pathological damage observed in the mice hearts compared with
the LPS group (Fig. 3F).
Ulinastatin decreases the levels of
LPS-induced autophagy
Myocardial tissues from the mice were obtained 12 h
after LPS induction. It was subsequently demonstrated that the
expression levels of LC3II were significantly increased in the LPS
and LPS + ulinastatin group compared with the control group
(Fig. 4A). However, the expression
levels of LC3II (Fig. 4A), Beclin 1
(Fig. 4B) and LAMP-1 (Fig. 4C) were all significantly decreased
following ulinastatin treatment compared with those in the LPS
group. In addition, it was revealed that the expression levels of
SQSTM1/p62 was significantly decreased in the LPS and LPS +
ulinastatin group compared with those in the control group,
ulinastatin significantly increased the expression levels of
SQSTM1/p62 further compared with those in the LPS group (Fig. 4D). Moreover, TEM was also used to
observe the number of autophagosomes formed in the heart of each
group. A normal mitochondrial structure and very few autophagosomes
were observed in the control group, whereas in the LPS group,
myocardial tissues exhibited a significantly increased number of
autophagosomes compared with the control group. Notably,
ulinastatin treatment was observed to significantly decrease this
LPS-induced increase in autophagosome formation (Fig. 4E).
 | Figure 4Effects of ulinastatin on autophagy in
mice with endotoxemia. LPS-induced endotoxemia mice were treated
with saline or ulinastatin, and the left ventricle tissues were
collected 12 h later for analysis. Western blotting was used to
analyze the protein expression levels of (A) LC3II, (B) Beclin 1,
(C) LAMP-1 and (D) SQSTM1/p62 in the myocardial tissues. GAPDH was
used as a loading control. (E) Transmission electron microscopy was
used to observe the number of autophagosomes formed in the cardiac
tissue. Magnification x30,000. Scale bar, 1 µm. Yellow arrow
indicates the autophagosomes, the white arrow indicates the
mitochondria and the red arrow indicates the lysosomes. Data are
presented as the mean ± SEM. Data were compared using a one-way
ANOVA, followed by a Student-Newman-Keuls post hoc test.
#P<0.05 vs. control group; *P<0.05 vs.
LPS group. LPS, lipopolysaccharide; LC3, microtubule-associated
protein light chain 3; LAMP, lysosomal-associated membrane protein
1; SQSTM1, sequestosome-1. |
Furthermore, the expression levels of the autophagy
associated proteins, LC3 and Beclin 1, were analyzed using confocal
microscopy. The expression levels of LC3 and Beclin 1 in the
control group were low, whereas under the same conditions, the
fluorescent intensities of LC3 and Beclin 1 in the LPS group were
significantly increased compared with the control group (Fig. 5). Notably, following the treatment
with ulinastatin, the expression levels of LC3 and Beclin 1 were
significantly decreased compared with the LPS group (Fig. 5).
Discussion
The present study demonstrated that ulinastatin may
improve survival rate and exert a protective effect against
LPS-induced cardiac dysfunction. It was suggested from the findings
of the present study that this protective effect may be associated
with the anti-inflammatory activity of ulinastatin and its ability
to inhibit autophagy. Therefore, the administration of ulinastatin
may exhibit a protective effect in the pathophysiology of
sepsis.
A previous study has reported the presence of
decreased serum levels of ulinastatin in patients with sepsis, and
the levels of ulinastatin were at their lowest during cases of
severe sepsis and septic shock (22). Therefore, treatment with ulinastatin
may increase the levels of ulinastatin in patients with severe
sepsis. The protective effect of ulinastatin in sepsis has been
confirmed in a number of previous studies; for example, ulinastatin
was observed to serve a protective role in the heart tissue of
septic rats, and its mechanism was attributed to its regulatory
effect over the stress response, cell signaling transduction,
energy metabolism, the immune response and other related genes
(23,24). Furthermore, a previous study revealed
that ulinastatin contributed to the recovery of cardiac function
following reperfusion by reducing mitochondrial dysfunction and
maintaining energy production (8).
However, the role that ulinastatin serves over the regulation of
autophagy during sepsis remains poorly understood. Therefore, in
the present study, endotoxemic mice were treated with ulinastatin
to investigate its underlying mechanism.
TNF-α and IL-6 are cytokines associated with the
inflammatory response (25). A
previous study indicated that ulinastatin reduced sepsis-related
inflammation by downregulating TNF-α expression levels (26). ELISA and western blotting data from
the present study revealed that the serum levels and protein
expression levels of TNF-α and IL-6 were significantly increased in
endotoxemia mice, and these LPS-induced increases could be
significantly decreased following ulinastatin treatment. Therefore,
the results of the present study indicated that ulinastatin may
protect against LPS-induced myocardial injury by inhibiting the
release of TNF-α and IL-6.
Ulinastatin has been proposed to serve as a
myocardial protective agent, and it has been reported that
ulinastatin improved the myocardial contractility, reduced the
myocardial infarct size and decreased the levels of creatine kinase
and cTnI in myocardial IR injury (16). cTnI is a highly sensitive and
specific marker of myocardial injury (27). Mitochondria are important organelles
for cardiomyocytes that not only provide the energy for cells, but
can also trigger cell apoptosis (28). Furthermore, decreases in the MMP have
been found to be the driving force for mitochondrial dysfunction
(29). In the present study,
endotoxemic mice were treated with ulinastatin and it was indicated
that ulinastatin exhibited a protective effect in the hearts of
mice with endotoxemia. This protective effect was demonstrated by
decreased cTnI levels, an increased MMP, as well as the presence of
an intact, well-ordered cardiac ultrastructure.
Autophagy serves an essential role in cell survival,
as well as in cell death (14).
Autophagy is a highly regulated intracellular degradation process,
by which cells remove cytosolic long-lived proteins and damaged
organelles (15). LC3 is the
mammalian homologue of yeast Atg8, and as a lipidated form of LC3,
LC3II has been widely used as a marker of autophagy by indicating
the number of autophagosomes formed (30). Moreover, Beclin 1 has been discovered
to serve an important role in autophagosome formation and
autolysosomal fusion (31), and
LAMP-1 is a lysosome marker that has been associated with the
increased accumulation of autophagic vacuoles (32). Furthermore, p62 is a marker of
autophagic flux and impaired autophagy is often accompanied by p62
accumulation, which results in large p62/ubiquitinated protein
aggregates (33). Previous studies
have demonstrated that the LPS-induced autophagic flux and
autophagy were at their highest levels 12 h after LPS
intraperitoneal injection (34).
Therefore, the present study chose to investigate the effect of
ulinastatin at this time point. Western blotting and TEM data
obtained from the present study revealed that the expression levels
of LC3II and the number of autophagosomes formed were significantly
decreased following ulinastatin treatment. Furthermore, it was
revealed that ulinastatin reduced the LPS-induced autophagosome
maturation, which was demonstrated through the decreased protein
expression levels of LAMP-1 and the increased expression levels of
SQSTM1/p62. Therefore, the present study results suggested that the
decreased activation of autophagy may be a critical protective
mechanism of ulinastatin in mice with endotoxemia.
In conclusion, the findings of the present study
suggested that ulinastatin may serve a cardioprotective role in
sepsis, which may be achieved through its ability to suppress
inflammation and autophagy. These findings may provide a clinical
basis for the use of ulinastatin as a novel therapeutic option for
the treatment of sepsis-related cardiac dysfunction. However, there
are several limitations to the present study. Whilst the present
study was able to observe changes in autophagy, there was a lack of
experiments using specific autophagy inhibitors 3-methyladenine or
autophagy agonists such as rapamycin. Therefore, future studies
should further investigate the effects of ulinastatin on
autophagy-related pathways. Additionally, the present study did not
investigate the effect of ulinastatin at multiple time points or in
in vitro cell models. As the present experimental set-up may
have also produced bias in the results, further extensive research
is required before solid conclusions can be made.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Foundation of
Shanxi Provincial [grant no. 2018SF-095(JK.K)].
Availability of materials and methods
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PZ and JKK designed the study. PZ, LZ, LFG, QD and
QY performed the experiments. Data were collated by LZ and the
results of data were discussed by LFG and QY. PZ prepared the
figures. PZ and LZ wrote the first draft of the manuscript. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures were approved by The Animal Ethics
Committees of The Fourth Military Medical University (Xi'an, China)
and were performed in accordance with the guidelines of The China
Council of Animal Care.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kumar A, Haery C and Parrillo JE:
Myocardial dysfunction in septic shock. Crit Care Clin. 16:251–287.
2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
You W, Min X, Zhang X, Qian B, Pang S,
Ding Z, Li C, Gao X, Di R, Cheng Y and Liu L: Cardiac-Specific
expression of heat shock protein 27 attenuated endotoxin-induced
cardiac dysfunction and mortality in mice through a
PI3K/Akt-dependent mechanism. Shock. 32:108–117. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fleischmann C, Scherag A, Adhikari NK,
Hartog CS, Tsaganos T, Schlattmann P, Angus DC and Reinhart K:
International Forum of Acute Care Trialists. Assessment of global
incidence and mortality of hospital-treated sepsis. Am J Respir
Crit Care Med. 193:259–272. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jönsson-Berling BM, Ohlsson K and
Rosengren M: Radioimmunological quantitation of the urinary trypsin
inhibitor in normal blood and urine. Biol Chem Hoppe Seyler.
370:1157–1161. 1989.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shin IW, Jang IS, Lee SM, Park KE, Ok SH,
Sohn JT, Lee HK and Chung YK: Myocardial protective effect by
ulinastatin via an anti-inflammatory response after regional
ischemia/reperfusion injury in an in vivo rat heart model. Korean J
Anesthesiol. 61:499–505. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Masuda T, Sato K, Noda C, Ikeda KM,
Matsunaga A, Ogura MN, Shimizu K, Nagasawa H, Matsuyama N and Izumi
T: Protective effect of urinary trypsin inhibitor on myocardial
mitochondria during hemorrhagic shock and reperfusion. Crit Care
Med. 31:1987–1992. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koga Y, Fujita M, Tsuruta R, Koda Y,
Nakahara T, Yagi T, Aoki T, Kobayashi C, Izumi T, Kasaoka S, et al:
Urinary trypsin inhibitor suppresses excessive superoxide anion
radical generation in blood, oxidative stress, early inflammation,
and endothelial injury in forebrain ischemia/reperfusion rats.
Neurol Res. 32:925–932. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yu Z, Rayile A, Zhang X, Li Y and Zhao Q:
Ulinastatin protects against lipopolysaccharide-induced cardiac
microvascular endothelial cell dysfunction via downregulation of
lncRNA MALAT1 and EZH2 in sepsis. Int J Mol Med. 39:1269–1276.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mizushima N: The ATG conjugation systems
in autophagy. Curr Opin Cell Biol. 31:1–10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cursio R, Colosetti P and Gugenheim J:
Autophagy and liver ischemia-reperfusion injury. Biomed Res Int.
2015(417590)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ho J, Yu J, Wong SH, Zhang L, Liu X, Wong
WT, Leung CC, Choi G, Wang MH, Gin T, et al: Autophagy in sepsis:
Degradation into exhaustion? Autophagy. 12:1073–1082.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gurusamy N and Das DK: Is autophagy a
double-edged sword for the heart? Acta Physiol Hung. 96:267–276.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
De Meyer GR and Martinet W: Autophagy in
the cardiovascular system. Biochim Biophys Acta. 1793:1485–1495.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiao J, Zhu X, Ji G, Yang Q, Kang B, Zhao
J, Yao F, Wu L, Ni X and Wang Z: Ulinastatin protects
cardiomyocytes against ischemiareperfusion injury by regulating
autophagy through mTOR activation. Mol Med Rep. 10:1949–1953.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao P, Kuai J, Gao J, Sun L, Wang Y and
Yao L: Delta opioid receptor agonist attenuates
lipopolysaccharide-induced myocardial injury by regulating
autophagy. Biochem Biophys Res Commun. 492:140–146. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang WK, Lu QH, Wang X, Wang B, Wang J,
Gong HP, Wang L, Li H and Du YM: Ulinastatin attenuates
diabetes-induced cardiac dysfunction by the inhibition of
inflammation and apoptosis. Exp Ther Med. 14:2497–2504.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lan R, Zhang Y, Xiang J, Zhang W, Wang GH,
Li WW, Xu LL and Cai DF: Xiao-Xu-Ming decoction preserves
mitochondrial integrity and reduces apoptosis after f ocal cerebral
ischemiaand reperfusion via the mitochondrial p53 pathway. J
Ethnopharmacol. 151:307–316. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qiu SQ, van Rooijen J, Nienhuis HH, van
derVegt B, Timmer-Bosscha H, van Leeuwen-Stok E, Walenkamp AME, van
Deurzen CHM, de Bock GH, et al: High hepatocyte growth factor
expression in primary tumor predicts better overall survival in
male breast cancer. Breast Cancer Res. 22(30)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin CM, Chen CR, Wu XQ, Ren JH, Chen SZ,
Luo XF, Mei XQ, Shen LY, Guo MX, Ma XD and Yang T: Effects of blood
purification on serum levels of inflammatory cytokines and cardiac
function in a rat model of sepsis. Blood Purif. 44:40–50.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lim YP, Bendelja K, Opal SM, Siryaporn E,
Hixson DC and Palardy JE: Correlation between mortality and the
levels of inter-alpha inhibitors in the plasma of patients with
severe sepsis. J Infect Dis. 188:919–926. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Han D, Shang W, Wang G, Sun L, Zhang Y,
Wen H and Xu L: Ulinastatin and thymosin α1-based immunomodulatory
strategy for sepsis: A meta-analysis. Int Immunopharmacol.
29:377–382. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang FY, Fang B, Qiang XH, Yu TO, Zhong
JR, Cao J and Zhou LX: The efficacy and immunomodulatory effects of
ulinastatinand thymosin α1 for sepsis: A systematic review and
meta-analysis. Biomed Res Int. 2016(9508493)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gogos CA, Drosou E, Bassaris HP and
Skoutelis A: Pro-Versus anti-inflammatory cytokine profile in
patients with severe sepsis: A marker for prognosis and future
therapeutic options. J Infect Dis. 181:176–180. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Chen X, Wang Y, Luo H, Luo Z, Liu L, Xu W,
Zhang T, Yang N, Long X, Zhu N, et al: Ulinastatin reduces urinary
sepsis-related inflammation by upregulating IL-10 and
downregulating TNF-α levels. Mol Med Rep. 8:29–34. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Varvarousis D, Goulas N, Polytarchou K,
Psychari SN, Paravolidakis K, Konstantinidou A, Tsoukalas D, Vlad
D, Bouki K and Kotsakis A: Biomarkers of myocardial injury and
inflammation after permanent pacemaker implantation: The lead
fixation type effect. J Atr Fibrillation. 10(1798)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shires SE and Gustafsson ÅB: Mitophagy and
heart failure. J Mol Med (Berl). 93:253–262. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Joiner ML, Koval OM, Li JD, He B,
Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, et al:
CaMKII determines mitochondrial stress responses in heart. Nature.
491:269–273. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nishida K, Kyoi S, Yamaguchi O, Sadoshima
J and Otsu K: The role of autophagy in the heart. Cell Death
Differ. 16:31–38. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Castillo K, Valenzuela V, Matus S, Nassif
M, Oñate M, Fuentealba Y, Encina G, Irrazabal T, Parsons G, Court
FA, et al: Measurement of autophagy flux in the nervous system in
vivo. Cell Death Dis. 4(e917)2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Eskelinen EL: Roles of LAMP-1 and LAMP-2
in lysosome biogenesis and autophagy. Mol Aspects Med. 27:495–502.
2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mathew R, Karp CM, Beaudoin B, Vuong N,
Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao P, Gao JJ, Jiang J, Peng X, Wu W,
Zheng L and Yao L: Myocardial cells and mitochondrial autophagy in
sepsis mice induced by lipopolysaccharide. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 32:177–181. 2016.PubMed/NCBI(In Chinese).
|