Introduction
Percutaneous catheter drainage (PCD) is an integral
part of the management of necrotic pancreatic collections in the
setting of acute pancreatitis (AP). The PANTER trial demonstrated
that a minimally invasive step-up approach utilizing percutaneous
catheter followed by minimally invasive retroperitoneal
necrosectomy reduced the rate of major complications as well as
mortality when compared to open necrosectomy (1). Among the patients assigned to the
step-up protocol, 35% of patients were able to be treated with
catheter drainage alone. Certain studies have reported a success
rate of PCD alone of as high as 50-60% (2-4). In
addition, a proactive or aggressive protocol of PCD is associated
with better outcomes (5-8).
The presence of solid, necrotic contents within the collection, as
suggested by a higher mean CT density of collection, is associated
with a lower success of PCD (9-11).
The present study proposed a modification of the PCD technique, the
‘kissing catheter’ technique, to achieve successful drainage of
these collections. This technique involves placing two catheters
side-by-side through a single cutaneous entry site. The aim is to
allow aggressive flushing (through one catheter) and aspiration
(through the other catheter) and remove as much necrotic tissue as
possible instead of just draining the fluid component of the
pancreatic collection.
Materials and methods
Patients and requirement for PCD
The present study was a retrospective evaluation of
a prospectively acquired database of patients with AP who underwent
PCD of necrotic pancreatic collections (acute necrotic
collections/walled-off necrosis) at the Postgraduate Institute of
Medical Education and Research, Chandigarh, between May and
December 2018. Patients who underwent PCD with the modified
technique (kissing catheter technique) were included for analysis.
The present study was approved by the institutional ethics
committee of the Postgraduate Institute of Medical Education and
Research, Chandigarh. As this was a retrospective study, informed
consent for inclusion in a study was not required from the
patients, but patients had given procedural consent. The diagnosis
of AP was based on the revised Atlanta criteria (12). Patients underwent an initial
contrast-enhanced CT between 3 and 7 days of pain onset. A modified
CT severity index (CTSI) was assigned. The mean CT density of the
collection was calculated by placing a circular region of interest
over multiple parts of the collection and taking a mean value. The
indications of the drainage were recorded.
Technical details
PCD was performed by an interventional radiologist
(PG) with five years' experience in performing abdominal
interventions using ultrasound or CT guidance. Catheter insertion
was performed using the Seldinger technique. Following the initial
image-guided puncture of the collection, a 0.035" stiff guidewire
(Amplatz Extra Stiff Wire Guide; Cook Medical) was placed into the
collection. After serial dilatations of the access tract using
stiff fascial dilators (Devon® Innovations Private
Ltd.), a single 12 or 14 French (F) catheter (Biomedical Health
care, India) was placed into the collection. Patients were
re-assessed for the resolution of organ failure and sepsis. A
repeat ultrasound was performed after three days to identify any
residual collection and determine the amount of solid debris. If a
patient had persistent or worsening organ failure and ongoing
sepsis and CT indicated a <50% reduction in the size of the
collection and attenuation >30 Hounsfield Units (HU), they were
subjected to the kissing catheter technique (11).
Details of the kissing catheter
technique
A total of two 0.035" stiff guidewires were placed
through the pre-existing catheter into the collection. The catheter
was then removed. Over one of the guidewires, a new 14 F catheter
was placed. Over the other guidewire, a 10-14 F catheter was placed
after fascial dilatation. Immediately following the placement of
the two catheters, flushing with normal saline and active
aspiration was performed by using one of the catheters for flushing
and the other catheter for active aspiration. The endpoint of
flushing was a clear aspirate from the two catheters without any
visible solid component. The flushing and aspiration were performed
daily by the interventional radiologist until the follow-up CT scan
indicated complete resolution of the collection and the organ
failure and sepsis were resolved. During this period, upsizing of
each catheter (16-24 F) was also performed based on the size of the
collection. After achieving resolution of the collection and until
the catheters had an output of <20 ml/day, the catheters were
clamped for 2 days and a repeat ultrasound was performed. If there
was no clinical deterioration, pericatheter leakage or residual
collection, the catheters were removed. The patients were subjected
to surgical necrosectomy if the collection persisted even after
three upsizing procedures (after the insertion of two catheters)
and organ failure or sepsis persisted.
Follow-up
Following catheter removal, patients were assessed
every week by the interventional radiologist for the 1st month
after discharge from the hospital. The following technical and
outcome parameters were recorded: The interval from onset of pain
to application of the kissing catheter technique, the total size of
the catheter combination, duration of catheter drainage,
complications, requirement for surgery, length of hospital stay,
length of intensive care unit (ICU) stay and death.
Results
Baseline characteristics
A total of 70 patients with moderately severe and
severe AP were managed with PCD during the study period. A total of
10 patients (14.2%) with a mean CT density of the pancreatic
collections of >30 HU who did not respond to the conventional
PCD method were subjected to the kissing catheter technique. The
cohort comprised 7 males and 3 females. The mean age was 30 years
(range, 17-45 years). The aetiology of AP was alcohol abuse (n=4),
gallstones (n=4) and idiopathic causes (n=2). All the patients
treated with this technique had severe AP. The CTSI ranged from
8-10 (mean CTSI 9) and the largest dimensions of the collections
ranged from 10-20 cm (mean size, 14.5 cm). The sites of the
collection were paracolic gutter (n=6) and lesser sac (n=4). The
mean CT density was 37 HU (range, 32-56).
Details of PCD
The indications of the initial PCD were suspected
infection (n=6), persistent or worsening organ failure (n=3) and
intra-abdominal hypertension (n=1). Intra-abdominal hypertension
was defined by an intra-abdominal pressure of >12 mmHg (13). The mean interval between the onset of
pain and PCD was 13.7 days (range, 7-26 days). The mean interval
between the initial PCD and the kissing catheter technique was 7.6
days (range, 3-14 days). The mean maximum size of the catheter
combination was 33.6 F (range, 28-48 F). The first catheter
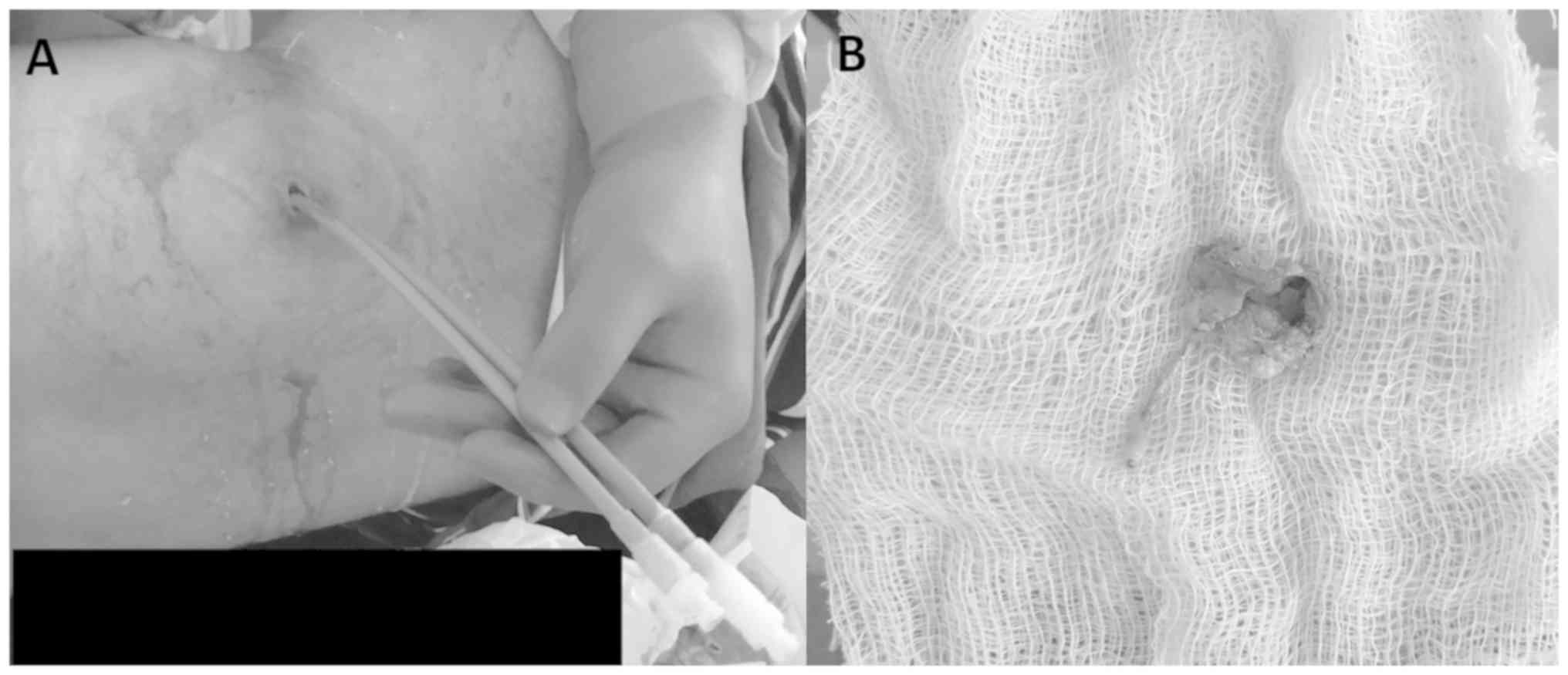
cultures were positive in 6 patients. Images of a representative
case are provided in Figs. 1 and
2. The complications associated with
this technique were catheter dislodgement (n=2), external
pancreatic fistula (n=1) and bleeding from the catheter site (n=1).
Catheter dislodgement was managed by placing new catheters through
the pre-existing tract. The external pancreatic fistula resolved
after transpapillary pancreatic stenting, while bleeding from the
catheter site required no active intervention. The mean total
duration of the PCD was 28.6 days (range, 12-43 days). None of the
patients underwent endoscopic drainage.
Clinical outcomes
The mean length of hospital stay was 32.4 days
(range, 10-59 days). A total of 3 patients required ICU admission.
The mean duration of ICU stay was 17 days (range, 11-45 days).
Furthermore, 2 patients underwent surgical necrosectomy, of which 1
patient died. Table I provides the
technical details and outcome parameters for all of the
patients.
 | Table IDetails of the subjects of the present
study. |
Table I
Details of the subjects of the present
study.
| Subject no. | Age (years) | Sex | Etiology | Location of
collections | Mean size of
collection (cm) | Indication | Interval between pain
onset and PCD drainage (days) | Interval between
initial PCD drainage and KCT (days) | Size of catheter
combination (F+F) | Duration of PCD
(days) | Complication | LOH | Length of ICU
stay | Surgical
intervention | Mortality |
|---|
| 1 | 38 | M | Alc | PP | 10 | OF | 7 | 5 | 18+14 | 19 | None | 10 | 0 | No | No |
| 2 | 43 | M | GS | PP | 11 | IN | 10 | 6 | 14+24 | 21 | None | 59 | 0 | No | No |
| 3 | 30 | M | Alc | PCG | 12 | OF | 12 | 6 | 14+18 | 34 | EPF | 36 | 0 | No | No |
| 4 | 20 | F | GS | PCG | 13 | IN | 12 | 3 | 24+24 | 38 | Dislodgement | 45 | 25 | Yes | Yes |
| 5 | 18 | M | Unk | PP | 12 | IAH | 14 | 14 | 12+18 | 42 | None | 58 | 45 | Yes | No |
| 6 | 24 | M | Alc | PCG | 6 | OF | 9 | 12 | 14+14 | 19 | None | 14 | 0 | No | No |
| 7 | 45 | F | GS | PP | 15 | IN | 13 | 3 | 14+14 | 12 | Minor bleeding | 24 | 0 | No | No |
| 8 | 42 | M | Alc | PCG | 20 | IN | 10 | 14 | 14+18 | 38 | None | 12 | 0 | No | No |
| 9 | 23 | F | GS | PP | 12 | IN | 24 | 7 | 18+18 | 20 | None | 31 | 11 | No | No |
| 10 | 17 | M | Unk | PP | 15 | IN | 26 | 6 | 14+18 | 43 | None | 35 | 0 | No | No |
Discussion
Necrotic fluid collection is the most critical local
complication of AP (14,15). An infected pancreatic collection is
responsible for significant morbidity and mortality associated with
acute necrotizing pancreatitis (1).
Open necrosectomy allows for effective removal of the necrotic
material and was previously the treatment of choice for necrotic
collections (16). However, it is
associated with significant morbidity and mortality (1). Image-guided percutaneous drainage of
pancreatic collections was initially advocated by Freeny et
al (17) in 1998. Multiple other
studies have reported high treatment success and lower morbidity
and mortality with the PCD (18-22).
The landmark PANTER trial in 2010 compared primary necrosectomy to
a ‘step-up approach’ in patients with infected necrotizing
pancreatitis (1). In this approach,
catheter drainage (percutaneous or endoscopic) was the initial
step. In patients who did not improve with catheter drainage
underwent video-assisted retroperitoneal drainage, and if required,
open necrosectomy. This trial indicated that 35% of patients could
be treated with catheter drainage alone. The high success rates of
PCD has also been confirmed in the two recent meta-analyses
(3,9).
One of the most important factors predicting the
failure of PCD is the amount of necrotic tissue within the
collection, as determined by the higher mean CT density and
heterogeneity (9-11).
The catheter drainage protocol adopted in various published studies
is effective in draining the fluid component of the collection
(17-22).
However, this PCD protocol is not efficient for collections that
contain a large amount of necrotic tissue. Certain studies have
reported that a proactive PCD protocol has increased efficacy in
treating pancreatic collections (5-8).
In all the published studies, a single percutaneous catheter is
placed in a collection. Active flushing and aspiration, integral to
the success of PCD, are challenging to achieve with a single
catheter, particularly in collections with a large amount of solid
component. The kissing catheter technique described in the present
study uses two catheters in combination, placed through a single
cutaneous opening. The two catheters also form a closed system for
flushing and active aspiration that allows for the removal of
necrotic debris. This approach prevents catheter blockade. It may
be argued that two catheters placed into the collection through
different sites or a single large catheter may function equally
well. The placement of two catheters through different sites may be
difficult given a limited acoustic window or bowel-free approach
for lesser sac collections. On the other hand, the placement of a
single large catheter does not allow for aggressive flushing and
aspiration. The mean interval from the onset of pain to PCD was
13.7 days. Although it is preferable to delay the interventions for
pancreatic collections as far as possible, recent studies have
indicated comparable outcomes of PCD for acute necrotic collections
and walled-off necrosis (4,8). A trial comparing postponed vs. early
drainage of infected necrotic collections (POINTER trial) is being
conducted by the Dutch pancreatitis group (23).
Potential complications of the technique include the
development of external pancreatic fistula and increased risk of
vascular and bowel injury due to large catheter sizes (24,25).
Even though one patient developed external pancreatic fistula, none
of the patients suffered any vascular or bowel injury. Additional
concerns include skin excoriation and infection (26). This mandates meticulous care of skin
with skin hygiene, regular dressing and use of emollients.
There are a few limitations to the present study.
First, the sample size was small. However, the present study aimed
to evaluate the novel PCD method. The outcomes and complications of
the kissing catheter technique were not compared with the standard
method of PCD. This was not possible given the small size of the
cohort.
In conclusion, the kissing catheter technique has
the potential to further improve the success rate of PCD of
necrotic collections containing a large amount of necrotic tissue.
Randomized controlled trials are required to confirm the utility of
this novel technique in the definitive management of necrotic
pancreatic collections.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
PG performed the interventions, PG and SK analyzed
the data and wrote the manuscript. JS, HM, VS, SKS, UD and RK were
involved in clinical care of patients and revised the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
ethics committee of the Postgraduate Institute of Medical Education
and Research, Chandigarh. As this was a retrospective study,
informed consent for inclusion in a study was not required from the
patients, but patients had given procedural consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Santvoort HC, Besselink MG, Bakker OJ,
Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF,
van Eijck CH, Bollen TL, et al: Dutch Pancreatitis Study Group: A
step-up approach or open necrosectomy for necrotizing pancreatitis.
N Engl J Med. 362:1491–1502. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ke L, Li J, Hu P, Wang L, Chen H and Zhu
Y: Percutaneous Catheter Drainage in Infected Pancreatitis
Necrosis: A Systematic Review. Indian J Surg. 78:221–228.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van Baal MC, van Santvoort HC, Bollen TL,
Bakker OJ, Besselink MG and Gooszen HG: Dutch Pancreatitis Study
Group. Systematic review of percutaneous catheter drainage as
primary treatment for necrotizing pancreatitis. Br J Surg.
98:18–27. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Mallick B, Dhaka N, Gupta P, Gulati A,
Malik S, Sinha SK, Yadav TD, Gupta V and Kochhar R: An audit of
percutaneous drainage for acute necrotic collections and walled off
necrosis in patients with acute pancreatitis. Pancreatology.
18:727–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van Grinsven J, Timmerman P, van Lienden
KP, Haveman JW, Boerma D, van Eijck CH, Fockens P, van Santvoort
HC, Boermeester MA and Besselink MG: Dutch Pancreatitis Study
Group. Proactive Versus Standard Percutaneous Catheter Drainage for
Infected Necrotizing Pancreatitis. Pancreas. 46:518–523.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sugimoto M, Sonntag DP, Flint GS, Boyce
CJ, Kirkham JC, Harris TJ, Carr SM, Nelson BD, Bell DA, Barton JG,
et al: Better Outcomes if Percutaneous Drainage Is Used Early and
Proactively in the Course of Necrotizing Pancreatitis. J Vasc
Interv Radiol. 27:418–425. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sugimoto M, Sonntag DP, Flint GS, Boyce
CJ, Kirkham JC, Harris TJ, Carr SM, Nelson BD, Barton JG and
Traverso LW: A percutaneous drainage protocol for severe and
moderately severe acute pancreatitis. Surg Endosc. 29:3282–3291.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gupta P, Gupta J, Kumar C, Samanta J,
Mandavdhare H, Sharma V, Sinha SK, Gupta V, Yadav TD, Dutta U, et
al: Aggressive Percutaneous Catheter Drainage Protocol for Necrotic
Pancreatic Collections. Dig Dis Sci: Feb 5, 2020 (Epub ahead of
print). doi: 10.1007/s10620-020-06116-6.
|
|
9
|
Hollemans RA, Bollen TL, van Brunschot S,
Bakker OJ, Ahmed Ali U, van Goor H, Boermeester MA, Gooszen HG,
Besselink MG and van Santvoort HC: Dutch Pancreatitis Study Group.
Predicting Success of Catheter Drainage in Infected Necrotizing
Pancreatitis. Ann Surg. 263:787–792. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Tong Z, Li W, Yu W, Geng Y, Ke L, Nie Y,
Sun J, Ni H, Wang X, Ye X, et al: Percutaneous catheter drainage
for infective pancreatic necrosis: Is it always the first choice
for all patients? Pancreas. 41:302–305. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guo Q, Li A and Hu W: Predictive factors
for successful ultrasound-guided percutaneous drainage in
necrotizing pancreatitis. Surg Endosc. 30:2929–2934.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group. Classification of acute
pancreatitis--2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Malbrain ML, Cheatham ML, Kirkpatrick A,
Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C,
Ivatury R, et al: Results from the International Conference of
Experts on Intra-abdominal Hypertension and Abdominal Compartment
Syndrome. I. Definitions. Intensive Care Med. 32:1722–1732.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gupta P, Jain R, Koshi S, Gulati A,
Samanta J, Mandavdhare H, Sharma V, Sinha SK, Dutta U, Sandhu MS,
et al: Radiation dose from computed tomography in patients with
acute pancreatitis: An audit from a tertiary care referral
hospital. Abdom Radiol (NY). 45:1517–1523, Epub ahead of print.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gupta P, Rana P, Bellam BL, Samanta J,
Mandavdhare H, Sharma V, Sinha SK, Dutta U and Kochhar R: Site and
size of extrapancreatic necrosis are associated with clinical
outcomes in patients with acute necrotizing pancreatitis.
Pancreatology. 20:9–15. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Beger HG, Büchler M, Bittner R, Oettinger
W, Block S and Nevalainen T: Necrosectomy and postoperative local
lavage in patients with necrotizing pancreatitis: Results of a
prospective clinical trial. World J Surg. 12:255–262.
1988.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Freeny PC, Hauptmann E, Althaus SJ,
Traverso LW and Sinanan M: Percutaneous CT-guided catheter drainage
of infected acute necrotizing pancreatitis: Techniques and results.
AJR Am J Roentgenol. 170:969–975. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fotoohi M, D'Agostino HB, Wollman B, Chon
K, Shahrokni S and vanSonnenberg E: Persistent pancreatocutaneous
fistula after percutaneous drainage of pancreatic fluid
collections: Role of cause and severity of pancreatitis. Radiology.
213:573–578. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baril NB, Ralls PW, Wren SM, Selby RR,
Radin R, Parekh D, Jabbour N and Stain SC: Does an infected
peripancreatic fluid collection or abscess mandate operation? Ann
Surg. 231:361–367. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bruennler T, Langgartner J, Lang S, Wrede
CE, Klebl F, Zierhut S, Siebig S, Mandraka F, Rockmann F,
Salzberger B, et al: Outcome of patients with acute, necrotizing
pancreatitis requiring drainage-does drainage size matter? World J
Gastroenterol. 14:725–730. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mortelé KJ, Girshman J, Szejnfeld D,
Ashley SW, Erturk SM, Banks PA and Silverman SG: CT-guided
percutaneous catheter drainage of acute necrotizing pancreatitis:
Clinical experience and observations in patients with sterile and
infected necrosis. AJR Am J Roentgenol. 192:110–116.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Rocha FG, Benoit E, Zinner MJ, Whang EE,
Banks PA, Ashley SW and Mortele KJ: Impact of radiologic
intervention on mortality in necrotizing pancreatitis: The role of
organ failure. Arch Surg. 144:261–265. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van Grinsven J, van Dijk SM, Dijkgraaf MG,
Boermeester MA, Bollen TL, Bruno MJ, van Brunschot S, Dejong CH,
van Eijck CH, van Lienden KP, et al: Dutch Pancreatitis Study
Group: Postponed or immediate drainage of infected necrotizing
pancreatitis (POINTER trial): Study protocol for a randomized
controlled trial. Trials. 20(239)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bansal A, Gupta P, Singh H, Samanta J,
Mandavdhare H, Sharma V, Sinha SK, Dutta U and Kochhar R:
Gastrointestinal complications in acute and chronic pancreatitis.
JGH Open. 3:450–455. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gupta P, Chayan Das G, Sharma V,
Mandavdhare H, Samanta J, Singh H, Kant Sinha S, Dutta U and
Kochhar R: Role of computed tomography in prediction of
gastrointestinal fistula in patients with acute pancreatitis. Acta
Gastroenterol Belg. 82:495–500. 2019.PubMed/NCBI
|
|
26
|
Sikora SS, Khare R, Srikanth G, Kumar A,
Saxena R and Kapoor VK: External pancreatic fistula as a sequel to
management of acute severe necrotizing pancreatitis. Dig Surg.
22:446–451; discussion 452. 2005.PubMed/NCBI View Article : Google Scholar
|