Introduction
The overexpression of human epidermal growth factor
receptor 2 (HER2) is reported in breast (1,2), gastric
(3), pancreatic (4), lung (5),
and colorectal cancers (6). This
expression is associated with poor clinical outcomes in patients
with HER2-positive breast cancer (1,2).
Humanized anti-HER2 monoclonal antibodies (mAbs) trastuzumab and
pertuzumab have been used in the treatment of HER2-positive breast
cancer (7-9).
Treatment with trastuzumab resulted in significant survival
benefits these patients (10). In
comparison to trastuzumab monotherapy, the combination of
trastuzumab and pertuzumab with chemotherapy has led to significant
improvements in overall survival (11).
Trastuzumab deruxtecan (DS-8201), a recently
developed drug, is comprised of three components, a novel
enzyme-cleavable linker, and a topoisomerase I inhibitor (12). Even in low-HER2-expressing tumors,
DS-8201 shows antitumor activity. This drug has several innovative
features: i) a highly potent, novel payload with a high
drug-to-antibody ratio, ii) good homogeneity, iii) a
tumor-selective cleavable linker, iv) a stable linker-payload in
circulation, and v) a cytotoxic agent with a short in vivo
half-life in vivo (13).
Furthermore, the cytotoxic payload can exert a bystander effect
(13).
The novel anti-HER2 mAb (H2Mab-19)
developed in this study was investigated for its antitumor
activities in mouse xenograft models of breast and oral cancers.
These properties have not been previously investigated with regard
to HER2 expression.
Materials and methods
Cell lines
Oral squamous carcinoma cell lines including Ca9-22
(derived from gingiva), HO-1-u-1 (mouth floor), HSC-2 (oral
cavity), and SAS (tongue) were obtained from the Japanese
Collection of Research Bioresources Cell Bank (Osaka, Japan). LN229
(glioblastoma cell line), MDA-MB-468 (breast cancer), BT-474
(breast cancer), and P3U1 (mouse myeloma) were obtained from the
American Type Culture Collection. LN229/HER2 cells were established
in a previous study (14). P3U1
cells were cultured in RPMI-1640 medium (Nacalai Tesque, Inc.,
Kyoto, Japan). LN229, LN229/HER2, MDA-MB-468, BT-474, Ca9-22,
HO-1-u-1, HSC-2, and SAS were cultured in Dulbecco's modified
Eagle's medium (DMEM; Nacalai Tesque, Inc.) supplemented with 10%
heat-inactivated fetal bovine serum (Thermo Fisher Scientific
Inc.), 100 units/ml of penicillin, 100 µg/ml streptomycin, and 25
µg/ml amphotericin B (Nacalai Tesque, Inc.) at 37°C in a humidified
atmosphere containing 5% CO2.
Animals
All animal experiments were performed in accordance
with relevant guidelines and regulations to minimize animal
suffering and distress in the laboratory. Animal experiments for
hybridoma production were approved by the Animal Care and Use
Committee of Tohoku University (permit no. 2016MdA-153). Animal
health was monitored daily. Animal studies for Antibody-Dependent
Cellular Cytotoxicity were approved by the institutional committee
for experiments of the Institute of Microbial Chemistry (permit no.
2019-066). Animal studies for antitumor activity were approved by
the institutional committee for experiments of the Institute of
Microbial Chemistry (permit no. 2019-014). Mice were monitored for
health and weight every 3 or 4 days. Experiment duration was three
weeks. A bodyweight loss exceeding 25% and a maximum tumor size
exceeding 3,000 mm3 were identified as humane endpoints.
Mice were euthanized by cervical dislocation, and the death was
verified by respiratory arrest and cardiac arrest.
Hybridoma production
One four-week-old female BALB/c mouse was purchased
from CLEA Japan and housed under specific pathogen-free conditions.
Anti-HER2 hybridoma cells were produced as described previously
(14). Briefly, the BALB/c animal
was immunized by intraperitoneal (i.p.) administration of 100 µg
recombinant HER2 extracellular domain along with Imject Alum
(Thermo Fisher Scientific Inc.). After several additional
immunizations, a booster dose was administered i.p. 2 days before
harvesting spleen cells. Mice were euthanized by cervical
dislocation, and the death was verified by respiratory arrest and
cardiac arrest. Spleen cells were then fused with P3U1 cells using
PEG1500 (Roche Diagnostics, Indianapolis, IN, USA). The resulting
hybridoma cells were grown in RPMI medium supplemented with
hypoxanthine, aminopterin, and thymidine selection medium (Thermo
Fisher Scientific, Inc.). Culture supernatants were screened using
enzyme-linked immunosorbent assays with recombinant HER2
extracellular domain. mAbs were purified from the supernatants of
hybridoma cells and cultured in Hybridoma-SFM medium (Thermo Fisher
Scientific, Inc.) using Protein G Sepharose 4 Fast Flow (GE
Healthcare UK Ltd.).
Flow cytometry
Hybridoma cells were harvested by brief exposure to
0.25% trypsin/1-mM ethylenediaminetetraacetic acid (EDTA; Nacalai
Tesque, Inc.). After washing with 0.1% bovine serum albumin in
phosphate-buffered saline (PBS), cells were treated with 1 µg/ml
anti-HER2 (H2Mab-19) for 30 min at 4°C and subsequently
with Alexa Fluor 488-conjugated anti-mouse IgG (1:1,000; Cell
Signaling Technology, Inc.). Fluorescence microscopy data were
collected using an EC800 Cell Analyzer (Sony Corp.).
Immunohistochemical analyses for
formalin-fixed paraffin-embedded (FFPE) tissues
Histologic sections (catalog no. T8235721-5; lot no.
B104066; BioChain Institute Inc.) were purchased in this study.
Four-µm histologic sections from paraffin blocks of resected
xenografts were also produced. These sections were deparaffinized
in xylene, then rehydrated and autoclaved in citrate buffer (pH
6.0; Agilent Technologies Inc.) for 20 min. Sections were incubated
with primary mAbs for 1 h at room temperature, then treated using
an Envision+ kit (Agilent Technologies Inc.) for 30 min. Color was
developed using 3,3-diaminobenzidine tetrahydrochloride (Agilent
Technologies Inc.) for 2 min, and sections were then counterstained
with hematoxylin (FUJIFILM Wako Pure Chemical Corporation).
Immunohistochemical analyses for
frozen tissues
Histologic sections (catalog no. T6235086-1,
BioChain Institute Inc.) were incubated with 1 µg/ml of primary
mAbs for 1 h at room temperature and were then treated using an
Envision+ kit (Agilent Technologies Inc.) for 30 min. Color was
developed using 3,3-diaminobenzidine tetrahydrochloride (Agilent
Technologies Inc.) for 2 min, and sections were then counterstained
with hematoxylin (FUJIFILM Wako Pure Chemical Corporation).
Determination of the binding
affinity
Cells were suspended in 100 µl serially diluted
H2Mab-19 (6 ng/ml-100 µg/ml), followed by the addition
of Alexa Fluor 488-conjugated anti-mouse IgG (1:200; Cell Signaling
Technology, Inc.). Fluorescence microscopy data were collected
using an EC800 Cell Analyzer (Sony Corp.). The dissociation
constant (KD) was obtained by fitting binding
isotherms to built-in one-site binding models in GraphPad PRISM 6
(GraphPad Software, Inc.).
Antibody-dependent cellular
cytotoxicity
Six six-week-old female BALB/c nude mice were
purchased from Charles River. After euthanization by cervical
dislocation, spleens were removed aseptically and single-cell
suspensions obtained by forcing spleen tissues through a stainless
steel mesh using a syringe. Erythrocytes were lysed with a 10-sec
exposure to ice-cold distilled water. Splenocytes were washed with
DMEM and resuspended in DMEM with 10% FBS and used as effector
cells. Target cells were labeled with 10-µg/ml Calcein AM (Thermo
Fisher Scientific, Inc.) and resuspended in the same medium. The
target cells (2x104 cells/well) were plated in 96-well
plates and mixed with effector cells, anti-HER2 antibodies, or
control IgG (mouse IgG2b) (Sigma-Aldrich Corp.). After a
4-h incubation, the Calcein AM release of supernatant from each
well was measured. Fluorescence intensity was determined using a
microplate reader (Power Scan HT) (BioTek Instruments) with an
excitation wavelength of 485 nm and an emission wavelength of 538
nm. Cytolytic activity (as % of lysis) was calculated as: %
lysis=(E-S)/(M-S) x100, where E is fluorescence of combined target
and effector cells, S is spontaneous fluorescence of target cells
only, and M is maximum fluorescence measured after lysing all cells
with a buffer containing 0.5% Triton X-100, 10 mM Tris-HCl (pH
7.4), and 10 mM of EDTA.
Complement-dependent cytotoxicity
Cells in DMEM supplemented with 10% FBS
(2x104 cells/well) were plated in 96-well plates., and
incubated for 5 h at 37°C with either anti-HER2 antibodies or
control IgG (mouse IgG2b) (Sigma-Aldrich Corp.) and 10%
of rabbit complement (Low-Tox-M Rabbit Complement) (Cedarlane
Laboratories). To assess cell viability, an MTS
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium;
inner salt] assay was performed using a CellTiter 96 AQueous assay
kit (Promega).
Antitumor activity of
H2Mab-19 in the xenografts of breast cancers
Sixteen six-week-old female BALB/c nude mice were
purchased from Charles River (Kanagawa, Japan) and used at 10 weeks
of age. BT-474 cells (0.3 ml of 1.33x108 cells/ml in
DMEM) were mixed with 0.5 ml BD Matrigel Matrix Growth Factor
Reduced (BD Biosciences). One hundred-µl of this suspension
(5x106 cells) was injected subcutaneously into the left
flank. After day 1, 100 µg H2Mab-19 and control mouse
IgG (Sigma-Aldrich Corp.) in 100 µl PBS were injected i.p. into
treated and control mice, respectively. Additional antibodies were
then injected on days 7 and 14. Eighteen days after cell
implantation, all mice were euthanized by cervical dislocation and
tumor diameters and volumes were determined as previously described
(15).
Antitumor activity of
H2Mab-19 in xenografts of oral cancers
Thirty-two six-week-old female BALB/c nude mice were
purchased from Charles River and used at 10 weeks of age. HSC-2 or
SAS cells in DMEM (0.3 ml with 1.33x108 cells/ml) were
mixed with 0.5 ml BD Matrigel Matrix Growth Factor Reduced (BD
Biosciences). A 100-µl suspension containing 5x106 cells
was injected subcutaneously into the left flank. After day 1, 100
µg H2Mab-19 and control mouse IgG (Sigma-Aldrich Corp.)
in 100 µl PBS were injected i.p. into treated and control mice,
respectively. Additional antibodies were then injected on days 6
and 14. Twenty days after cell implantation, all mice were
euthanized by cervical dislocation. Tumor diameters and volumes
were determined as previously described (15).
Statistical analyses
All data were expressed as mean ± SEM. Statistical
analysis used ANOVA and Tukey-Kramer's test with GraphPad Prism 6
(GraphPad Software, Inc.). P<0.05 was considered to indicate
a statistically significant difference.
Results
Production of anti-HER2 mAb
One mouse was immunized with the recombinant
extracellular domain of HER2(16),
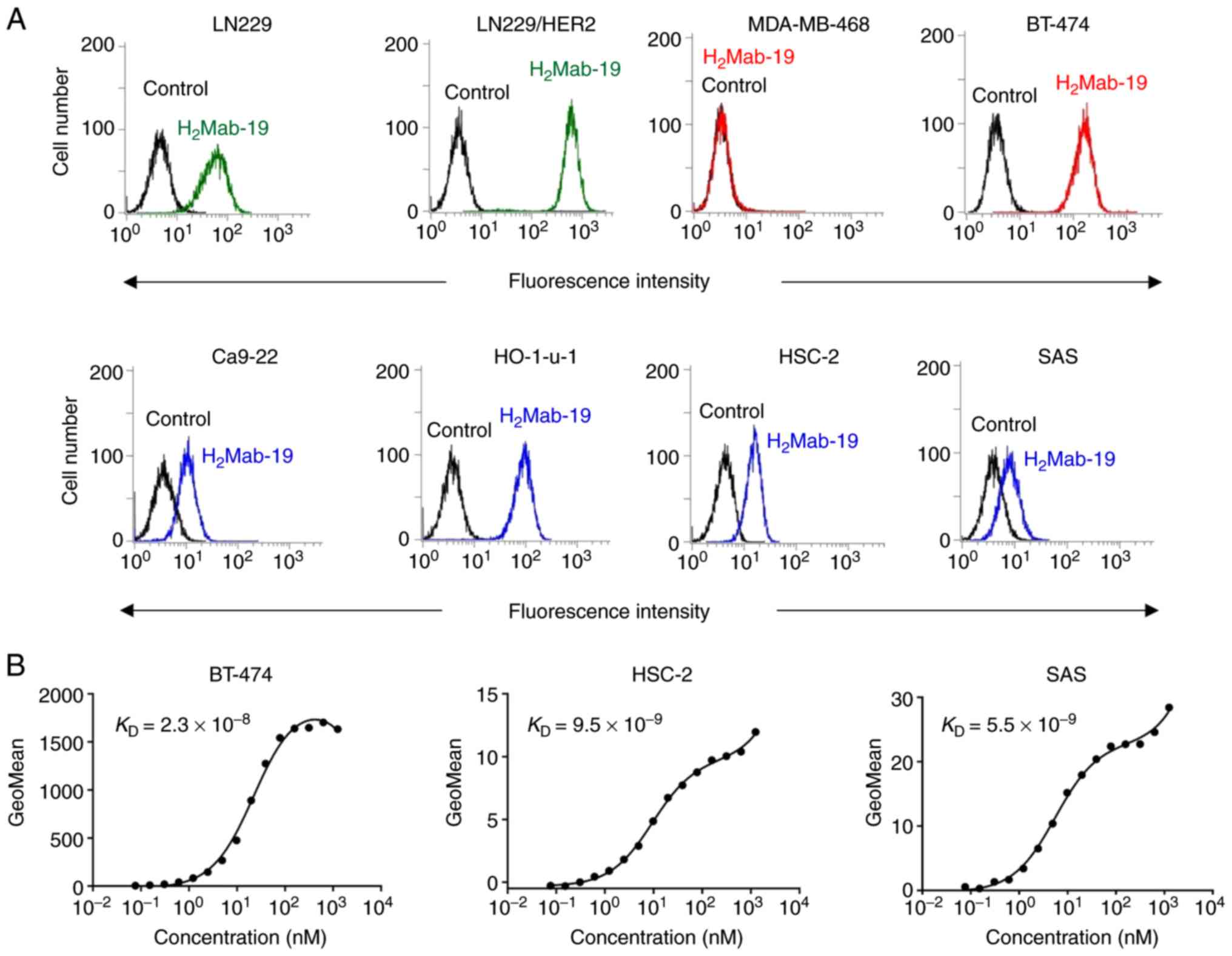
purified using the MAP tag system (17). Flow cytometry was performed to check
reactions with the LN229 cells (glioblastoma) and
HER2-overexpressing LN229 cells (LN229/HER2). LN229 cells
endogenously express HER2 and some reaction with these cells was
expected. The overexpression of HER2 in LN229/HERS2 cells would
produce a stronger reaction. One IgG2b subclass clone of
H2Mab-19 was obtained, though almost all mAbs were in
the mouse IgG1 subclass. H2Mab-19 reacted
with LN229/HER2 and weakly reacted with LN229 cells (Fig. 1A), indicating that
H2Mab-19 is specific to HER2.
Characterization of
H2Mab-19
H2Mab-19 recognized endogenous HER2 in a
breast cancer cell line, BT-474, which is HER2-positive (18), but did not react with a breast cancer
cell line, MDA-MB-468, which is HER2-negative (18) (Fig.
1A). Further, H2Mab-19 strongly reacted with
endogenous HER2 in HO-1-u-1 cells (oral cancer) and only weakly
reacted with other oral cancer cell lines, Ca9-22, HSC-2, and SAS
(Fig. 1A). Using flow cytometry,
binding affinities (KD) of H2Mab-19 to
BT-474, HSC-2, and SAS cell lines were 2.3x10-8,
9.5x10-9 and 5.5x10-9 M, respectively. These
results indicate that H2Mab-19 maintains high affinity
across HER2-expressing cell lines. H2Mab-19 did not
stain FFPE-breast cancer tissues (Fig.
S1). In contrast, H2Mab-19 reacted with frozen
breast cancer tissues although the sensitivity of
H2Mab-77 was better than that of H2Mab-19
(Fig. S2).
ADCC and CDC activities against breast
and oral squamous cell carcinoma cell lines
This study examined whether H2Mab-19
induced ADCC and CDC in HER2-expressing breast or OSCC cell lines.
H2Mab-19 was a mouse IgG2b subclass antibody
that could possess both ADCC and CDC. H2Mab-19 exhibited
high ADCC activity against BT-474, HSC-2, and SAS cells (Fig. 2A). High CDC activity was also
observed in BT-474, HSC-2, and SAS cells (Fig. 2B), suggesting that
H2Mab-19 might exert antitumor activity in
vivo.
Antitumor activity of
H2Mab-19 in mouse xenografts of breast cancers
To study the antitumor activity of
H2Mab-19 on cell growth in vivo, BT-474 cells
were implanted subcutaneously in the flanks of nude mice.
H2Mab-19 and control mouse IgG were injected i.p. three
times (days 1, 7, and 14 after cell injection) into treated and
control mice, respectively. Tumor formation was observed in mice in
both H2Mab-19-treated and control groups.
H2Mab-19 treatment significantly reduced tumor
development compared to development in control mice on days 5, 7,
12, 15, and 18 (Fig. 3A, upper).
Weights of tumors from H2Mab-19-treated mice were
significantly less than for tumors from IgG-treated control mice
(Fig. 3B, upper). BT-474 xenografts
on day 18 are shown in Fig. S3A.
Resected tumors are depicted in Fig.
S3B. Total body weight was not significantly different between
the two groups (Fig. S3C). We could
not show the histological data about the liver and kidney in this
study. HER2 was highly expressed in all cancer cells of
H2Mab-19-treated BT-474 and control xenografts (Fig. S4).
Antitumor activities of
H2Mab-19 in the mouse xenografts of oral cancers
H2Mab-19 possessed antitumor activity in
mouse xenografts of breast cancers. Whether this activity extended
to xenografts of oral cancers was also assessed. BT-474 cells
expressed high levels of HER2 (Fig.
1A); HER2 levels were however low in HSC-2 and SAS cells
(Fig. 1B). Nevertheless, HSC-2 and
SAS are useful for investigation of antitumor activity in
vivo (16). Thus, HSC-2 and SAS
were used for mouse xenografts of oral cancers.
Initially, HSC-2 cells were implanted subcutaneously
into the flanks of nude mice. H2Mab-19 and mouse IgG
were injected i.p. three times (on days 1, 6, and 14 after cell
injections into treated and control mice, respectively. Tumor
formation was observed in mice in both groups. In comparison to
control mice, H2Mab-19-treated mice showed significantly
reduced tumor development on days 6, 10, 14, 17 and 20 (Fig. 3A, middle). Weights of tumors from
H2Mab-19-treated mice were significantly less than for
tumors from control mice (Fig. 3B,
middle). HSC-2 xenograft mice are shown on day 20 in Fig. S5A and resected tumors are depicted
in Fig. S5B. Total body weights
were not significantly different between the two groups (Fig. S5C). We could not show the
histological data about the liver and kidney in this study. HER2
was not expressed in cancer cells of H2Mab-19-treated or
control groups (Fig. S6). HER2
expression was diminished in HSC-2 xenografts, and
H2Mab-19 did not exert effective antitumor activity.
For the second xenograft model of oral cancers, SAS
cells were subcutaneously implanted into the flanks of nude mice.
H2Mab-19 and mouse IgG were injected i.p. thrice, on
days 1, 6, and 14 after cell injections into the mice, into treated
and control mice, respectively. Tumor formation was observed in
mice in both treated and control groups. In comparison to
IgG-treated control mice, H2Mab-19 significantly reduced
tumor development on days 14, 17, and 20 (Fig. 3A, lower). Weights of tumors from
H2Mab-19-treated mice were significantly less than
tumors from IgG-treated control Mice (Fig. 3B, lower). The SAS xenografts on day
20 are shown in Fig. S7A and
resected tumors are depicted in Fig.
S7B. Total body weights were not significantly different
between the two groups (Fig. S7C).
We could not show the histological data about the liver and kidney
in this study. HER2 was not expressed in cancer cells of
H2Mab-19-treated and control groups (Fig. S8). HER2 expression was diminished in
SAS xenografts, and H2Mab-19 did not exert effective
antitumor activity.
Discussion
Using CasMab technology (19), several anti-HER2 mAbs, including
H2Mab-77(14),
H2Mab-119(20), and
H2Mab-139(16) were
identified. These antibodies are useful for flow cytometry, western
blot, and immunohistochemical analyses. Because the subclass of
these mAbs is mouse IgG1, they do not possess
antibody-dependent cellular cytotoxicity (ADCC) or
complement-dependent cytotoxicity (CDC).
The first objective of this study was the
development of an anti-HER2 mAb in either IgG2a or
IgG2b subclasses using CasMab technology. Both
IgG2a (21) and
IgG2b antibodies (22)
show ADCC and CDC activity. The second objective was to investigate
anti-HER2 activity using oral cancer cell lines; anti-HER2 mAbs
have not been investigated for their activity against oral cancers.
The first objective was met through isolation of
H2Mab-19 from the IgG2b subclass (Fig. 1). This antibody could then be used to
investigate ADCC and CDC activity in vitro and antitumor
activity in vivo. H2Mab-19 showed both ADCC and
CDC activity against breast or oral cancer cell lines (Fig. 2). Further, H2Mab-19
exerted antitumor activity against both breast cancer and oral
cancer xenografts (Fig. 3). These
results demonstrated two important issues: i) anti-HER2 mAbs from
the IgG2b subclass could be developed using our original
CasMab technology, and ii) anti-HER2 mAbs from IgG2b
subclass could possess ADCC, CDC, and antitumor activities.
Recently, Fiedler et al reported that TrasGEX, an
ADCC-enhanced version of trastuzumab, showed antitumor activity in
50% of evaluated patients from a phase I study (23). They showed that TrasGEX exhibited
similar pharmacokinetics to those of trastuzumab and was safe and
well-tolerated by patients with solid tumors. These data are
consistent with the designation of HER2 as a promising target for
the treatment of HER2-amplified tumors. Trastuzumab and TrasGEX are
known as beneficial anti-HER2 mAbs for targeting breast or stomach
cancers, H2Mab-19 could be also a useful tool for
investigating ADCC, CDC, antitumor activities for oral cancers.
Further investigation of the mechanism of antitumor activity by
H2Mab-19, and the development of antibody-engineered
antibodies, including chimeric or humanized H2Mab-19 or
its single chain (sc) Fv, are aims for future studies.
Oral cancer accounts for approximately 2% of all
cancer cases worldwide (24).
Annually, more than 350,000 individuals are diagnosed with oral
cancer and these diseases prove fatal for 170,000 of these people.
Major risk factors for oral cancer are the use of tobacco and
alcohol (25). Decreased smoking and
drinking has resulted in a decline in the incidence of oral cancer.
However, recent studies have reported an increase in the number of
young patients diagnosed with these diseases (26,27).
More than 50% of oral cancers occur in tongue tissue
and on the floor of the mouth. Other locations include the buccal
mucosa, gingiva, lip and palate (28). HER2 expression was assessed in four
oral cancer cell lines of different origin, including Ca9-22
(gingiva), HO-1-u-1 (mouth floor), HSC-2 (oral cavity), and SAS
(tongue). HER2 expression was observed in all cell lines (Fig. 1), indicating that expression is
independent of location in the oral cavity.
Oral cancers display several histological tumor
types, including squamous cell carcinoma (SCC), adenocarcinoma,
mucoepidermoid carcinoma, adeno cystic carcinoma and osteosarcoma.
SCC is most common, accounting for over 90% of all disease
(29). Treatment of oral SCC (OSCC)
depends for the most part on stage. Early stages (stage-I and -II)
are treated via surgery or radiotherapy (RT) alone. Advanced stages
(stage-III and -IV) require a combination of surgery, RT and
chemotherapy (CT) (30). Cisplatin
(CDDP) is mainly used for CT of OSCCs, often combined with
5-fluorouracil (5-FU) and docetaxel (31,32).
Other anticancer agents such as carboplatin, paclitaxel, and
methotrexate (MTX) can be useful (33), but useful drugs with specific
molecular targets are limited.
Cetuximab, a mouse-human chimeric antibody
(IgG1) that targets epidermal growth factor receptor
(EGFR), was recently approved for treatment of oral cancer. Several
studies report its effectiveness against locoregionally advanced
head and neck cancer and recurrent or metastatic squamous cell
carcinoma of the head and neck (34-36).
Advances in diagnosis and therapeutic techniques have improved the
overall 5-year survival rate to 70%. However, the 5-year survival
rate in stage IV is only 40% (37)
and further treatments need to be developed. In this study, HER2 is
shown to be expressed in oral cancers, and anti-HER2 mAbs have
useful for antitumor activity. Thus, anti-HER2 therapies using
trastuzumab could be valuable for oral cancer treatment.
Immunohistochemically, HER2 expressed was reported in only 1.4%
(38) of oral cancer, though it is
expressed in 10.4% of breast cancers (39). Thus, targeting only HER2 may not be
sufficient for treating oral cancers. Despite the low HER2
overexpression/amplification rate of only 1-2%, those few patients
may possibly benefit from anti-HER2 therapy because an antitumor
effect of combined gefitinib and trastuzumab or cetuximab and
trastuzumab treatment on HNSCC in vitro were demonstrated
(40,41). Pursuing multiple targets, such as
EGFR and HER2, may be needed for effective therapy.
Supplementary Material
Immunohistochemical analyses of
paraffin sections of breast cancers using H2Mab-19 and
H2Mab-77. Sections of breast cancers were incubated with
H2Mab-19 (10 μg/ml) or H2Mab-77 (1
μg/ml) Sections were counterstained with hematoxylin. Scale
bar=100 μm.
Immunohistochemical analyses of frozen
sections of breast cancers using H2Mab-19 or
H2Mab-77. Sections of breast cancers were incubated with
H2Mab-19 (1 μg/ml) or H2Mab-77 (1
μg/ml). Sections were then counterstained with hematoxylin.
Scale bar=100 μm.
Evaluation of antitumor activity of
H2Mab-19 in BT-474 xenografts. (A) BT-474 xenografts on
day 18. (B) Resected tumors of BT-474 xenografts (day 18). (C) Body
weights of the mice with the BT-474 xenografts. n.s., not
significant. Scale bar=1 cm.
Immunohistochemical analyses and
hematoxylin & eosin (HE) staining of resected tissues in BT-474
xenografts. Sections were incubated with H2Mab-19 (10
μg/ml) or H2Mab-77 (10 μg/ml). Sections
were then counterstained with hematoxylin. HE staining was also
performed. Scale bar=100 μm.
Evaluation of antitumor activity of
H2Mab-19 in HSC-2 xenografts. (A) HSC-2 xenografts on
day 20. (B) Resected tumors of HSC-2 xenografts (day 20). (C) Body
weights of mice with HSC-2 xenografts. n.s., not significant. Scale
bar=1 cm.
Immunohistochemical analyses and
hematoxylin & eosin (HE) staining of resected tissues in HSC-2
xenografts. Sections were incubated with H2Mab-19 (10
μg/ml) or H2Mab-77 (10 μg/ml). Then,
sections were counterstained with hematoxylin. HE staining was also
performed. Scale bar=100 μm.
Evaluation of antitumor activity of
H2Mab-19 in SAS xenografts. (A) SAS xenografts on day
20. (B) Resected tumors of SAS xenografts (day 20). (C) Body
weights of mice with the SAS xenografts. n.s., not significant.
Scale bar=1 cm.
Immunohistochemical analyses and
hematoxylin & eosin (HE) staining of resected tissues in SAS
xenografts. Sections were incubated with H2Mab-19 (10
μg/ml) or H2Mab-77 (10 μg/ml). Then,
sections were counterstained with hematoxylin. HE staining was also
performed. Scale bar=100 μm.
Acknowledgements
The authors would like to thank Ms. Akiko Harakawa
(Institute of Microbial Chemistry (BIKAKEN), Numazu, Microbial
Chemistry Research Foundation) for technical assistance of animal
experiments, and Mr. Takuro Nakamura, Ms. Miyuki Yanaka, Ms. Saori
Handa, Ms. Saki Okamoto, and Mr. Yu Komatsu (Department of Antibody
Drug Development, Tohoku University Graduate School of Medicine)
for technical assistance of in vitro experiments.
Funding
This research was supported in part by Japan Agency
for Medical Research and Development (AMED) under (grant nos.
JP19am0401013, JP19am0101078 and JP19ae0101028), and by Japan
Society for the Promotion of Science (JSPS) Grants-in-Aid for
Scientific Research (KAKENHI; grant nos. 17K07299 and
19K07705).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JT and TO performed experiments. MKK analyzed
experimental data. MK, HH, and YK designed the current study and
wrote the manuscript.
Ethics approval and consent to
participate
Animal experiments described in the hybridoma
production were approved by the Animal Care and Use Committee of
Tohoku University (permit no. 2016MdA-153). Animal studies for ADCC
were approved by the institutional committee for experiments of the
Institute of Microbial Chemistry (permit no. 2019-066). Animal
studies for the antitumor activity were approved by the
institutional committee for experiments of the Institute of
Microbial Chemistry (permit no. 2019-014).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howlader N, Altekruse SF, Li CI, Chen VW,
Clarke CA, Ries LA and Cronin KA: US incidence of breast cancer
subtypes defined by joint hormone receptor and HER2 status. J Natl
Cancer Inst. 106(dju055)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xie S, Zhang H, Wang X, Ge Q and Hu J: The
relative efficacy and safety of targeted agents used in combination
with chemotherapy in treating patients with untreated advanced
gastric cancer: A network meta-analysis. Oncotarget. 8:26959–26968.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harder J, Ihorst G, Heinemann V, Hofheinz
R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S,
et al: Multicentre phase II trial of trastuzumab and capecitabine
in patients with HER2 overexpressing metastatic pancreatic cancer.
Br J Cancer. 106:1033–1038. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim EK, Kim KA, Lee CY and Shim HS: The
frequency and clinical impact of HER2 alterations in lung
adenocarcinoma. PLoS One. 12(e0171280)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Seo AN, Kwak Y, Kim DW, Kang SB, Choe G,
Kim WH and Lee HS: HER2 status in colorectal cancer: Its clinical
significance and the relationship between HER2 gene amplification
and expression. PLoS One. 9(e98528)2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lambert JM and Chari RV: Ado-trastuzumab
emtansine (T-DM1): An antibody-drug conjugate (ADC) for
HER2-positive breast cancer. J Med Chem. 57:6949–6964.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: Mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Amiri-Kordestani L, Wedam S, Zhang L, Tang
S, Tilley A, Ibrahim A, Justice R, Pazdur R and Cortazar P: First
FDA approval of neoadjuvant therapy for breast cancer: Pertuzumab
for the treatment of patients with HER2-positive breast cancer.
Clin Cancer Res. 20:5359–5364. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Swain SM, Kim SB, Cortés J, Ro J,
Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A,
Knott A, et al: Pertuzumab, trastuzumab, and docetaxel for
HER2-positive metastatic breast cancer (CLEOPATRA study): Overall
survival results from a randomised, double-blind,
placebo-controlled, phase 3 study. Lancet Oncol. 14:461–471.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Doi T, Shitara K, Naito Y, Shimomura A,
Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N,
et al: Safety, pharmacokinetics, and antitumour activity of
trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug
conjugate, in patients with advanced breast and gastric or
gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet
Oncol. 18:1512–1522. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nakada T, Sugihara K, Jikoh T, Abe Y and
Agatsuma T: The latest research and development into the
antibody-drug conjugate, [fam-] trastuzumab deruxtecan (DS-8201a),
for HER2 cancer therapy. Chem Pharm Bull (Tokyo). 67:173–185.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Itai S, Fujii Y, Kaneko MK, Yamada S,
Nakamura T, Yanaka M, Saidoh N, Chang YW, Handa S, Takahashi M, et
al: H2Mab-77 is a sensitive and specific anti-HER2 monoclonal
antibody against breast cancer. Monoclon Antib Immunodiagn
Immunother. 36:143–148. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kato Y, Kunita A, Abe S, Ogasawara S,
Fujii Y, Oki H, Fukayama M, Nishioka Y and Kaneko MK: The chimeric
antibody chLpMab-7 targeting human podoplanin suppresses pulmonary
metastasis via ADCC and CDC rather than via its neutralizing
activity. Oncotarget. 6:36003–36018. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kaneko MK, Yamada S, Itai S and Kato Y:
Development of an anti-HER2 monoclonal antibody H2Mab-139 against
colon cancer. Monoclon Antib Immunodiagn Immunother. 37:59–62.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fujii Y, Kaneko MK and Kato Y: MAP tag: A
novel tagging system for protein purification and detection.
Monoclon Antib Immunodiagn Immunother. 35:293–299. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Al-Saden N, Lam K, Chan C and Reilly RM:
Positron-emission tomography of HER2-positive breast cancer
xenografts in mice with 89Zr-labeled trastuzumab-DM1: A
comparison with 89Zr-labeled trastuzumab. Mol Pharm.
15:3383–3393. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kato Y and Kaneko MK: A cancer-specific
monoclonal antibody recognizes the aberrantly glycosylated
podoplanin. Sci Rep. 4(5924)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yamada S, Itai S, Nakamura T, Chang YW,
Harada H, Suzuki H, Kaneko MK and Kato Y: Establishment of
H2Mab-119, an anti-human epidermal growth factor
receptor 2 monoclonal antibody, against pancreatic cancer. Monoclon
Antib Immunodiagn Immunother. 36:287–290. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kaneko MK, Nakamura T, Honma R, Ogasawara
S, Fujii Y, Abe S, Takagi M, Harada H, Suzuki H, Nishioka Y and
Kato Y: Development and characterization of anti-glycopeptide
monoclonal antibodies against human podoplanin, using
glycan-deficient cell lines generated by CRISPR/Cas9 and TALEN.
Cancer Med. 6:382–396. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ogasawara S, Kaneko MK and Kato Y:
LpMab-19 recognizes sialylated O-Glycan on Thr76 of human
podoplanin. Monoclon Antib Immunodiagn Immunother. 35:245–253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fiedler W, Stoeger H, Perotti A, Gastl G,
Weidmann J, Dietrich B, Baumeister H, Danielczyk A, Goletz S,
Salzberg M and De Dosso S: Phase I study of TrasGEX, a
glyco-optimised anti-HER2 monoclonal antibody, in patients with
HER2-positive solid tumours. ESMO Open. 3(e000381)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hashibe M, Brennan P, Chuang SC, Boccia S,
Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova
E, et al: Interaction between tobacco and alcohol use and the risk
of head and neck cancer: Pooled analysis in the international head
and neck cancer epidemiology consortium. Cancer Epidemiol
Biomarkers Prev. 18:541–550. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tota JE, Anderson WF, Coffey C, Califano
J, Cozen W, Ferris RL, St John M, Cohen EEW and Chaturvedi AK:
Rising incidence of oral tongue cancer among white men and women in
the United States, 1973-2012. Oral Oncol. 67:146–152.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hussein AA, Helder MN, de Visscher JG,
Leemans CR, Braakhuis BJ, de Vet HCW and Forouzanfar T: Global
incidence of oral and oropharynx cancer in patients younger than 45
years versus older patients: A systematic review. Eur J Cancer.
82:115–127. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bagan J, Sarrion G and Jimenez Y: Oral
cancer: Clinical features. Oral Oncol. 46:414–417. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rivera C: Essentials of oral cancer. Int J
Clin Exp Pathol. 8:11884–11894. 2015.PubMed/NCBI
|
|
30
|
Guneri P and Epstein JB: Late stage
diagnosis of oral cancer: Components and possible solutions. Oral
Oncol. 50:1131–1136. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Vokes EE: Induction chemotherapy for head
and neck cancer: Recent data. Oncologist. 15 (Suppl
3)(S3-S7)2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Marcazzan S, Varoni EM, Blanco E, Lodi G
and Ferrari M: Nanomedicine, an emerging therapeutic strategy for
oral cancer therapy. Oral Oncol. 76:1–7. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Furness S, Glenny AM, Worthington HV,
Pavitt S, Oliver R, Clarkson JE, Macluskey M, Chan KK and Conway
DI: Interventions for the treatment of oral cavity and
oropharyngeal cancer: Chemotherapy. Cochrane Database Syst Rev.
CD006386:2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vermorken JB, Mesia R, Rivera F, Remenar
E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol
D, et al: Platinum-based chemotherapy plus cetuximab in head and
neck cancer. N Engl J Med. 359:1116–1127. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Naruse T, Yanamoto S, Matsushita Y,
Sakamoto Y, Morishita K, Ohba S, Shiraishi T, Yamada SI, Asahina I
and Umeda M: Cetuximab for the treatment of locally advanced and
recurrent/metastatic oral cancer: An investigation of distant
metastasis. Mol Clin Oncol. 5:246–252. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Amit M, Yen TC, Liao CT, Chaturvedi P,
Agarwal JP, Kowalski LP, Ebrahimi A, Clark JR, Kreppel M, Zöller J,
et al: Improvement in survival of patients with oral cavity
squamous cell carcinoma: An international collaborative study.
Cancer. 119:4242–4248. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hanken H, Gaudin R, Gröbe A, Fraederich M,
Eichhorn W, Smeets R, Simon R, Sauter G, Grupp K, Izbicki JR, et
al: Her2 expression and gene amplification is rarely detectable in
patients with oral squamous cell carcinomas. J Oral Pathol Med.
43:304–308. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yan M, Schwaederle M, Arguello D, Millis
SZ, Gatalica Z and Kurzrock R: HER2 expression status in diverse
cancers: Review of results from 37,992 patients. Cancer Metastasis
Rev. 34:157–164. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kondo N, Ishiguro Y, Kimura M, Sano D,
Fujita K, Sakakibara A, Taguchi T, Toth G, Matsuda H and Tsukuda M:
Antitumor effect of gefitinib on head and neck squamous cell
carcinoma enhanced by trastuzumab. Oncol Rep. 20:373–378.
2008.PubMed/NCBI
|
|
41
|
Kondo N, Tsukuda M, Sakakibara A,
Takahashi H, Hyakusoku H, Komatsu M, Niho T, Nakazaki K and Toth G:
Combined molecular targeted drug therapy for EGFR and HER-2 in head
and neck squamous cell carcinoma cell lines. Int J Oncol.
40:1805–1812. 2012.PubMed/NCBI View Article : Google Scholar
|