Introduction
Uric acid (UA) is the end product of purine
metabolism in the human body. The incidence of hyperuricemia is
gradually increasing worldwide as the result of various factors,
such as high-protein diets, environmental pollution and genetic
factors. Hyperuricemia is strongly associated with metabolic
syndrome (1), cardiovascular
diseases (2) and cerebrovascular
diseases (3). Obesity (4) and diabetes (5) can be independent risk factors for these
diseases. As an exogenous inflammatory stimulator, UA can cause a
systemic inflammatory response that ultimately upregulates
pro-inflammatory cytokines through activation of the MAPK signaling
pathway and the nuclear factor-κB (NF-κB) signal pathways. These,
in turn, contribute to vascular endothelial cell dysfunction and
the subsequent development of cardiovascular diseases (6).
Oxidative stress is a state in which reactive oxygen
species (ROS) are excessively produced and exceed the clearance
capacity of the body. In inflammation (7), toxicity (8), ischemia-reperfusion (9) and other pathological conditions,
several ROS are produced and the ability to scavenge ROS is
inhibited, leading to an imbalance between oxidation and
antioxidation (9,10). While NADPH oxidase-derived ROS are
essential for innate immunity and microbial killing, excess
production of ROS induces prolonged inflammatory reactions that
contribute to cellular dysfunction and inflammatory diseases
(7,10-12).
ROS can be generated in numerous ways, from a variety of molecules,
and participate in pathophysiological processes during disease
(7,9,10). A
previous study suggested that UA is one of the most important
antioxidants in circulation and endothelial cells can benefit from
its antioxidant effects (13).
However, a growing number of studies have demonstrated that the
antioxidant effects of UA are far less than its oxidant effects.
Thus, UA-activated oxidative stress can in turn exacerbate the
inflammatory response (6,14). UA-induced ROS derived from NADPH
oxidase can promote the phosphorylation of MAPK/ERK and PI3-K/AKT
and sequentially promote the downstream NF-κB signal pathway,
thereby enhancing inflammatory responses (15,16).
This pathological mechanism of action was previously identified in
endothelial cells (14), adipocytes
(17) and smooth muscle cells
(18). In addition, Sautin et
al (17) previously demonstrated
that inflammation induced by high levels of UA was associated with
oxidative stress.
NADPH oxidase consists of the cytosolic subunits
p47phox, p67phox, p40phox and Rac2, as well as transmembrane
subunits p22phox and gp91phox. The fundamental mechanism of NADPH
oxidase activation is through the transfer of p47phox to the
membrane, which drives other cytoplasmic subunits together and
transfers them towards the membrane to complete cell assembly.
Lipoxins are endogenously produced arachidonic acid
metabolites, which can act as potent anti-inflammatory agents that
suppress the expression of inflammation-related genes and attenuate
the activation of inflammatory cells (19). As such, lipoxins are known as the
‘brake signal’ of inflammation. A previous study suggested that the
overexpression of lipoxins is fundamental to the control of
inflammation in vivo (20). Lipoxin
A4 (LXA4), a subtype that best represents the biological activities
of lipoxins, restrains ROS generation to reduce the inflammatory
response and prevent damage to host cells (21,22).
However, to the best of the authors' knowledge, whether LXA4 can
inhibit UA-induced oxidative stress in human umbilical vein
endothelial cells (HUVECs) has not been previously reported.
Therefore, the aim of the present study was to
investigate the impact of LXA4 on the oxidative stress induced by
UA in HUVECs, as well as to examine the possible underlying
mechanisms in vitro. LXA4 inhibited the release of NADPH
oxidase-derived ROS in HUVECs stimulated by UA. A potential
mechanism of action underlying this effect could be LXA4-mediated
suppression of NADPH oxidase activity, leading to inhibition of
p47phox translocation from the cytoplasm to the cell membrane.
Materials and methods
Cell culture and treatment
The HUVEC line was purchased from The American Type
Culture Collection and maintained in RPMI-1640 complete medium
(Hyclone; GE Healthcare Life Sciences) supplemented with 0.4 µl/ml
vascular endothelial growth factor, 100 U/ml penicillin, 100 U/ml
streptomycin and 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences) at 37˚C in a humidified atmosphere of 5%
CO2 and 95% atmospheric air. HUVECs were digested with
0.25% trypsin when cells presented their typical morphology (a
paving stone shape, clear nucleus, clear membrane and no obvious
antennae) and reseeded for further growth. Cells in logarithmic
phase were used for experiments. HUVECs were seeded into 6-well
plates at a density of 2.5x105 cells/well, then
serum-starved for 12 h before further experiments.
HUVECs were incubated for 12 h in the initial
experiments with varying concentrations of UA (Sigma-Aldrich; Merck
KGaA; 0, 6, 12 or 16 mg/dl) or incubated with 12 mg/dl UA for
varying time periods (0, 3, 6, 12, 24 or 48 h). Appropriate
concentrations of UA were used for further experiments. Similar to
the aforementioned procedure, HUVECs were pre-treated with various
concentrations of LXA4 (Cayman Chemical Company; 0, 1, 10 or 100
nM) and time periods (0, 15, 30, 60 and 120 min). A concentration
of 100 nM LXA4 was used for further experiments for 1 h.
Furthermore, cells were pre-treated for the same time period when
pre-treated with diphenyleneiodonium chloride (DPI; 10 µM; Cayman
Chemical Company), indomethacin (3 mM; Sigma-Aldrich, Merck KGaA)
and rotenone (1 µM; Sigma-Aldrich; Merck KGaA) with LXA4 before the
addition of UA under serum-free conditions.
Measurement of intracellular ROS
Total intracellular ROS in HUVECs was determined
using the peroxide-sensitive probe 2',7'-dichlorodihydrofluorescein
diacetate (DCFH2-DA; Sigma-Aldrich; Merck KGaA) dye as
previously described (23). The
non-fluorescent form of DCFH2-DA is oxidized by intracellular ROS
and forms the highly fluorescent form of DCFH2-DA. The intensity of
fluorescence is proportional to the levels of intracellular ROS.
HUVECs were cultured and treated according to grouping
requirements, and subsequently were washed with PBS and incubated
for 30 min with DCFH2-DA at a final concentration of 10 µmol/l.
Fluorescence was then measured using a fluorometer (Thermo Fisher
Scientific, Inc.) with excitation and emission wavelengths of 480
and 520 nm, respectively. Images were captured using a Nikon
fluorescence microscope (magnification, x40; Nikon
Corporation).
Measurement of NADPH oxidase
activity
The activity of NADPH oxidase was measured using
lucigenin-enhanced chemiluminescence. NADPH oxidase was activated
to transfer electrons from NADPH to O2, forming
O2-. Lucigenin is luminescent when
interacting with O2-, with the amount of
luminescent electrons indirectly representing the activity of NADPH
oxidase, as described previously (24). HUVECs were treated as described, then
washed three times with ice-cold PBS (pH 7.4) and centrifuged at
2,000 x g for 5 min at 4˚C. The pellet was resuspended in cell
lysis buffer (20 µM K2HPO4; 1 mM EDTA; 1 mM
PMSF; 10 µg/ml aprotinin). Cells were lysed and the collected
proteins were resuspended to a final concentration of 1 mg/ml. A
total of 100 µl of the samples were mixed with 900 µl reaction
buffer (50 nM K2HPO4; 1 nM EDTA; 150 µM
sucrose; 5 µM lucigenin; 100 µM NADPH). A chemiluminescence
analyzer (Thermo Fisher Scientific, Inc.) gathered photon emission
every 60 sec for 20 min, with a 5-sec signal integration time.
p47phox small interfering (si)RNA and
p22phox siRNA transfection
Transfection of siRNA into HUVECs was performed as
previously described (25). The
siRNAs (100 nM; Sangon Biotech Co, Ltd.) used in the present study
were: p47phox siRNA, 5'-GGACCCAGAACCCAACUAUGCAGGT-3' and
5'-ACCUGCAUAGUUGGGUUCUGGGUCCUC-3'; p22phox siRNA,
5'-GAAGGGCUCCACCAUGGAGTT-3' and 5'-UCCAUGGUGGAGCCCUUCTT-3';
siRNA-scramble, 5'-GCUGCAGTAUGAGGAG-3' and 5'-CGACGC
CATUCCGTAGC-3'. Transfections were carried out using
Lipofectamine® 2000 transfection reagent (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol, where
subsequent experimentation was performed 48 h after transfection.
The mRNA expression of p47phox and p22phox in transfected cells was
determined by reverse transcription-quantitative PCR (RT-qPCR).
RT-qPCR
Total RNA was extracted from HUVECs using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.)
according to manufacturer's protocol, cDNA was synthesized from
total RNA by Transcriptor First Strand cDNA Synthesis kit (Roche
Diagnostics). The temperature protocol was as follows: 10 min at
25˚C, followed by 55˚C for 30 min. The expression of p47phox
(forward, 5'-ACGAGAGTGGTTGGTGGTTC-3' and reverse,
5'-TGTAGGCTTTGATGGTGACG-3') and p22phox (forward,
5'-TGCTTGTGGGTAAACCAAGGCCGGTG-3' and reverse,
5'-AACACTGAGGTAAGTGGGGGTGGCTCCTGT-3') were measured using the 2X
SYBR-Green Abstart Mix kit (cat. no. B110031; Sangon Biotech Co,
Ltd.). GADPH (forward, 5'-GAAGGTGAAGGTCGGAGTC-3' and reverse,
5'-GAAGATGGTGATGGGATTTC-3') as endogenous control. The qPCR
reaction system consisted of 10 µl SYBR® Green Master
Mix, 0.5 µl upstream and downstream primers, 1 µl cDNA and 8 µl
ddH2O. The thermocycling conditions were as follows:
Initial denaturation at 95˚C for 3 min, followed by 40 cycles of
95˚C for 30 sec and 58˚C for 1 min. The relative expression of
p47phox RNA and p22phox RNA were evaluated using
2-∆∆Cq method (26). GADPH was used as a control for
normalization.
Cell membrane and cytoplasm
fractionation
HUVECs were cultured and treated as required and
subsequently washed three times with ice-cold PBS (pH 7.4). The
cells were lysed in RIPA Lysis buffer (Beyotime Institute of
Biotechnology) supplemented with protease inhibitor cocktail
(Beyotime Institute of Biotechnology). Membrane and cytoplasmic
proteins were extracted using a Membrane and Cytosol Protein
Extraction kit (Beyotime Institute of Biotechnology) according to
the manufacturer's protocol.
Western blot analysis
The concentration of cytoplasmic and membrane
proteins extracted from HUVECs was determined using a bicinchoninic
acid protein assay kit (Beyotime Institute of Biotechnology) and
concentrations were normalized to be electrophoresed. Equivalent
masses (5 µg/ml) of protein were electrophoresed on 10% SDS
polyacrylamide gels and transferred onto polyvinylidene fluoride
membranes (Beyotime Institute of Biotechnology). The transformed
membrane was blocked using 5% non-fat milk at 4˚C for 1 h and
incubated with primary antibodies against p47phox (cat. no.
ab166930; 1:500; Abcam), GAPDH (cat. no. AG019; 1:500; Beyotime
Institute of Biotechnology) and Caveolin-1 (cat. no. AF0087; 1:500;
Beyotime Institute of Biotechnology) at 4˚C overnight. The
hybridized membrane was washed three times with Tris-buffered
saline containing 0.05% Tween-20 (TBST) for 10 min and subsequently
incubated with horseradish peroxidase-conjugated goat anti-mouse
IgG (cat. no. A0216; 1:5,000; Beyotime Institute of Biotechnology)
at 4˚C for 2 h. The membrane was washed three times with TBST and
the signal was detected using Beyo ECL Plus kit (Beyotime Institute
of Biotechnology), densitometric analysis was performed using
Image-Pro Plus V software (v7.0; Media cybernetics, Inc.).
Statistical analysis
The SPSS v13.0 statistical software package (SPSS,
Inc.) was used for statistical analysis. The results are presented
as the mean ± SD. Multigroup comparisons of the means were carried
out by ANOVA, followed by post hoc correction with Tukey's test.
All experiments were repeated three times. P<0.05 was considered
to indicate a statistically significant difference.
Results
UA induces oxidative stress and ROS
production by HUVECs, which are reversed by LXA4
UA stimulated HUVECs were treated in a time and
concentration-dependent manner to produce ROS. The relative
dichlorofluorescein (DCF) fluorescence increased with time and
concentration. The maximum signal was reached at 24 h and with 12
mg/dl UA (Fig. 1). In order to
determine the effect of LXA4 on ROS generation, HUVECs were
incubated with LXA4 for different periods of time and
concentrations, then treated with UA. The inhibitory effect of LXA4
was observed after 15 min of pre-incubation and the influence
remained significant, with a maximal inhibition reached at 1 h.
Pre-incubation with varying concentrations of LXA4 caused a
significant reduction in UA-induced ROS generation and a maximum
effect was observed for 100 nM (Fig.
1).
 | Figure 1UA-induced oxidative stress induces
ROS production and LXA4 affects this response. (A) ROS production
was detected in HUVECs following treatment with different
concentrations of UA (0, 4, 8, 12 and 16 mg/dl). n=3. (B) ROS
production was detected in HUVECs following treatment with UA for
varying time periods (0, 3, 6, 12, 24 and 48 h). n=3. (C) ROS were
detected in HUVECs incubated with various concentrations of LXA4
(0, 1, 10 and 100 nM) for 1 h following treatment with 12 mg/dl UA
for 24 h. The control group was treated with normal saline for 24
h. n=3. (D) ROS were detected in HUVECs incubated with LXA4 for
various time periods (0, 15, 30, 60 and 120 min) following
treatment with UA. n=3. *P<0.05 vs. 0 mg/dl;
&P<0.05 vs. 0 h; #P<0.05 vs.
treatment with UA alone; ^P<0.05 vs. 0 min. HUVEC,
human umbilical vein endothelial cell; ROS, reactive oxidative
species; UA, uric acid; LXA4, lipoxin A4; DCF,
dichlorofluorescein. |
LXA4 suppresses UA-induced increase in
generation of ROS in a NADPH oxidase-dependent manner
The present study adopted various inhibitors which
altered the basal superoxide generation when pre-incubated with
HUVECs to determine whether the inhibitory effect of LXA4 on ROS
generation was NADPH oxidase dependent. HUVECs were incubated with
specific inhibitors for cyclooxygenase (indomethacin), mitochondria
complex I (rotenone), NADPH oxidase (DPI) and LXA4, results
demonstrated that indomethacin and rotenone had a marginal
inhibitory effect on ROS generation, although this was not
significant. However, DPI abrogated the UA-induced increase in
generation of ROS to the same extent as LXA4. This suggested that
NADPH oxidase was a major source for ROS production during
UA-induced oxidative stress and LXA4 downregulated ROS generation
in HUVECs (Fig. 2).
 | Figure 2Effect of various inhibitors on the
generation of ROS induced by UA in HUVECs. ROS levels were measured
in HUVECs incubated with Ind, Rot, DPI or LXA4 following treatment
with UA. Data are presented as the mean ± SD. n=3.
&P<0.05 vs. control; *P<0.05 vs. UA
treatment. Magnification, x40. HUVEC, human umbilical vein
endothelial cell; ROS, reactive oxidative species; UA, uric acid;
LXA4, lipoxin A4; Ind, indomethacin; Rot, Rotenone; DPI,
diphenyleneiodium chloride; DCF, dichlorofluorescein. |
LXA4 suppresses UA-induced activation
of NADPH oxidase
Having demonstrated that NADPH oxidase was a major
source for ROS in the oxidative stress response, NADPH oxidase
activity was then measured. Cells were incubated with 100 nM LXA4
for 1 h then with UA (12 mg/dl) for 12 h, and subsequently the
NADPH oxidase activity was measured. Compared with the control
group, NADPH oxidase activity in HUVECs significantly increased
following UA treatment, consistent with the ROS generation.
However, when cells were incubated with LXA4 before UA stimulation,
a significant reduction in NADPH oxidase activity was observed,
compared with that in the UA group (Fig.
3). Thus, LXA4 pre-treatment inhibits NADPH oxidase activity
following UA stimulation.
Transfection of p47phox siRNA
attenuates UA-induced increase generation of ROS and activation of
NADPH oxidase
The mRNA expression levels of p47phox and p22phox
were examined by RT-qPCR in transfected HUVECs. The levels of
p47phox and p22phox mRNA in HUVECs transfected with p47phox or
p22phox siRNA were significantly lower than the blank group and the
scrambled group (Fig. 4).
Activated NADPH oxidase is a membrane-bound enzyme,
which depends on the translocation of activated cytoplasmic
subunits to the membrane to form the functional complex (26). The cytoplasmic subunit p47phox plays
a crucial role in NADPH oxidase activation (27). To ascertain that p47phox is required
for the UA-induced oxidative stress response in HUVECs, cells were
transfected with p47phox siRNA before exposure to UA (12 mg/dl) for
12 h. Subsequently, ROS generation and NADPH oxidase activity was
measured. UA-induced ROS generation and NADPH oxidase activity were
both significantly reduced in HUVECs transfected with p47phox
siRNA. It was hypothesized that LXA4 suppression of the activation
of NADPH oxidase may be interfered with p47phox (Fig. 5).
Transfer of p47phox to the cell
membrane to activate NADPH oxidase
No apparent change in the total protein expression
levels of p47phox was observed in HUVECs in the presence or absence
of UA (Fig. 6A). In addition, a
previous study demonstrated that p47phox was transferred from the
cytoplasm to the membrane and associated with p22phox to activate
NADPH oxidase (28). Thus, it was
hypothesized an analogous mechanism allowed p47phox to bind to
p22phox in HUVECs stimulated with UA. Cells were transfected with
p22phox siRNA followed by addition of UA (12 mg/dl) for 12 h.
Membrane proteins were extracted and p47phox was detected using
western blotting. UA-stimulation demonstrated a marked elevation in
the expression of p47phox on the membrane of HUVECs. Cells
transfected with p22phox siRNA showed a significant reduction in
the expression levels of p47phox on the membrane compared with
cells treated with only UA. These findings suggested that UA
activated NADPH oxidase by promoting the transfer of p47phox to the
membrane, rather than by modulating the expression levels of
p47phox (Fig. 6B).
LXA4 inhibits NADPH oxidase activity
by preventing p47phox translocation
Since UA activated NADPH oxidase by promoting the
transfer of p47phox from the cytoplasm to the membrane, the present
study aimed to determine whether LXA4 suppressed NADPH oxidase
activation by preventing the translocation of p47phox in HUVECs
treated with UA. Cells were incubated with 100 nM LXA4 for 1 h,
then with UA (12 mg/dl) for 24 h. The cytoplasmic and membrane
proteins were extracted and p47phox expression levels were measured
using western blotting. The expression levels of p47phox in the
cytoplasm significantly decreased in HUVECs stimulated by UA,
compared with that of the control cells. However, in cells
pre-incubated with LXA4, translocation of p47phox from the
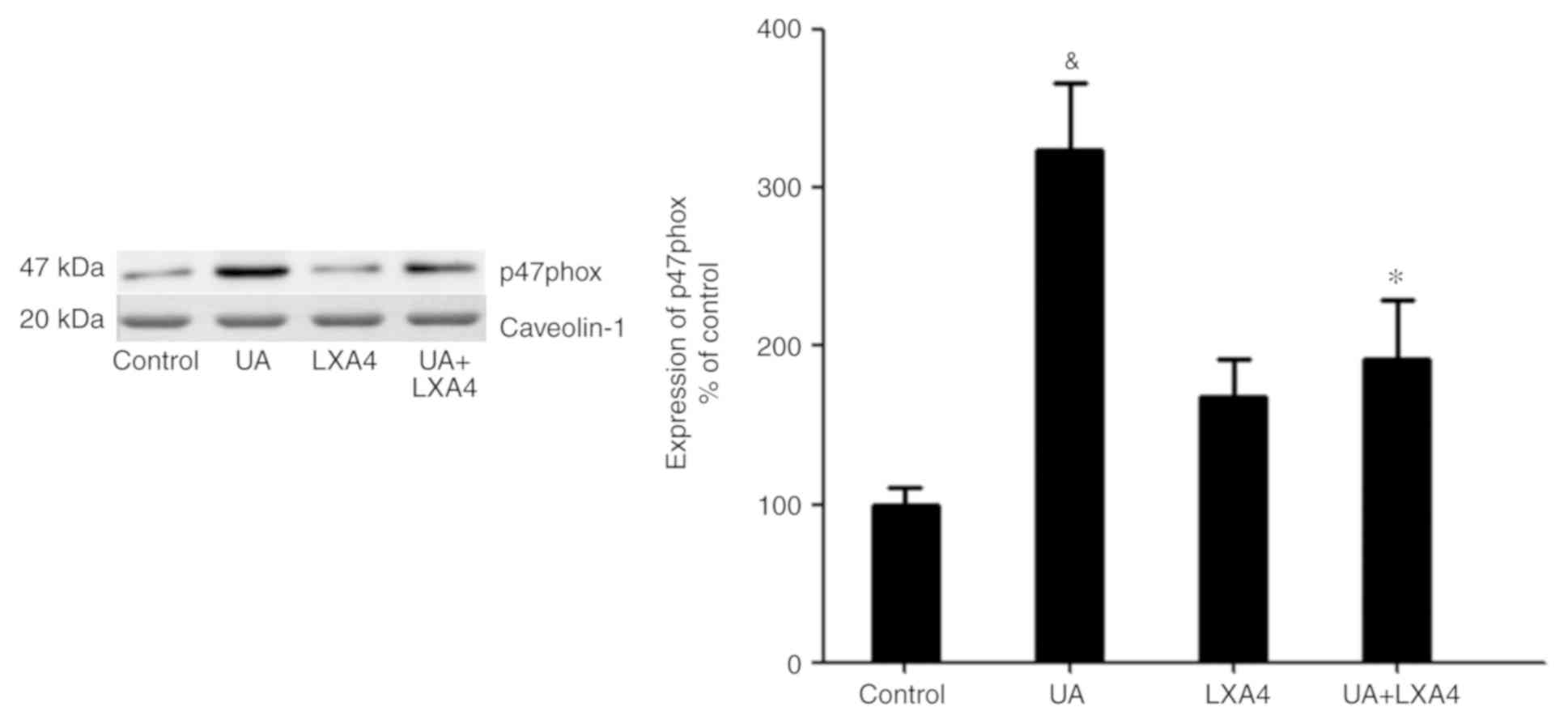
cytoplasm was significantly reduced (Fig. 7). Notably, UA treatment increased the
levels of p47phox on the membrane and LXA4 interrupted this trend
in HUVECs (Fig. 8). These results
suggested that LXA4 inhibited the translocation of p47phox from the
cytoplasm to the membrane, thereby suppressing NADPH oxidase
activity in HUVECs following UA stimulation.
Discussion
In the present study, the impact of LXA4 on
oxidative stress induced by UA in HUVECs and the potential
mechanism of action underlying the regulation of NADPH oxidase were
determined. The primary finding of the present study was that
UA-stimulated HUVECs exhibited greater oxidative stress, which
contributed to the generation of intracellular ROS, and treatment
with LXA4 could protect against this response. It was demonstrated
that NADPH oxidase was the dominant source of ROS following
treatment with UA in HUVECs. Moreover, LXA4 suppressed the increase
in the generation of ROS and this was achieved in an NADPH
oxidase-dependent manner. The mechanism of action underlying
LXA4-mediated inhibition of NADPH oxidase activation was
investigated. It was found that the cytoplasmic subunits of p47phox
translocated to the membrane, which resulted in the activation of
NADPH oxidase. Furthermore, NADPH oxidase activity was suppressed
as result of LXA4 inhibiting the translocation of p47phox. These
results provided a mechanistic link between UA and the antioxidant
effects of LXA4 in HUVECs.
A previous study demonstrated that UA exerted an
antioxidant effect on endothelial cells (13). However, a number of previous studies
also suggested that the oxidant properties of UA far exceeded its
antioxidant effects in endothelial cells (14), adipocytes (17) and smooth muscle cells (18). Previous studies have demonstrated
that high serum levels of UA in vivo for extended periods
positively correlated with the incidence of metabolic (1,29),
cerebrovascular (3,30) and cardiovascular diseases (2,31), owing
to endothelial cell dysfunction and pathological vascular
remodeling. A recent study also indicated that prolonged
hyperuricemia could reduce peroxide stress and inflammation in body
(32). In the present study, UA
significantly increased ROS generation in HUVECs, suggesting that
UA could promote oxidative stress in these cells. Oxidative stress
is involved in several pathophysiological processes (7-10).
This is especially the case in inflammatory responses, in which
oxidative stress-derived ROS can activate signaling pathways, such
as the MAPK/ERK, PI3-K/AKT and NF-κB pathways to upregulate the
transcription of genes related to inflammation (10,15,16,33).
Thus, the present study aimed to investigate the mechanism of
oxidative stress and its prevention, and to identify potential
therapeutic targets for inflammatory diseases.
LXA4 is an endogenous anti-inflammatory protein and
acts as a ‘brake signal’ for inflammation (19). A variety of inflammation-related
genes and inflammatory cytokines, such as tumor necrosis factor-α
(TNF-α), interleukin (IL)-β and IL-6 are downregulated by
LXA4(34). Li et al (35), reported that LXA4 inhibited the
LPS-induced expression of monocyte chemoattractant protein and
macrophage colony-stimulating factor in hepatocellular carcinoma
cells by switching off the ROS, MAPK and NF-κB pathways and
controlling the extent of oxidative stress. Previous studies
suggested that LXA4 protected endothelial cells from inflammatory
damage, which was associated with the suppression of oxidative
stress (22). It is evident from the
present study that LXA4 suppressed the generation of ROS induced by
UA in HUVECs. ROS have extensive sources in mammalian cells, such
as the mitochondrial respiratory chain (36), cyclooxygenases (37) and NADPH oxidase (30,37). The
present study examined different inhibitors for mitochondria,
cyclooxygenase and NADPH oxidase to explore the main source for ROS
in this model. Several inhibitors decreased ROS production, but
only to a limited extent. However, both the NADPH oxidase inhibitor
DPI and LXA4 significantly decreased ROS production. This suggested
that NADPH oxidase was the main source of ROS in this model.
NADPH oxidase is a complex regulator of oxidative
stress that is highly expressed in endothelial cells, vascular
smooth muscle cells and kidney cells (26). Activated NADPH oxidase provides an
electron to O2, creating O2-.
Superoxide dismutase catalyzes the conversion of the high
concentration OO2-, generating
H2O2. H2O2 eventually
converts to further ROS (26). In
tissues, the excessive production of NADPH oxidase-derived ROS
induces tissue inflammation and fibrosis, leading to stress damage
(22). NADPH oxidase embedded on the
vascular endothelial cell membrane is activated to generate ROS,
which triggers multiple pathways downstream of ERK, including
generation of NADPH oxidase-derived ROS, transfer of
transcriptional activator protein AP-1 to the nucleus and reduction
of the levels of the inflammatory cytokine, IL-10(38). Another previous study in BV2
microglia cells suggested that LXA4 acted as an NADPH oxidase
inhibitor, which prevented the production of intracellular ROS
(39). The present study identified
that LXA4 significantly suppressed UA-stimulated NADPH oxidase
activity in HUVECs, further strengthening the evidence that LXA4
inhibits ROS in an NADPH oxidase-dependent response.
NADPH oxidase is a multicomponent enzyme system that
be activated through the assembly of its cytosolic subunits,
p47phox, p67phox, p40phox and Rac2 with the transmembrane subunits
p22phox and gp91phox (26). p47phox
is considered to be the principal subunit for NADPH oxidase.
p47phox transfers to the membrane, and drives other cytoplasmic
subunits together and promotes their translocation to the membrane
to finish assembly of NADPH oxidase (38,40).
Recently, a study have investigated the function of p47phox and the
role of NADPH oxidase activation, and it has been identified that
ROS generation depends on p47phox regulating the nuclear
translocation of FOXO in ischemia cardiac cells. Targeted silencing
by p47phox siRNA mitigates this domino effect (41). In p47phox (-/-) murine endothelial
cells, NADPH oxidase-derived ROS production was largely suppressed
(42). In addition, the p47phox is
predominantly involved in the activation of other NADPH oxidase
subunits (26). It was hypothesized
that LXA4 may suppress the activation of NADPH oxidase through
interactions with p47phox. The present study demonstrated that
transfection of p47phox siRNA attenuated UA-induced increases in
the generation of ROS and the activation of NADPH oxidase. p47phox
translocates to the membrane following binding with the membrane
subunit p22phox (28). p22phox has a
terminal proline-rich tail, which could be used to bind with the
cytoplasmic subunits and co-immunoprecipitation assays have
demonstrated that p47phox binds with p22phox on the membrane in
activated endothelial cells (28,43). In
the present study, since UA failed to raise the total protein
expression levels of p47phox, it was hypothesized that UA might
promote the translocation of p47phox to the membrane from the
cytoplasm to activate NADPH oxidase. Moreover, p22phox siRNA was
transfected into HUVECs prior to treatment with UA. The expression
of p47phox on the membrane significantly decreased in cells
transfected with p22phox siRNA. Binding between p22phox and p47phox
anchors p47phox at the cell membrane (43). Therefore, it was hypothesized that UA
activates NADPH oxidase by promoting the transfer of p47phox to the
membrane rather than through the upregulation of p47phox.
Whether LXA4 interfered with the binding of p22phox
with the translocated p47phox to suppress UA-induced activation of
NADPH oxidase in HUVECs is unclear. Several factors can attenuate
NADPH oxidase activity by preventing the assembly of NADPH oxidase,
including its p47phox and p22phox subunits (44). Carlo et al (45), demonstrated that the number of
complexes of p47phox-p22phox were significantly decreased in
activated neutrophils treatment with 15-Epi-lipoxin A4, and
suggested that the assembly between p47phox and p22phox might be an
effective target for the inhibition of NADPH oxidase activity. In
the present study, the expressions of p47phox on the cytoplasm and
the membrane were detected, demonstrating that the levels of
p47phox significantly decreased in the cytoplasm and significantly
increased on the membrane of HUVECs stimulated by UA. In addition,
LXA4 could reverse this alteration. It was observed that LXA4 was
able to indirectly interfere with the translocation of p47phox from
the cytoplasm to the membrane. This mechanism may lead to
suppression of NADPH oxidase activation in HUVECs following UA
stimulation.
In conclusion, the results of the present study
suggested that LXA4 inhibited the UA-induced release of NADPH
oxidase-derived ROS in HUVECs. A possible mechanism of action
behind this effect involved the suppression NADPH oxidase by
preventing the translocation of p47phox from the cytoplasm to the
membrane (Fig. 9). Considering the
presence of oxidative stress and inflammatory responses downstream
in patients with hyperuricemia, the assembly of NADPH oxidase may
be considered as a potential therapeutic target to treat
hyperuricemia or related diseases and LXA4 may be a therapeutic
agent to achieve this effect. However, the present study has
limitations in that in vitro experiments were used, which could not
fully replicate the internal environment of human tissues. Further
research is necessary to follow-up these studies in vivo to
investigate the relevance of the observations of the present study
for therapeutic intervention.
Acknowledgements
Not applicable.
Funding
The present was supported by The Research Project of
Sichuan Provincial Health Planning Commission (grant no.
17PJ067).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ was a major contributor in writing the
manuscript, performed all experiments and data analysis. HY and AZ
performed cell culture and the western blot analyses. XJ designed
the present study. ZP, GX and MZ performed data analysis. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu CW, Chen KH, Tseng CK, Chang WC, Wu YW
and Hwang JJ: The dose-response effects of uric acid on the
prevalence of metabolic syndrome and electrocardiographic left
ventricular hypertrophy in healthy individuals. Nutr Metab
Cardiovasc Dis. 29:30–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Puddu P, Puddu GM, Cravero E, Vizioli L
and Muscari A: Relationships among hyperuricemia, endothelial
dysfunction and cardiovascular disease: Molecular mechanisms and
clinical implications. J Cardiol. 59:235–242. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li M, Hou W, Zhang X, Hu L and Tang Z:
Hyperuricemia and risk of stroke: A systematic review and
meta-analysis of prospective studies. Atherosclerosis. 232:265–270.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ogura T, Matsuura K, Matsumoto Y, Mimura
Y, Kishida M, Otsuka F and Tobe K: Recent trends of hyperuricemia
and obesity in Japanese male adolescents, 1991 through 2002.
Metabolism. 53:448–453. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mortada I: Hyperuricemia, type 2 diabetes
mellitus, and hypertension: An emerging association. Curr Hypertens
Rep. 19(69)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mishima M, Hamada T, Maharani N, Ikeda N,
Onohara T, Notsu T, Ninomiya H, Miyazaki S, Mizuta E, Sugihara S,
et al: Effects of uric acid on the NO production of HUVECs and its
restoration by urate lowering agents. Drug Res (Stuttg).
66:270–274. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lepetsos P and Papavassiliou AG:
ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys
Acta. 1862:576–591. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rasheed NO, Ahmed LA, Abdallah DM and
El-Sayeh BM: Nephro-toxic effects of intraperitoneally injected
EGCG in diabetic mice: Involvement of oxidative stress,
inflammation and apoptosis. Sci Rep. 7(40617)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Minutoli L, Puzzolo D, Rinaldi M, Irrera
N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, et
al: ROS-mediated NLRP3 inflammasome activation in brain, heart,
kidney, and testis ischemia/reperfusion injury. Oxid Med Cell
Longev. 2016(2183026)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kadowaki D, Sakaguchi S, Miyamoto Y,
Taguchi K, Muraya N, Narita Y, Sato K, Chuang VT, Maruyama T,
Otagiri M, et al: Direct radical scavenging activity of
benzbromarone provides beneficial antioxidant properties for
hyperuricemia treatment. Biol Pharm Bull. 38:487–492.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matsuzawa A, Saegusa K, Noguchi T,
Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K
and Ichijo H: ROS-dependent activation of the TRAF6-ASK1-p38
pathway is selectively required for TLR4-mediated innate immunity.
Nat Immunol. 6:587–592. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Chen Z, Zhou Q, Zou D, Tian Y, Liu B,
Zhang Y and Wu Z: Chloro-benzoquinones cause oxidative DNA damage
through iron-mediated ROS production in Escherichia coli.
Chemosphere. 135:379–386. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kuzkaya N, Weissmann N, Harrison DG and
Dikalov S: Interactions of peroxynitrite with uric acid in the
presence of ascorbate and thiols: Implications for uncoupling
endothelial nitric oxide synthase. Biochem Pharmacol. 70:343–354.
2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xie H, Sun J, Chen Y, Zong M, Li S and
Wang Y: EGCG attenuates uric acid-induced inflammatory and
oxidative stress responses by medicating the NOTCH pathway. Oxid
Med Cell Longev. 2015(214836)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Z, Sheng Y, Liu C, Li K, Huang X, Huang
J and Xu K: Nox4 has a crucial role in uric acid-induced oxidative
stress and apoptosis in renal tubular cells. Mol Med Rep.
13:4343–4348. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ives A, Nomura J, Martinon F, Roger T,
LeRoy D, Miner JN, Simon G, Busso N and So A: Xanthine
oxidoreductase regulates macrophage IL1β secretion upon NLRP3
inflammasome activation. Nat Commun. 6(6555)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sautin YY, Nakagawa T, Zharikov S and
Johnson RJ: Adverse effects of the classic antioxidant uric acid in
adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am
J Physiol Cell Physiol. 293:C584–C596. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tang L, Xu Y, Wei Y and He X: Uric acid
induces the expression of TNF-α via the ROS-MAPK-NF-κB signaling
pathway in rat vascular smooth muscle cells. Mol Med Rep.
16:6928–6933. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chandrasekharan JA and Sharma-Walia N:
Lipoxins: Nature's way to resolve inflammation. J Inflamm Res.
8:181–192. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Weiss GA, Troxler H, Klinke G, Rogler D,
Braegger C and Hersberger M: High levels of anti-inflammatory and
pro-resolving lipid mediators lipoxins and resolvins and declining
docosahexaenoic acid levels in human milk during the first month of
lactation. Lipids Health Dis. 12(89)2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu C, Guan H, Cai C, Li F and Xiao J:
Lipoxin A4 suppresses osteoclastogenesis in RAW264.7 cells and
prevents ovariectomy-induced bone loss. Exp Cell Res. 352:293–303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nascimento-Silva V, Arruda MA,
Barja-Fidalgo C and Fierro IM: Aspirin-triggered lipoxin A4 blocks
reactive oxygen species generation in endothelial cells: A novel
antioxidative mechanism. Thromb Haemost. 97:88–98. 2007.PubMed/NCBI
|
|
23
|
Peshavariya HM, Taylor CJ, Goh C, Liu GS,
Jiang F, Chan EC and Dusting GJ: Annexin peptide Ac2-26 suppresses
TNFα-induced inflammatory responses via inhibition of
Rac1-dependent NADPH oxidase in human endothelial cells. PLoS One.
8(e60790)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Griendling KK, Minieri CA, Ollerenshaw JD
and Alexander RW: Angiotensin II stimulates NADH and NADPH oxidase
activity in cultured vascular smooth muscle cells. Circ Res.
74:1141–1148. 1994.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maeda M, Hayashi T, Mizuno N, Hattori Y
and Kuzuya M: Intermittent high glucose implements stress-induced
senescence in human vascular endothelial cells: Role of superoxide
production by NADPH oxidase. PLoS One. 10(e0123169)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Δ Δ C(T)) Method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jaquet V, Scapozza L, Clark RA, Krause KH
and Lambeth JD: Small-molecule NOX inhibitors: ROS-generating NADPH
oxidases as therapeutic targets. Antioxid Redox Signal.
11:2535–2552. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Belambri SA, Rolas L, Raad H,
Hurtado-Nedelec M, Dang PM and El-Benna J: NADPH oxidase activation
in neutrophils: Role of the phosphorylation of its subunits. Eur J
Clin Invest. 48 (Suppl 2)(e12951)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mohandas R, Sautina L, Beem E, Schuler A,
Chan WY, Domsic J, McKenna R, Johnson RJ and Segal MS: Uric acid
inhibition of dipeptidyl peptidase IV in vitro is dependent on the
intracellular formation of triuret. Exp Cell Res. 326:136–142.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Song C and Zhao X: Uric acid promotes
oxidative stress and enhances vascular endothelial cell apoptosis
in rats with middle cerebral artery occlusion. Biosci Rep.
38(BSR20170939)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Choi YJ, Yoon Y, Lee KY, Hien TT, Kang KW,
Kim KC, Lee J, Lee MY, Lee SM, Kang DH, et al: Uric acid induces
endothelial dysfunction by vascular insulin resistance associated
with the impairment of nitric oxide synthesis. FASEB J.
28:3197–3204. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou Y, Zhao M, Pu Z, Xu G and Li X:
Relationship between oxidative stress and inflammation in
hyperuricemia: Analysis based on asymptomatic young patients with
primary hyperuricemia. Medicine (Baltimore).
97(e13108)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xia F, Wang C, Jin Y, Liu Q, Meng Q, Liu K
and Sun H: Luteolin protects HUVECs from TNF-α-induced oxidative
stress and inflammation via its effects on the Nox4/ROS-NF-κB and
MAPK pathways. J Atheroscler Thromb. 21:768–783. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kantarci A, Aytan N, Palaska I, Stephens
D, Crabtree L, Benincasa C, Jenkins BG, Carreras I and Dedeoglu A:
Combined administration of resolvin E1 and lipoxin A4 resolves
inflammation in a murine model of Alzheimer's disease. Exp Neurol.
300:111–120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Y, Cai L, Wang H, Wu P, Gu W, Chen Y,
Hao H, Tang K, Yi P, Liu M, et al: Pleiotropic regulation of
macrophage polarization and tumorigenesis by formyl peptide
receptor-2. Oncogene. 30:3887–3899. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Biala AK, Dhingra R and Kirshenbaum LA:
Mitochondrial dynamics: Orchestrating the journey to advanced age.
J Mol Cell Cardiol. 83:37–43. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Uddin MJ, Werfel TA, Crews BC, Gupta MK,
Kavanaugh TE, Kingsley PJ, Boyd K, Marnett LJ and Duvall CL:
Fluorocoxib A loaded nanoparticles enable targeted visualization of
cyclooxygenase-2 in inflammation and cancer. Biomaterials.
92:71–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lian S, Xia Y, Khoi PN, Ung TT, Yoon HJ,
Kim NH, Kim KK and Jung YD: Cadmium induces matrix
metalloproteinase-9 expression via ROS-dependent EGFR, NF-кB, and
AP-1 pathways in human endothelial cells. Toxicology. 338:104–116.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang YP, Wu Y, Li LY, Zheng J, Liu RG,
Zhou JP, Yuan SY, Shang Y and Yao SL: Aspirin-triggered lipoxin A4
attenuates LPS-induced pro-inflammatory responses by inhibiting
activation of NF-κB and MAPKs in BV-2 microglial cells. J
Neuroinflammation. 8(95)2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Park IH, Hwang HM, Jeon BH, Kwon HJ, Hoe
KL, Kim YM and Ryoo S: NADPH oxidase activation contributes to
native low-density lipoprotein-induced proliferation of human
aortic smooth muscle cells. Exp Mol Med. 47(e168)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ter Horst EN, Hahn NE, Geerts D, Musters
RJP, Paulus WJ, van Rossum AC, Meischl C, Piek JJ, Niessen HWM and
Krijnen PAJ: p47phox-dependent reactive oxygen species stimulate
nuclear translocation of the foxO1 transcription factor during
netabolic inhibition in cardiomyoblasts. Cell Biochem Biophys.
76:401–410. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen JX, Zeng H, Lawrence ML, Blackwell TS
and Meyrick B: Angiopoietin-1-induced angiogenesis is modulated by
endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol.
291:H1563–H1572. 2006.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chakraborti S, Sarkar J and Chakraborti T:
Role of PLD-PKCζ signaling axis in p47phox phosphorylation for
activation of NADPH oxidase by angiotensin II in pulmonary artery
smooth muscle cells. Cell Biol Int. 43:678–694. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Macías Pérez ME, Hernández Rodríguez M,
Cabrera Pérez LC, Fragoso-Vázquez MJ, Correa-Basurto J,
Padilla-Martínez II, Méndez Luna D, Mera Jiménez E, Flores Sandoval
C, Tamay Cach F, et al: Aromatic regions govern the recognition of
NADPH oxidase inhibitors as diapocynin and its analogues. Arch
Pharm (Weinheim). 350(1700041)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Carlo T, Kalwa H and Levy BD:
15-Epi-lipoxin A4 inhibits human neutrophil superoxide anion
generation by regulating polyisoprenyl diphosphate phosphatase 1.
FASEB J. 27:2733–2741. 2013.PubMed/NCBI View Article : Google Scholar
|