Introduction
Sepsis is defined as a life-threatening organ
dysfunction caused by a host's dysregulation of infection. In
sepsis, the immune response elicited by invading pathogens does not
return to homeostasis, ultimately leading to pathological syndrome
characterized by persistent excessive inflammation and
immunosuppression (1). Sepsis causes
a systemic dysregulated inflammatory response characterized by
excessive proinflammatory mediators (2). Therefore, it can be prevented by
controlling systemic inflammation. Nuclear factor-κB (NF-κB) acts
as a transcription factor regulating the transcription of different
genes, including pro-inflammatory cytokines, chemokines, adhesion
molecules and growth factors (3).
Regulation of transcription factor NF-κB activation may be a
potential treatment for sepsis. Lentinus edodes polysaccharide is a
cell wall glucan extracted from the fruiting body of Lentinus
edodes with significant anticancer and antitumor and
immunomodulatory activities (4).
However, the anti-inflammatory mechanism of lentinan is still
unclear, and the role of lentinan in sepsis is not clear. In this
study, rats with scald and endotoxin challenged were used as models
to simulate clinical burn sepsis. The influence of lentinan on
NF-κB activity and plasma inflammatory cytokine expression was
observed. The activity of lentinan on NF-κB in vivo was
investigated. The influence and regulation of systemic inflammatory
response in sepsis provide a theoretical basis for future research
on the role of lentinan in sepsis.
Materials and methods
Main reagents and equipment
Lipopolysaccharide (LPS) (O55:B5) (L2880;
Sigma-Aldrich; Merck KGaA), lentinan (Shanxi Senfu Natural Products
Co., Ltd.), hematoxylin-yen dyeing solution (G1005; Wuhan Google
Biotechnology), nuclear transcription factor NF-κB antibody
(bs-2695R; Beijing Boaosen Bio), secondary antibody, histological
kit DAB chromogenic reagent (K5007; DAKO), interleukin-4 (IL-4),
IL-6, IL-10, tumor necrosis factor-α (TNF-α) detection kit (Beijing
Sizhengbai Biotechnology Co., Ltd.), dehydrator (JJ-12J; Wuhan
Junjie Electronics Co., Ltd.), embedding machine (JB-P5; Wuhan
Junjie Electronics Co., Ltd.), pathology slicer (RM2235; Shanghai
Leika Instrument Co., Ltd.), upright fluorescence microscope (Nikon
Eclipse TI-SR; Nikon).
Animal grouping and model
building
Seventy-two healthy male SD rats, 8 weeks old,
weighing 250-270 g (purchased from Guangdong Medical Animal
Center), with a feeding environment of 12 h light and 12 h darkness
(animal experiments were conducted in accordance with the
provisions of the National Institutes of Health guidelines for the
care and use of experimental animals and the requirements of
general recommendations, and approved by the Ethics Committee of
Experimental Animal Welfare, Plastic Surgery Hospital, Chinese
Academy of Medical Sciences, Beijing, China). Rats were randomly
divided into 6 groups (12 in each group): Normal control group,
burn sepsis group, positive drug control group, burn septic
lentinan low concentration group (50 mg/kg), medium concentration
group (100 mg/kg) and high concentration group (200 mg/kg). Model
establishment: Preoperative fasting for 24 h, free drinking water,
10% chloral hydrate (0.3 g/kg) intraperitoneal (i.p.) injection
anesthesia prior to cervical dislocation, there was no sign of
peritonitis, pain or discomfort found after injection. The general
indications are slow breathing, eyes are insensitive to light, the
painful contraction when pinching its toes with tweezers completely
disappear. After full anesthesia, the rats had regular breathing,
slow eyelid reflexes and the contractile reflexes disappeared.
After back hair removal, the rats given a stage III scald at 30˚C
for 12 sec, and then they were immediately given i.p. injection of
4 mg endotoxin (O55: B5) to simulate burn sepsis and anti-shock
with 100 ml of normal saline. The rats were raised in separete
cages. Each group was intraperitoneally injected with normal
saline, anti-inflammatory drugs, low concentration of lentinan (50
mg/kg), medium concentration (100 mg/kg) and high concentration
(200 mg/kg) 30 min before injury. The rats were monitored regularly
during and after the operation. If abnormal symptoms such as
convulsion or severe pain were found in the rats, the operation was
stopped immediately and the neck was cut off for euthanasia. Blood
collection was conducted 24 h after the model, 10% chloral hydrate
(0.3 g/kg) was intraperitoneally injected for anesthesia and the
toe pinching test showed that contractile reflexes disappeared. The
rats were sacrificed immediately by cervical dislocation after the
blood was collected from the abdominal aorta. The rats were judged
dead after respiratory arrest following cervical dislocation. Some
of the fresh livers were immediately fixed in 4% paraformaldehyde,
and the other part of the fresh livers were preserved at -80˚C for
subsequent western blot protein determination.
Tissue paraffin embedded slice
The fresh tissue was fixed in 4% paraformaldehyde
for 24 h, then placed in a hanging basket and dehydrated and dipped
in a dewatering machine. The wax-impregnated tissue was embedded in
an embedding machine, and after the wax was solidified, the wax
block was taken out from the embedding frame and the wax block was
trimmed. The trimmed wax block was placed on a paraffin slicer and
sliced to a thickness of 4 µm. The slices were floated on a
spreader at 40˚C. The tissue was flattened in warm water, the
tissue was picked up with a glass slide, and placed in a 60˚C oven.
After the water-baked dry wax was roasted, it was taken out and
stored at room temperature for later use.
Hematoxylin and eosin (H&E)
staining
Paraffin sections were dewaxed in water and sliced
into Harris hematoxylin for 5 min. Washed with tap water, 1%
hydrochloric acid alcohol was differentiated for several seconds,
rinsed in tap water, 0.6% ammonia turned water blue, and was rinsed
again. The sections were stained for 3 min in Hematoxylin-Ihong
dyeing solution. After dehydration, the film was placed under a
microscope for microscopic examination and image collection and
analysis.
Western blot for the analysis of
protein content of liver tissue
The fresh liver tissue homogenate was taken. The
liver tissues of each group were dissolved in cleavage buffer and
the protein concentration was measured by BCA method. The tissue
lysis products were separated by 10% polyacrylamide gel
electrophoresis, then transferred to PVDF membrane, and then sealed
with 5% skim milk for 2 h. Then incubated with the first antibody
NF-κB (ab131546; Abcam) overnight at 4˚C. The second antibody was
incubated at 37˚C for 1 h, rinsed with TBST buffer three times, ECL
luminescent solution was reacted at room temperature for 30 sec,
and pressed in the dark. GAPDH was used as an internal
reference.
Plasma inflammatory factor
enzyme-linked immunosorbent assay (ELISA)
Twenty-four hours after the last dose, the rats were
given 10% chloral hydrate i.p. injection. After full anesthesia, ~5
ml of blood was collected from the abdominal aorta by anticoagulant
blood vessel. The rats were sacrificed immediately after the blood
collection, the supernatant of the collected blood was centrifuged
at 4˚C and 1,000 x g for 15 min and stored at -20˚C until use. The
serum levels of IL-4, IL-6, IL-10 and TNF-α in rats were detected
by ELISA. The experimental method was performed according to the
kit instructions.
Statistical analysis
Data were analyzed by one-way ANOVA using SPSS 16.0,
and the Tukey test was used for pairwise comparison between groups.
Results are expressed as mean ± standard deviation (mean ± SD).
P<0.05 was considered statistically significant.
Results
Lentinan treatment can significantly
reduce liver damage in burns and sepsis
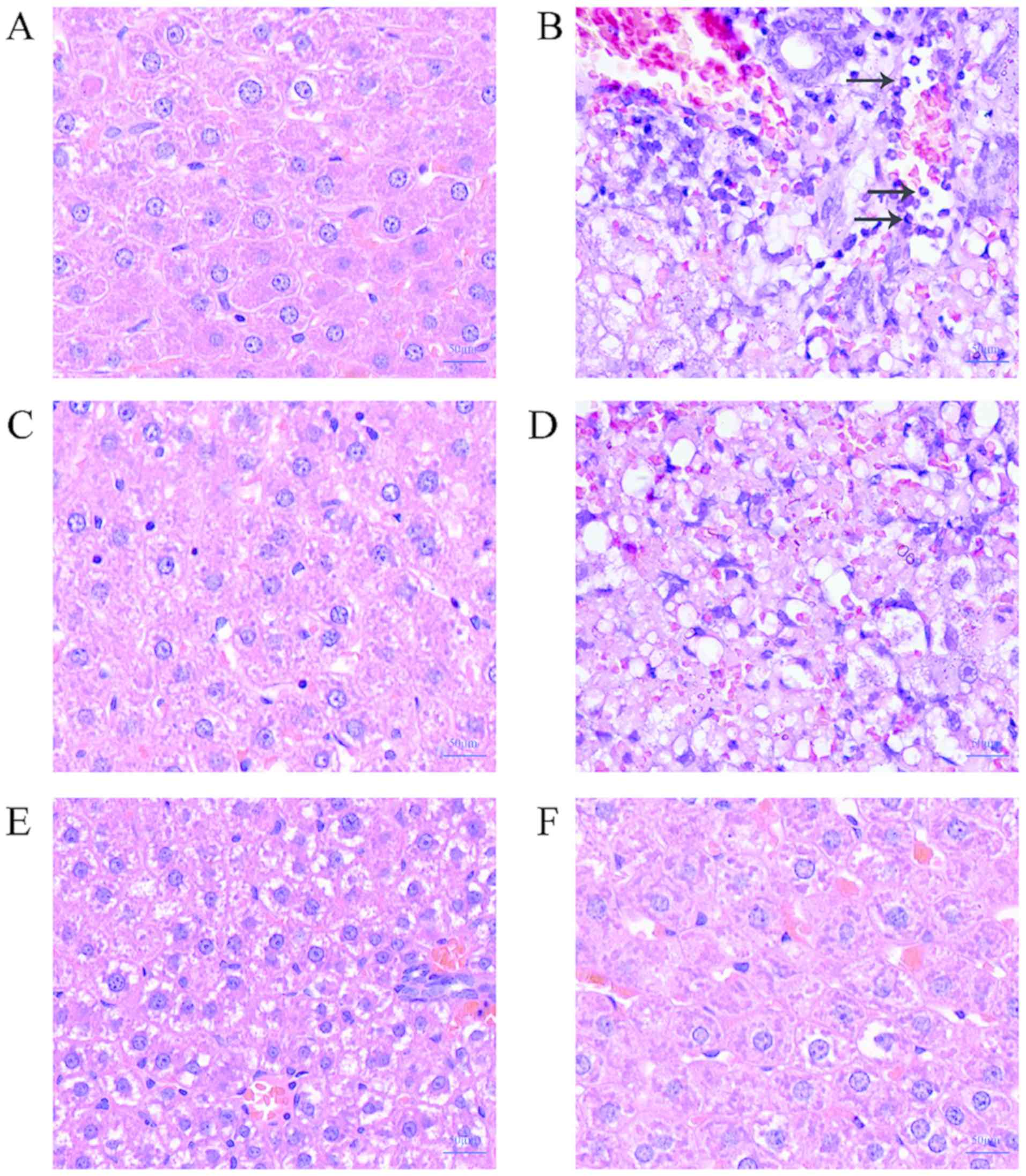
H&E staining results show that the liver cells
of the control group have clear boundaries and complete morphology.
Normal control group liver tissue was basically normal (Fig. 1A). The burnt sepsis model group had
blurred liver cell boundaries, severe vacuolar degeneration, and
hepatic cord disorder. There were a large number of inflammatory
cells (Fig. 1B), mild inflammatory
cell infiltration in the low-concentration lentinan-treated group,
mild vacuolar degeneration (Fig.
1D), and medium- and high-concentration lentinan-treated group
compared with the model group. The inflammatory cell infiltration
was obviously relieved, the hepatic cord was clear, and the
morphology was intact, and there was no obvious difference from the
positive drug control group (Figs.
1C, E and F). This result suggests that lentinan can
reduce the damage of LPS on rat liver in the burn sepsis model.
Lentinan treatment can significantly
reduce the expression of NF-κB in liver tissue
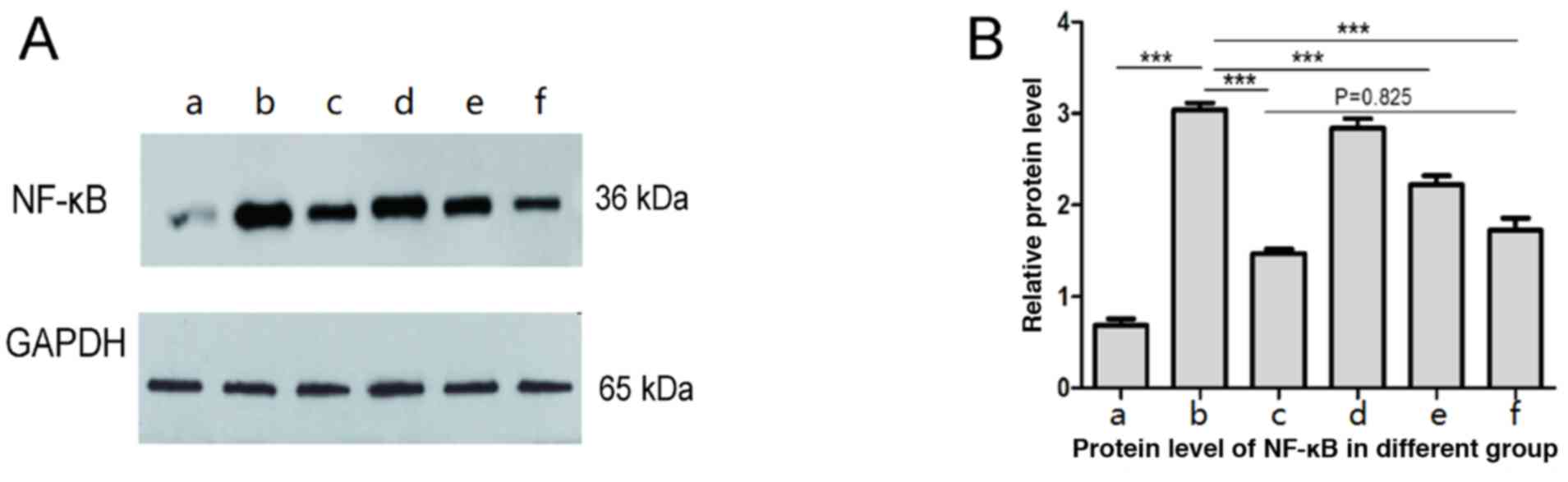
Western blot results showed that the expression of
NF-κB in liver tissue of burn sepsis group was significantly
enhanced (Fig. 2b) compared with
normal control group (Fig. 2a),
while in burn septic lentinan treatment group, the expression of
NF-κB in liver tissue was weakened compared with the burn sepsis
group (Figs. 2d-f). High
concentrations of lentinan treatment significantly reduced NF-κB
expression (Fig. 2f), and there was
no statistical significance compared with the positive control
group (P>0.05) (Fig. 2c). The
results suggest that the protective effect of lentinan on the liver
of burned sepsis model rats is achieved by inhibiting the NF-κB
inflammatory pathway, so the levels of inflammatory factors in
plasma of each group were further tested.
Lentinan treatment can reverse the
increase of plasma inflammatory factors in burn sepsis model
By measuring the levels of plasma inflammatory
factors in each group, plasma IL-4, IL-6, IL-10 were detected in
burned sepsis model rats. The level of TNF-α was significantly
increased (P<0.05), while the high concentration of lentinan
treatment significantly reduced plasma IL-4 (P<0.05), and IL-6
(P<0.05), TNF in burned sepsis model rats, TNF-α (P<0.05) and
IL-10 (P<0.05) levels, while low-middle concentration also
showed a downward trend, showing concentration-dependent, high
concentration of lentinan treatment had good anti-inflammatory
effect (Table I).
 | Table IConcentration of plasma IL-4, IL-6,
IL-10 and TNF-α in different groups. |
Table I
Concentration of plasma IL-4, IL-6,
IL-10 and TNF-α in different groups.
| Group | IL-4 (pg/ml) | IL-6 (pg/ml) | IL-10 (pg/ml) | TNF-α (pg/ml) |
|---|
| Normal control
group |
60.07±11.90a |
41.32±11.62a |
17.19±4.34a |
114.50±16.43a |
| Burn sepsis
group | 106.97±13.93 | 68.99±10.06 | 36.62±4.78 | 200.61±20.78 |
| Positive drug control
group |
83.27±14.11a |
41.55±6.34a | 24.31±4.68 |
117.85±13.08a |
| Low concentration
groups |
82.05±18.17a | 67.20±8.34 | 30.40±2.74 |
165.15±14.19a |
| Medium concentration
group |
75.17±10.74a | 62.13±12.81 | 26.48±5.12 |
140.78±17.54a |
| High concentration
group |
60.64±8.84a |
44.86±8.63a |
24.71±4.01a |
117.36±14.34a |
Discussion
Sepsis is a serious clinical syndrome with a
complicated path mechanism. Systemic inflammation and immune
response are important factors in the formation of sepsis (5). The function of different organ systems
of the body is impaired when the infection develops beyond the
compensatory capacity of the body. In general, the respiratory
system is the first system to malfunction, followed by the liver,
kidneys, heart, and so on. Organ failure is closely related to
mortality in patients with sepsis. The results of this study show
that lentinan can reduce the damage of sepsis to the liver to a
certain extent, and provide a theoretical basis for the application
of lentinan in sepsis.
The inflammatory response is the main cause of
sepsis. Therefore, controlling inflammation within an effective
range is a key method for the treatment of sepsis. The inflammatory
response to sepsis is mainly caused by overproduction of
pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6. In
addition, inhibition of secretion of these cytokines can delay and
reduce the incidence of sepsis and mortality in patients or animal
models of sepsis (6). Our results
are consistent with previous studies. The inflammatory factors such
as TNF-α and IL-6 in the plasma of the model group were
significantly increased. The results show that the treatment of
lentinan can greatly reduce the expression levels of these
inflammatory factors in plasma. This result suggests that lentinan
can reduce the damage of liver caused by sepsis, which may be
achieved by inhibiting the expression level of inflammatory
factors. It points the way for further study of its specific
mechanism.
NF-κB is involved in the transcriptional expression
of a variety of genes, including pro-inflammatory cytokines,
chemokines, adhesion molecules and other inducing factors, and is a
very common nuclear transcription factor in cells (7). Normally located in the cytoplasm and
inhibited by IκB-α, IκB-α binds to NF-κB to form a polymer when the
cells are not stimulated. At this time, NF-κB is inactive and
stimulated by TNF-α, IκB-α is degraded by the ubiquitin system, and
NF-κB is activated into the nucleus to participate in the
expression of inflammatory factors, forming a vicious circle.
Williams et al (8) found that
when NF-κB was activated early, sepsis mortality would be
positively correlated. Controlling NF-κB is obviously helpful for
the treatment of sepsis (9). The
results of this experiment indicate that lentinan reduces the
damage of sepsis to the liver by inhibiting the nuclear
transcription factor NF-κB.
Lentinus eddoes polysaccharide is a kind of
β-glucan, which has antitumor, anti-bacterial, anti-viral and
immunomodulatory effects, and is extracted from the fruiting body
of the mushroom (10-12),
and the effect of lentinan on inflammatory reaction has also been
reported (13). A study found that
Lentinus edodes polysaccharide can downregulate TLR4-mediated NF-κB
signaling pathway and IL-13, CD30L and expression of other
cytokines under conditions of lipopolysaccharide-induced
inflammatory response (14). TLR4 is
distributed in tissues such as spleen, liver and lung, and is an
important molecule for cell recognition of LPS. The expression of
TLR4 is closely related to the response of cells to LPS. Inhibition
of TLR4 and its downstream signaling pathways, including
overexpression of inflammatory factors, is considered as a
treatment for sepsis (15). Our
experimental evidence also suggests that lentinan can downregulate
the expression of NF-κB and further confirmed that the mushroom
polysaccharide treatment can inhibit the excessive increase of
IL-4, IL-6, IL-10 and TNF-α levels in plasma.
In conclusion, the results of this study suggest
that lentinan may reduce the content of serum IL-4, IL-6, IL-10 and
TNF-α by downregulating the expression of NF-κB in liver tissue,
thereby reducing burn sepsis and causing acute damage to the liver.
The results of this study suggest that lentinan administration may
be an adjuvant treatment for sepsis treatment, laying the
foundation for further research.
Acknowledgements
The authors would like to thank Yujia Biotechnology
Co., Ltd., for providing us with the laboratory where some of the
experiments were performed.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL made substantial contributions to the design of
the study and wrote the manuscript. YG and YQ were responsible for
immunohistochemistry and ELISA. ZL and GEM contributed to
observation indexes analysis. The final version was read and
adopted by all the authors. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Plastic Surgery Hospital, Chinese Academy of Medical Sciences and
Peking Union Medical College (Beijing, China). Animal experiments
were conducted in accordance with the National Institutes of Health
Laboratory Animal Care and Use Guidelines (16).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van der Poll T, van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wiersinga WJ, Leopold SJ, Cranendonk DR
and van der Poll T: Host innate immune responses to sepsis.
Virulence. 5:36–44. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hoffmann A, Levchenko A, Scott ML and
Baltimore D: The IkappaB-NF-kappaB signaling module: Temporal
control and selective gene activation. Science. 298:1241–1245.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fang N, Li Q, Yu S, Zhang J, He L, Ronis
MJ and Badger TM: Inhibition of growth and induction of apoptosis
in human cancer cell lines by an ethyl acetate fraction from
shiitake mushrooms. J Altern Complement Med. 12:125–132.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Venet F and Monneret G: Advances in the
understanding and treatment of sepsis-induced immunosuppression.
Nat Rev Nephrol. 14:121–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aikawa N, Takahashi T, Fujimi S, Yokoyama
T, Yoshihara K, Ikeda T, Sadamitsu D, Momozawa M and Maruyama T: A
phase II study of polyclonal anti-TNF-α (AZD9773) in Japanese
patients with severe sepsis and/or septic shock. J Infect
Chemother. 19:931–940. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu SF and Malik AB: NF-kappa B activation
as a pathological mechanism of septic shock and inflammation. Am J
Physiol Lung Cell Mol Physiol. 290:L622–L645. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Williams DL, Ha T, Li C, Kalbfleisch JH
and Ferguson DA Jr: Early activation of hepatic NFkappaB and NF-IL6
in polymicrobial sepsis correlates with bacteremia, cytokine
expression, and mortality. Ann Surg. 230:95–104. 1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jin LY, Li CF, Zhu GF, Wu CT, Wang J and
Yan SF: Effect of siRNA against NF-κB on sepsis induced acute lung
injury in a mouse model. Mol Med Rep. 10:631–637. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu Q, Dong L, Li H, Yuan J, Peng Y and
Dai S: Lentinan mitigates therarubicin-induced myelosuppression by
activating bone marrow-derived macrophages in an
MAPK/NF-κB-dependent manner. Oncol Rep. 36:315–323. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu W, Gu J, Qi J, Zeng XN, Ji J, Chen ZZ
and Sun XL: Lentinan exerts synergistic apoptotic effects with
paclitaxel in A549 cells via activating ROS-TXNIP-NLRP3
inflammasome. J Cell Mol Med. 19:1949–1955. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang Y, Li Q, Wang J, Cheng F, Huang X,
Cheng Y and Wang K: Polysaccharide from Lentinus edodes combined
with oxaliplatin possesses the synergy and attenuation effect in
hepatocellular carcinoma. Cancer Lett. 377:117–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ahn H, Jeon E, Kim JC, Kang SG, Yoon SI,
Ko HJ, Kim PH and Lee GS: Lentinan from shiitake selectively
attenuates AIM2 and non-canonical inflammasome activation while
inducing pro-inflammatory cytokine production. Sci Rep.
7(1314)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Zhao J, Zhao Y, Zong S, Tian Y,
Chen S, Li M, Liu H, Zhang Q, Jing X, et al: Therapeutic effects of
lentinan on inflammatory bowel disease and colitis-associated
cancer. J Cell Mol Med. 23:750–760. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Savva A and Roger T: Targeting toll-like
receptors: Promising therapeutic strategies for the management of
sepsis-associated pathology and infectious diseases. Front Immunol.
4(387)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Vogel HG, Vogel WH, Schölkens BA, Sandow
J, Müller PG and Vogel WF: Guidelines for the care and use of
laboratory animals. Thromb Haemost. 58:1078–1084. 1987.
|