Introduction
Rheumatoid arthritis (RA) is a common systemic
autoimmune disease (1). The primary
pathological manifestations of RA are chronic synovium inflammation
and pannus formation, which can lead to swelling and deformities in
the joints of patients (2). These
symptoms can later lead to disability, which may cause a loss of
work time, which can create a burden on society and the families of
patients (3). The pathogenesis of RA
is complex, involving many types of cells, including macrophages, T
and B cells, fibroblasts, chondrocytes and dendritic cells
(4). Despite study into the role of
many genes and mechanisms underlying the development of RA, there
is still no clear predisposing factor (5-7).
Chemokines are small protein cytokines, and their
main function is to induce leukocytes aggregation to form
inflammatory lesions, via directional migration, for participation
in the inflammatory response (8).
Previous studies have demonstrated that many chemokines are highly
expressed in the joint synovial fluid or peripheral blood of
patients with RA, which suggests that chemokines may be associated
with RA pathogenesis (9,10).
C-X-C motif chemokine ligand 12 (CXCL12) is mainly
produced by stromal cells and is a key factor for the activation
and migration of inflammatory cells to synovial tissues (11). CXC receptor 4 (CXCR4) is a natural
receptor of CXCL12(12). The
chemokine CXCL12 can participate in the immune response to RA by
mediating the migration and activation of T and B cells in immune
cells (13). CXCL12 can also be
secreted and produced by joint synovial cells, while CXCR4 can be
expressed on the surface of articular chondrocytes (14,15). The
activation of CXCR4 and CXCL12 can induce the secretion of a
variety of inflammatory factors from articular chondrocytes,
leading to apoptosis and destruction of chondrocytes (16,17).
Previous studies have demonstrated that CXCR4 and CXCL12 together
can serve an important role in lupus erythematosus (18-20).
These aforementioned studies indicated that CXCR4 and CXCL12 are
closely associated with autoimmune diseases.
Although previous studies have indicated that the
expression of CXCL12 in the joint synovial membranes was
significantly higher in the patients with RA compared with healthy
controls (21), there are relatively
few studies on the relationship between CXCR4 and CXCL12, and
disease activity in patients with RA. Therefore, the present study
investigated the expression levels of CXCR4 and CXCL12 in the serum
and joint synovial fluid of patients with RA, and correlation
analyses was performed to examine this data with clinical
indicators. In addition, the present study investigated the roles
of CXCR4 and CXCL12 in the occurrence and development of RA, and
the relationship between CXCR4, CXCL12 and disease activity, to
identify accurate evaluation indicators for use in patients with
RA.
Materials and methods
Patient data
Using a random number table method, 60 patients
(male patients, 34; female patients, 26) with RA were recruited and
randomly selected as the study group from the Rheumatology and
Immunology Department of First People's Hospital of Jingzhou from
January 1 to December 31, 2018. The age distribution was 32-60
years old. The average age of all patients was 54.31±5.89 years.
Another 60 patients (male patients, 32; female patients, 28) with
osteoarthritis, recruited from The First People's Hospital of
Jingzhou hospital were selected as the control group.
Patients were selected based on inclusion and
exclusion criteria. Patients who met the RA diagnostic criteria
revised by The American College of Rheumatology in 1987(22) were included in the study group, and
patients who met the guidelines on management of osteoarthritis of
the hand, hip and knee by the American College of Rheumatology
(23) were included in the control
group. Patients were excluded if they had any joint infection,
severe liver or kidney dysfunction, cognitive or communication
disorders, other autoimmune diseases or malignant tumors, or those
who declined to participle. All patients agreed to participate in
the experiment and signed an informed consent agreement. This
experiment has been approved by the Hospital Ethics Committee of
First People's Hospital of Jingzhou.
Specimen extraction
A total of 5 ml fasting venous blood was obtained
from all patients and centrifuged at 1,500 x g for 10 min at 4˚C.
The serum was collected following centrifugation (1,500 x g for 10
min at 4˚C) and stored at -80˚C. A total of 500 µl joint fluid
specimens were collected by outpatient joint punctures or knee
joint surgery, and stored at -80˚C for subsequent analysis. Western
blotting (WB) and ELISA were used to detect the expression levels
of CXCR4 and CXCL12, respectively, in serum and joint fluid.
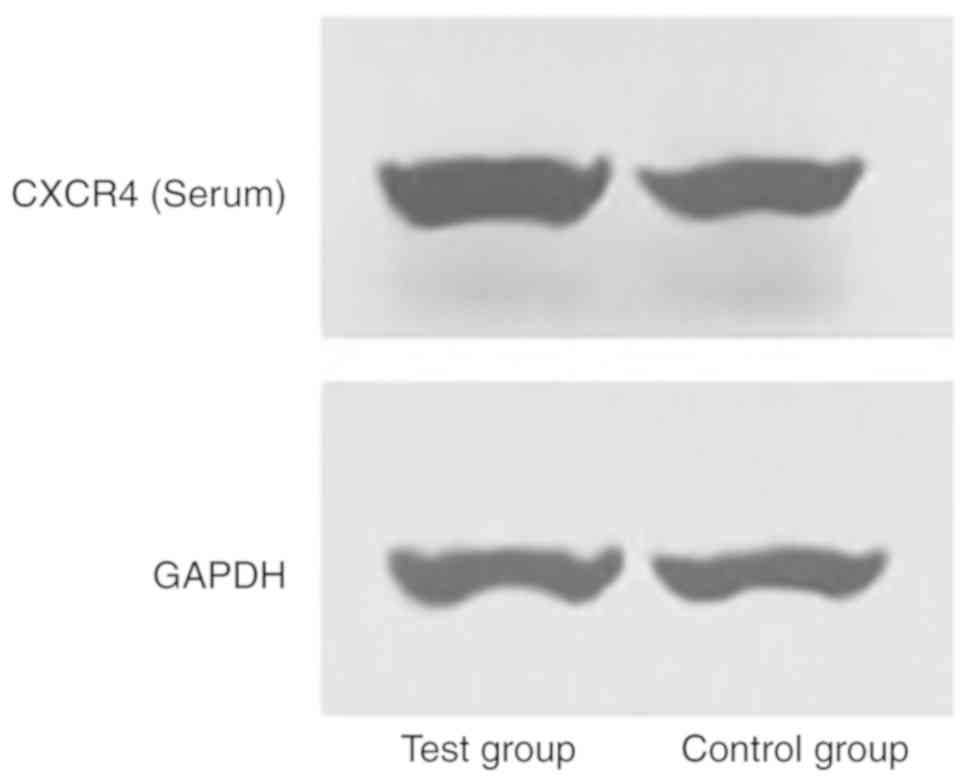
Detection of CXCR4 expression level
using WB
200 µl Radioimmunoprecipitation assay buffer (Cell
Signaling Technology, Inc.) was added to the serum and joint fluid
specimens collected from patients. The cells were lysed and the
total protein of each group was collected. BCA assays were used to
determine the protein concentration. After collection, the protein
was boiled in water for 8 min at 100˚C. A total of 20 µg protein
was loaded into the 10% SDS-PAGE gel and run at 140 V for 2 h.
Subsequently, the protein was transferred to a PVDF membrane at 300
mA for 2 h, and PVDF membrane was blocked with 5% skimmed milk
powder for 2 h at room temperature. The membranes were probed with
the primary antibody targeting CXCR4 (1:1,000; Abcam; cat. no.
ab227767) incubated overnight at 4˚C. The antibody GAPDH (1:500;
Wuhan Fine Biotech Co., Ltd.; cat. no. FNab03342) was added and
incubated overnight at 4˚C, then the membrane was probed with
horseradish peroxidase (HRP)-labeled goat anti-rabbit secondary
antibodies (1:5,000; Abcam; cat. no. ab97080) at room temperature
for 2 h. Subsequently, an enhanced chemiluminescence (ECL)
developer (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
develop the protein bands. Filter paper was used to absorb excess
liquid from the film and protein bands were developed for
film-imaged using ECL. The protein bands were scanned and analyzed
using Quantity One software (v4.6.6; Bio-Rad Laboratories, Inc.),
where the relative expression level of the protein = the gray value
of the target protein band/the grey value of the GAPDH protein
band.
Detection of CXCL12 expression in
serum and joint fluid of patients using ELISA
The ELISA kit was purchased from Quanzhou Ruixin
Biotechnology Co., Ltd. (cat. no. 13236). A total of 50 µl of the
diluted standard and samples were added into each reaction well,
followed by 50 µl biotin-labeled antibodies (all provided as part
of the aforementioned kit). These samples were incubated at 37˚C
for 1 h and then washed using the wash buffer, 4 times for 5 min
each time. Streptomycin-HRP conjugate from the aforementioned kit
was added and incubated for 30 min at room temperature.
Subsequently, a total of 50 µl substrates provided by the
aforementioned kit were added and incubated for 10 min at 37˚C.
Subsequently, 50 µl terminating solution, provided by the
aforementioned kit, was added to each well and the OD values were
measured at 450 nm.
Detection of routine detection indexes
in patients with RA
The erythrocyte sedimentation rate (ESR) from the
patients with RA was detected using the Westergren's method
(24) using an automatic erythrocyte
sedimentation rate tester (Plyson cat. no. LBY-XC40B). C-reactive
protein (CRP) and the rheumatoid factor (RF) were detected using
rate scatter nephelometry (25)
using an IMMAGE rate scattering turbidity analyzer (UniCel DxC800;
Beckman Coulter, Inc.).
Outcome measures
The outcome measures were as follows: i) The levels
of CXCR4 and CXCL12 in serum and joint fluid were compared between
the study and control groups; ii) patients with RA were divided
based on their disease activity score 28 (DAS28) scores (13) into the remission group (28 cases;
≤2.6 points) and the active group (32 cases; >2.6 points), which
were evaluated from four aspects, including the number of tender
joints, the number of swollen joints and the erythrocyte
sedimentation rate. DAS28 values range from 2.0-10.0(13). A DAS28 <2.6 is interpreted as
'remission'; and iii) CXCR4 and CXCL12 expression levels in the
serum and joint fluid of patients were compared. Correlations were
analyzed between CXCR4 and CXCL12 expression levels, and ESR, CRP,
DAS28 scores and RF in patients with RA.
Statistical analysis
SPSS 18.0 statistical software (SPSS Inc.) was used
to analyze the experimental data. Data are presented as the mean ±
standard deviation. A χ2 test was used to analyze
categorical data. An independent t-test was used for comparison
between two groups. One-way ANOVA with a post-hoc LSD test was used
for comparison among three groups. Spearman's correlation was used
to analyze the correlation between CXCR4 and CXCL12 expression
levels. The figures in this study were drawn using Graph Pad Prism
6 software (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient data
There were no significant differences between sex,
age or body mass index in the two groups (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Study group N=60
(%) | Control group N=60
(%) |
χ2/t | P-value |
|---|
| Sex | | | 0.090 | 0.764 |
|
Male | 34 (56.67) | 32 (53.33) | | |
|
Female | 26 (43.33) | 28 (46.67) | | |
| Age | | | 0.022 | 0.882 |
|
≥54 | 31 (51.67) | 30 (50.00) | | |
|
<54 | 29 (48.33) | 30 (50.00) | | |
| BMI,
kg/m2 | | | 0.023 | 0.881 |
|
≤22 | 27 (45.00) | 26 (43.33) | | |
|
>22 | 33 (55.00) | 34 (56.67) | | |
| Smoking | | | 1.808 | 0.179 |
|
No | 39 (65.00) | 38 (63.33) | | |
|
Yes | 21 (35.00) | 22 (36.67) | | |
| Drinking volume,
ml | | | 0.023 | 0.879 |
|
<70 | 37 (61.67) | 36 (60.00) | | |
|
≥70 | 23 (38.33) | 14 (40.00) | | |
| Coagulation
function | | | | |
|
APTT,
sec | 28.23±1.35 | 28.19±1.41 | 0.131 | 0.896 |
|
PT, sec | 11.31±1.15 | 11.29±1.14 | 0.078 | 0.938 |
|
FIB,
g/l | 3.16±0.21 | 3.19±0.19 | 0.659 | 0.512 |
| Renal function
index (µmol/l) | | | | |
|
Creatinine | 57.63±4.45 | 58.01±4.52 | 0.280 | 0.705 |
|
Urea | 5.29±0.45 | 5.31±0.57 | 0.182 | 0.856 |
|
Uric
acid | 291.34±11.35 | 293.14±11.45 | 0.707 | 0.481 |
CXCR4 and CXCL12 expression levels
among the study and control groups
The serum CXCR4 expression levels of the study group
were 5.12±1.04 and 3.62±0.54, and the serum CXCL12 expression
levels were 8.28±1.23 and 7.31±1.69. The joint fluid CXCR4
expression levels of the control group were 3.05±1.11 and
2.13±0.63, and the joint fluid CXCL12 expression levels were
5.14±1.12 and 4.54±1.25. The present results suggested that CXCR4
and CXCL12 expression levels in the serum and articular fluid of
the study group were significantly higher compared with the control
group (P<0.05; Tables II and
III; Figs. 1 and 2).
 | Table IICXCR4 expression levels in the serum
and joint fluid of the study and control groups. |
Table II
CXCR4 expression levels in the serum
and joint fluid of the study and control groups.
| Factor | Study group
N=60 | Control group
N=60 | t | P-value |
|---|
| Serum | 3.12±1.04 | 3.05±1.11 | 8.704 | <0.001 |
| Joint fluid | 3.62±0.54 | 2.13±0.63 | 11.67 | <0.001 |
 | Table IIICXCL12 expression levels in the serum
and joint fluid of the study and control groups. |
Table III
CXCL12 expression levels in the serum
and joint fluid of the study and control groups.
| Factor | Study group
N=60 | Control group
N=60 | t | P-value |
|---|
| Serum | 8.28±1.23 | 5.14±1.12 | 11.75 | <0.001 |
| Joint fluid | 7.31±1.69 | 4.54±1.25 | 7.947 | <0.001 |
CXCR4 and CXCL12 expression levels
among the RA, remission and control groups
The serum CXCR4 expression levels of the RA-active
group were 7.85±1.21 and 6.71±0.69, and the serum CXCL12 expression
levels were 10.82±1.33 and 9.46±1.59. CXCR4 expression levels of
the RA-remission group were 3.33±1.05 and 2.41±0.57, and CXCL12
expression levels were 5.18±1.15 and 4.74±1.39. The joint fluid
CXCR4 expression levels of the control group were 3.05±1.11 and
2.13±0.63, and the joint fluid CXCL12 expression levels were
5.14±1.12 and 4.54±1.25. Therefore, the present results suggested
that the expression levels of CXCR4 and CXCL12 in the RA-active
group were significantly higher compared with the RA-remission
(P<0.05) and control groups (P<0.05; Tables IV and V; Figs. 3
and 4).
 | Table IVCXCR4 expression levels in the serum
and joint fluid of the active, remission, and control groups. |
Table IV
CXCR4 expression levels in the serum
and joint fluid of the active, remission, and control groups.
| Factor | Active group
N=32 | Remission group
N=28 | Control group
N=60 | F | P-value |
|---|
| Serum | 7.85±1.21 |
3.33±1.05a |
3.05±1.1a | 156.7 | <0.001 |
| Joint fluid | 6.71±0.69 |
2.41±0.57a |
2.13±0.63a | 507.2 | <0.001 |
 | Table VCXCL12 expression levels in the serum
and joint fluid of the active, remission and control groups. |
Table V
CXCL12 expression levels in the serum
and joint fluid of the active, remission and control groups.
| Factor | Active group
N=32 | Remission group
N=28 | Control group
N=60 | F | P-value |
|---|
| Serum | 10.82±1.33 |
5.18±1.15a |
5.14±1.12a | 226.5 | <0.001 |
| Joint fluid | 9.46±1.59 |
4.74±1.39a |
4.54±1.25a | 118.8 | <0.001 |
Correlations between CXCR4 and CXCL12
expression levels and ESR, CRP, RF and DAS28 scores
The expression levels of CXCR4 and CXCL12 had a
positive correlation with the ESR, CRP, RF and DAS28 scores (data
not shown) of patients with RA (P<0.05; Tables VI and VII;
Figs. 5-8).
 | Table VICorrelation between CXCR4 and CXCL12
expression in serum from the patients with RA and ESR, CRP, RF and
DAS28 scores. |
Table VI
Correlation between CXCR4 and CXCL12
expression in serum from the patients with RA and ESR, CRP, RF and
DAS28 scores.
| | CXCR4 | CXCL12 |
|---|
| Parameter | r | P-value | r | P-value |
|---|
| ESR | 0.766 | <0.05 | 0.798 | <0.05 |
| CRP | 0.836 | <0.05 | 0.879 | <0.05 |
| RF | 0.76 | <0.05 | 0.781 | <0.05 |
| DAS28 | 0.751 | <0.05 | 0.799 | <0.05 |
 | Table VIICorrelation between CXCR4 and CXCL12
expression in joint fluid from the patients with RA and ESR, CRP,
RF and DAS28 scores. |
Table VII
Correlation between CXCR4 and CXCL12
expression in joint fluid from the patients with RA and ESR, CRP,
RF and DAS28 scores.
| | CXCR4 | CXCL12 |
|---|
| Score | r | P-value | r | P-value |
|---|
| ESR | 0.703 | <0.05 | 0.743 | <0.05 |
| CRP | 0.85 | <0.05 | 0.858 | <0.05 |
| RF | 0.788 | <0.05 | 0.727 | <0.05 |
| DAS28 | 0.731 | <0.05 | 0.809 | <0.05 |
Correlation between the expression of
CXCR4 and CXCL12 with disease activity
Compared with the RA-active group, the expression of
CXCR4 and CXCL12 in the serum of the RA remission group was
significantly lower. Therefore, the present results suggested that
the expression levels of CXCR4 and CXCL12 were positively
correlated with the changes in RA disease activity (CXCR4, r=0.858,
P<0.001; CXCL12, r=0.864, P<0.001; Fig. 9).
Discussion
RA is a chronic systemic immune disease and its
pathogenesis has yet to be elucidated, but abnormal immunity is
considered to contribute to its development (26). Previous studies have demonstrated
that over the course of RA, inflammatory cells migrate from
peripheral blood to synovial tissues and secrete a variety of
inflammatory factors, resulting in the destruction of the synovial
membrane (27,28). Chemokines serve an important role in
the infiltration and migration of inflammatory cells (29). CXCL12 is the only chemokine that
binds to the receptor CXCR4(30).
CXCL12 and CXCR4 promote the migration of monocytes and T
lymphocytes, and serve an important role in the process of
inflammation (31). Previous studies
have indicated that the expression levels of CXCL12 in the synovial
tissue and joint fluid of patients with RA was increased, and that
CXCR4 expression was also significantly increased in T lymphocytes
(32-34).
CXCL12 can promote the secretion of matrix
metalloproteins (MMPs) via chondrocytes, including MMP-1 and
MMP-13(35). CXCR4 receptor
antagonists have been used to reduce the expression levels of MMP-1
and MMP-13 in synovial fluid in patients with RA (36). In addition, CXCL12 and CXCR4 can
further induce injury of the articular cartilage by promoting MMP
secretion (37). Previous studies
have demonstrated that CXCL12 and CXCR4 are closely associated with
synovial cell inflammation in patients with RA (17,38), but
to the best of our knowledge, no previous reports have discussed
the correlation between CXCL12 and CXCR4, or disease activity in
patients with RA.
The present results suggested that the expression
levels of CXCR4 and CXCL12 in the RA-active group were
significantly higher compared with the RA-remission and control
groups (P<0.05), but there was no significant difference between
the latter two groups. Therefore, the present results indicated
that the expression levels of CXCR4 and CXCL12 may be closely
associated with RA pathogenesis. The correlations between CXCR4 and
CXCL12 expression levels, and ESR, CRP, RF and DAS28 scores in
patients with RA were also investigated. The present results
suggested that CXCR4 and CXCL12 expression levels were strongly
positively correlated with ESR, CRP, RF and DAS28 scores
(P<0.05). Therefore, these results indicated that CXCR4 and
CXCL12 expression levels exhibited an effect on the disease
activity of patients with RA and may be used as an index to
evaluate this activity.
Previous studies have revealed that when CXCR4 and
CXCL12 expression levels were increased, chemotaxis and activating
effects were induced in immune cells, which leads to overactivation
of immune cells and the subsequent secretion of additional
inflammatory factors and their corresponding antibodies (39-41).
This effect may explain the results of the present study, in which
the CXCR4 and CXCL12 expression levels of patients with RA were
significantly increased. Previous studies have indicated that
synovial fibroblasts in patients with RA may produce high levels of
CXCL12 compared with patients with osteoarthritis (21,42).
This is primarily due to the hypomethylation of DNA in synovial
fibroblasts in patients with RA compared with patients with
osteoarthritis, which may affect CXCL12 expression (43). The results from these previous
studies are in line with the results of the present study. In
addition, previous studies have indicated that high expression
levels of CXCL12 in patients with RA are associated with a variety
of pathogenic events, and that the expression of CXCL12 was
positively correlated with the expression of CRP, which is
consistent with results from the present study (44,45).
Moreover, other studies have used immunohistochemistry scoring to
study synovial tissues of patients with RA and revealed that CXCL12
expression level was significantly correlated with DAS28 scores,
and that the expression levels of CXCL12 and CXCR4 were closely
associated with disease activity and joint destruction (46,47).
Previous studies indicated that CXCL12/CXCR4 expression were
positively correlated with RF expression (48-50),
which was consistent with the results of the present study.
In conclusion, the results of the present study
suggested that CXCL12 and CXCR4 are highly expressed in patients
with RA, and that these levels are positively correlated with the
ESR, CRP, RF and DAS28 scores. Collectively, the results of the
current study suggested that these factors may be used as indexes
to evaluate disease activity in patients with RA.
However, there are some limitations to the present
study. Firstly, the present results indicated that CXCL12 and CXCR4
expression levels were increased in patients with RA, but it is
unknown whether the continuous increase of CXCL12 and CXCR4
expression levels were related to disease severity. Correlational
analyses were conducted between the expression levels of CXCL12 and
CXCR4, and the related indexes of disease activity, but further
analysis was not performed. Secondly, a close relationship was
revealed between CXCL12 and CXCR4 and inflammation in patients with
RA, but the correlation between CXCL12 and CXCR4, and the
expression of related inflammatory factors was not analyzed.
Inflammatory factors serve an important role in the pathogenesis of
RA and are important for the clinical evaluation of the prognosis
of patients with RA (51). In
addition, due to the random array method for sample selection and
the limited sample size, there may be a certain deviation between
the research group and the control group. The present study
recruited patients with osteoarthritis as the control group, rather
than healthy individuals, as it is possible to extract bone and
joint fluid during the examination of patients with osteoarthritis
but not healthy individuals. However, the patients with
osteoarthritis did not have rheumatic diseases, thus the selection
may result in some bias. Future studies will further increase the
sample size and recruit a more suitable selection of individuals as
controls.
Acknowledgements
Not applicable.
Funding
This work was supported by Scientific Research
Project of Health Department of Hubei Province (grant no.
JX6C-29).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LP and BW conceived the study and designed the
experiments. NZ, JM and LH contributed to the data collection and
performed the experiments. YY, ZZ and LW performed the data
analysis and interpreted the results. LP and NZ wrote the
manuscript. BW contributed to the critical revision of article. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients and their families agreed to
participate in the experiment and signed an informed consent
agreement. This experiment was approved by the Hospital Ethics
Committee of First People's Hospital of Jingzhou.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lowin T and Straub RH: Synovial
fibroblasts integrate inflammatory and neuroendocrine stimuli to
drive rheumatoid arthritis. Expert Rev Clin Immunol. 11:1069–1071.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ
and Xu J: Rheumatoid arthritis: Pathological mechanisms and modern
pharmacologic therapies. Bone Res. 6(15)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lehmann J, Jüngel A, Lehmann I, Busse F,
Biskop M, Saalbach A, Emmrich F and Sack U: Grafting of fibroblasts
isolated from the synovial membrane of rheumatoid arthritis (RA)
patients induces chronic arthritis in SCID mice-A novel model for
studying the arthritogenic role of RA fibroblasts in vivo. J
Autoimmun. 15:301–313. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leblond A, Allanore Y and Avouac J:
Targeting synovial neoangiogenesis in rheumatoid arthritis.
Autoimmun Rev. 16:594–601. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Picerno V, Ferro F, Adinolfi A, Valentini
E, Tani C and Alunno A: One year in review: The pathogenesis of
rheumatoid arthritis. Clin Exp Rheumatol. 33:551–558.
2015.PubMed/NCBI
|
|
6
|
Andrii IR and James D: The three
dimensions of somatic evolution: Integrating the role of genetic
damage, life history traits and aging in carcinogenesis. Evol Appl:
Mar 9, 2020 (Epub ahead of print). doi: 10.1111/eva.12947.
|
|
7
|
Foretz M, Guigas B and Viollet B:
Understanding the glucoregulatory mechanisms of metformin in type 2
diabetes mellitus. Nat Rev Endocrinol. 15:569–589. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Braunersreuther V, Viviani GL, Mach F and
Montecucco F: Role of cytokines and chemokines in non-alcoholic
fatty liver disease. World J Gastroenterol. 18:727–735.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kotrych D, Dziedziejko V, Safranow K,
Drozdzik M and Pawlik A: CXCL9 and CXCL10 gene polymorphisms in
patients with rheumatoid arthritis. Rheumatol Int. 35:1319–1323.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Antonelli A, Rotondi M, Fallahi P,
Romagnani P, Ferrari SM, Barani L, Ferrannini E and Serio M:
Increase of interferon-gamma-inducible CXC chemokine CXCL10 serum
levels in patients with active Graves' disease, and modulation by
methimazole therapy. Clin Endocrinol (Oxf). 64:189–195.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rosengren S, Kalunian KC, Kavanaugh A and
Boyle DL: CXCL13 as a marker for outcome of rheumatoid arthritis:
Comment on the article by Meeuwisse et al. Arthritis Rheum.
63:3646–3647. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu F, Xie Y, Wang Y, Peng ZH, Li J and
Oupický D: Chloroquine-containing HPMA copolymers as polymeric
inhibitors of cancer cell migration mediated by the CXCR4/SDF-1
chemokine axis. ACS Macro Lett. 5:342–345. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nanki T, Takada K, Komano Y, Morio T,
Kanegane H, Nakajima A, Lipsky PE and Miyasaka N: Chemokine
receptor expression and functional effects of chemokines on B
cells: Implication in the pathogenesis of rheumatoid arthritis.
Arthritis Res Ther. 11(R149)2009.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Brown MP, Dymock DC, Merritt KA and
Trumble TN: Stromal cell-derived factor-1 (SDF-1) validation using
equine serum, plasma, and synovial fluid. Osteoarthritis Cartilage.
22:S71. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kanbe K, Takemura T, Takeuchi K, Chen Q,
Takagishi K and Inoue K: Synovectomy reduces stromal-cell-derived
factor-1 (SDF-1) which is involved in the destruction of cartilage
in osteoarthritis and rheumatoid arthritis. J Bone Joint Surg Br.
86:296–300. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiang Y, Li Y, Yang L, He Y, Jia D and Hu
X: miR-142-5p as a CXCR4-targeted MicroRNA attenuates SDF-1-induced
chondrocyte apoptosis and cartilage degradation via inactivating
MAPK signaling pathway. Biochem Res Int.
2020(4508108)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Janssens R, Struyf S and Proost P:
Pathological roles of the homeostatic chemokine CXCL12. Cytokine
Growth Factor Rev. 44:51–68. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang A, Guilpain P, Chong BF, Chouzenoux
S, Guillevin L, Du Y, Zhou XJ, Lin F, Fairhurst AM, Boudreaux C, et
al: Dysregulated expression of CXCR4/CXCL12 in subsets of patients
with systemic lupus erythematosus. Arthritis Rheum. 62:3436–3446.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhao LD, Liang D, Wu XN, Li Y, Niu JW,
Zhou C, Wang L, Chen H, Zheng WJ, Fei YY, et al: Contribution and
underlying mechanisms of CXCR4 overexpression in patients with
systemic lupus erythematosus. Cell Mol Immunol. 14:842–849.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Karimabad MN, Khoramdelazad H and
Hassanshahi G: Genetic variation, biological structure, sources,
and fundamental parts played by CXCL12 in pathophysiology of type 1
diabetes mellitus. Int J Diabetes Dev Ctries. 37:229–239. 2017.
|
|
21
|
Karouzakis E, Rengel Y, Jüngel A, Kolling
C, Gay RE, Michel BA, Tak PP, Gay S, Neidhart M and Ospelt C: DNA
methylation regulates the expression of CXCL12 in rheumatoid
arthritis synovial fibroblasts. Genes Immun. 12:643–652.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Medina YF, Ruíz-Gaviria RE, Buitrago-Lopez
A and Villota C: Physical articular examination in the activity of
rheumatoid arthritis: a systematic review of the literature :
Systematic review of the literature regarding physical examination
in rheumatoid arthritis. Clin Rheumatol. 37:1457–1464.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Van Thillo A, Vulsteke JB, Van Assche D,
Verschueren P and De Langhe E: Physical therapy in adult
inflammatory myopathy patients: A systematic review. Clin
Rheumatol. 38:2039–2051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Khalid S, Javad Y and Amir R: Study of
erythrocyte sedimentation rate and anti-cyclic citrullinated
peptide antibodies in rheumatoid arthritis. Pak Armed Forces Med J.
67:283–286. 2017.
|
|
25
|
Varun CN, Raju R, Venkataswamy MM, Ravi V
and Varambally S: Procalcitonin and C-reactive protein as
peripheral inflammatory markers in antipsychotic drug-free
schizophrenia patients. Asian J Psychiatr. 35:11–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liao KP: Cardiovascular disease in
patients with rheumatoid arthritis. Trends Cardiovasc Med.
27:136–140. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Albus E, Sinningen K, Winzer M, Thiele S,
Baschant U, Hannemann A, Fantana J, Tausche AK, Wallaschofski H,
Nauck M, et al: Milk fat globule-epidermal growth factor 8 (MFG-E8)
is a novel anti-inflammatory factor in rheumatoid arthritis in mice
and humans. J Bone Miner Res. 31:596–605. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Harlow L, Rosas IO, Gochuico BR, Mikuls
TR, Dellaripa PF, Oddis CV and Ascherman DP: Identification of
citrullinated hsp90 isoforms as novel autoantigens in rheumatoid
arthritis-associated interstitial lung disease. Arthritis Rheum.
65:869–879. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Crijns H, Vanheule V and Proost P:
Targeting chemokine-glycosaminoglycan interactions to inhibit
inflammation. Front Immunol. 11(483)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cutolo P, Basdevant N, Bernadat G,
Bachelerie F and Ha-Duong T: Interaction of chemokine receptor
CXCR4 in monomeric and dimeric state with its endogenous ligand
CXCL12: Coarse-grained simulations identify differences. J Biomol
Struct Dyn. 35:399–412. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Guo F, Wang Y, Liu J, Mok SC, Xue F and
Zhang W: CXCL12/CXCR4: A symbiotic bridge linking cancer cells and
their stromal neighbors in oncogenic communication networks.
Oncogene. 35:816–826. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chung SH, Seki K, Choi BI, Kimura KB, Ito
A, Fujikado N, Saijo S and Iwakura Y: CXC chemokine receptor 4
expressed in T cells plays an important role in the development of
collagen-induced arthritis. Arthritis Res Ther.
12(R188)2010.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Armas-González E, Domínguez-Luis MJ,
Díaz-Martín A, Arce-Franco M, Castro-Hernández J, Danelon G,
Hernández-Hernández V, Bustabad-Reyes S, Cantabrana A, Uguccioni M,
et al: Role of CXCL13 and CCL20 in the recruitment of B cells to
inflammatory foci in chronic arthritis. Arthritis Res Ther.
20(114)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ding L, Amendola A, Wolf B, Bollier M,
Albright J, Wang Q, Wu M, Wang X, Song H, Pedersen D, et al:
Association of chemokine expression in anterior cruciate ligament
deficient knee with patient characteristics: Implications for
post-traumatic osteoarthritis. Knee. 27:36–44. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wei F, Moore DC, Wei L, Li Y, Zhang G, Wei
X, Lee JK and Chen Q: Attenuation of osteoarthritis via blockade of
the SDF-1/CXCR4 signaling pathway. Arthritis Res Ther.
14(R177)2012.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
De Buck M, Gouwy M, Struyf S, Opdenakker G
and Van Damme J: The ectoenzyme-side of matrix metalloproteinases
(MMPs) makes inflammation by serum amyloid A (SAA) and chemokines
go round. Immunol Lett. 205:1–8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lin C, Liu L, Zeng C, Cui ZK, Chen Y, Lai
P, Wang H, Shao Y, Zhang H, Zhang R, et al: Activation of mTORC1 in
subchondral bone preosteoblasts promotes osteoarthritis by
stimulating bone sclerosis and secretion of CXCL12. Bone Res.
7(5)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kircher M, Herhaus P, Schottelius M, Buck
AK, Werner RA, Wester HJ, Keller U and Lapa C: CXCR4-directed
theranostics in oncology and inflammation. Ann Nucl Med.
32:503–511. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ren C and Duan G: The expression of SDF-1α
and CXCR4 in the peripheral blood patients with rheumatoid
arthritis. West Chin Med J, 2018.
|
|
40
|
Leiblein M, Ponelies N, Johnson T, Marzi
J, Kontradowitz K, Geiger E, Marzi I and Henrich D: Increased
extracellular ubiquitin in surgical wound fluid provides a
chemotactic signal for myeloid dendritic cells. Eur J Trauma Emerg
Surg. 46:153–163. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Milenkovic VM, Stanton EH, Nothdurfter C,
Rupprecht R and Wetzel CH: The role of chemokines in the
pathophysiology of major depressive disorder. Int J Mol Sci.
20(20)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Karouzakis E, Gay RE, Michel BA, Gay S and
Neidhart M: DNA hypomethylation in rheumatoid arthritis synovial
fibroblasts. Arthritis Rheum. 60:3613–3622. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Doody KM, Bottini N and Firestein GS:
Epigenetic alterations in rheumatoid arthritis fibroblast-like
synoviocytes. Epigenomics. 9:479–492. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kanbe K, Takagishi K and Chen Q:
Stimulation of matrix metalloprotease 3 release from human
chondrocytes by the interaction of stromal cell-derived factor 1
and CXC chemokine receptor 4. Arthritis Rheum. 46:130–137.
2002.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Nanki T, Hayashida K, El-Gabalawy HS,
Suson S, Shi K, Girschick HJ, Yavuz S and Lipsky PE: Stromal
cell-derived factor-1-CXC chemokine receptor 4 interactions play a
central role in CD4+ T cell accumulation in rheumatoid
arthritis synovium. J Immunol. 165:6590–6598. 2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kanbe K, Chiba J, Inoue Y, Taguchi M and
Yabuki A: SDF-1 and CXCR4 in synovium are associated with disease
activity and bone and joint destruction in patients with rheumatoid
arthritis treated with golimumab. Mod Rheumatol. 26:46–50.
2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Diamond P, Labrinidis A, Martin SK,
Farrugia AN, Gronthos S, To LB, Fujii N, O'Loughlin PD, Evdokiou A
and Zannettino AC: Targeted disruption of the CXCL12/CXCR4 axis
inhibits osteolysis in a murine model of myeloma-associated bone
loss. J Bone Miner Res. 24:1150–1161. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kamezaki K, Kikushige Y, Numata A,
Miyamoto T, Takase K, Henzan H, Aoki K, Kato K, Nonami A, Kamimura
T, et al: Rituximab does not compromise the mobilization and
engraftment of autologous peripheral blood stem cells in
diffuse-large B-cell lymphoma. Bone Marrow Transplant. 39:523–527.
2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zou S, Zhang D, Xu Z, Wen X and Zhang Y:
JMJD3 promotes the epithelial-mesenchymal transition and migration
of glioma cells via the CXCL12/CXCR4 axis. Oncol Lett.
18:5930–5940. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li XQ, Zhang ZL, Tan WF, Sun XJ and Ma H:
Down-regulation of CXCL12/CXCR4 expression alleviates
ischemia-reperfusion-induced inflammatory pain via inhibiting glial
TLR4 activation in the spinal cord. PLoS One.
11(e0163807)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
McInnes IB and Schett G: Pathogenetic
insights from the treatment of rheumatoid arthritis. Lancet.
389:2328–2337. 2017.PubMed/NCBI View Article : Google Scholar
|