Introduction
Chronic kidney disease (CKD) is a leading cause of
end-stage renal disease worldwide and is a global public health
concern (1). A previous study has
demonstrated that autophagy is associated with renal tubular
epithelial cell (RTEC) damage, which eventually results in CKD
(2). Dysregulated autophagy may
occur in response to either intracellular or extracellular factors,
such as endoplasmic reticulum stress, oxidative stress or pathogen
infection (3,4). Consistent with those findings, our
previous study revealed that advanced oxidation protein products
(AOPP), a toxic protein product produced in patients with CKD,
induced RTEC injury by inhibiting cell autophagy (5). Therefore, enhancing RTEC autophagy may
suppress the progression of CKD.
Mesenchymal stem cells (MSCs) are mesodermal stem
cells with self-renewal properties that can differentiate into a
number of mesodermal cell lineages. Compared with MSCs from other
sources, human umbilical cord-derived MSCs (hUC-MSCs) have received
particular attention due to their abundant sources, simple
extraction, good growth capacity, lower immunogenicity and
decreased potential for harm to mothers and newborns (6). MSC transplantation may be an effective
treatment modality in acute and chronic kidney disease (7); however, the underlying mechanisms
remain unclear. Moreover, MSCs were reported to enhance autophagy
in the nervous (8), digestive
(9), respiratory (10) and endocrine (11) systems. However, whether hUC-MSCs
increase renal cell autophagy to serve a protective role remains
unknown.
Evidence suggests that MSCs promote the repair of
damaged organs via a paracrine mechanism (12), including the secretion of growth
factors such as hepatocyte growth factor (HGF), vascular
endothelial growth factor and epidermal growth factor. HGF is an
antifibrotic cytokine and has been reported to attenuate organ
fibrosis, including hepatic (13)
and renal (14) fibrosis. Liu et
al (15) found that MSCs
promoted the regeneration of damaged neurons through the secretion
of HGF in a model of Parkinson's disease. Lan et al
(16) reported that HGF secreted
from oncostatin M-preconditioned MSCs alleviated lung fibrosis in
mice. Eom et al (17)
demonstrated that HGF induced the expression microtubule-associated
protein 1 light chain 3B (LC3B) II, an autophagy marker, in bone
marrow-derived MSCs. However, whether HGF secreted from hUC-MSCs
serves protective roles by enhancing RTEC autophagy in CKD requires
further investigation.
During autophagy, the biosynthesis of LC3II/LC3I and
beclin 1 increases, while upregulated expression of p62 inhibits
autophagy (18). Studies have
revealed that the PI3K/AKT/mTOR signaling pathway is an important
negative modulator of autophagy (19,20). Our
previous study revealed that AOPP inhibited HK-2 cell autophagy by
activating the PI3K/AKT/mTOR signaling pathway (5).
The present study investigated the role of hUC-MSCs
in AOPP-mediated inhibition of autophagy in human RTECs in CKD.
Furthermore, the effect of HGF secreted from hUC-MSCs in
hUC-MSC-enhanced autophagy, as well as the underlying mechanism in
HK-2 cells, were examined.
Materials and methods
Materials and reagents
LC3B, Beclin 1, p62, phosphorylated (p)-mTOR, mTOR,
p-AKT, AKT, PI3K antibodies and Ly294002, an inhibitor of the
PI3K/AKT/mTOR signal pathway, were obtained from Cell Signaling
Technology, Inc. GAPDH antibody was obtained from Bioworld
Technology, Inc. BSA was obtained from Sigma-Aldrich; Merck KGaA.
Hypochlorous acid (HOCl) was purchased from Fluka Chemie AG
(Sigma-Aldrich; Merck KGaA). Tivantinib, a competitive inhibitor of
HGF, and insulin-like growth factor 1 (IGF-1), an inducer of the
PI3K/AKT/mTOR signal pathway, were acquired from APeXBIO Technology
LLC. Recombinant human HGF (rhHGF, an analogs of HGF) was obtained
from PeproTech, Inc., and the HGF ELISA kit was purchased from
MultiSciences (Lianke) Biotech Co., Ltd. The Cell Counting Kit-8
(CCK-8) was purchased from Dojindo Molecular Technologies, Inc.
AOPP preparation
AOPP was prepared as previously described (21). Briefly, HOCl (200 mmol/l) was added
to a BSA solution for 30 min at room temperature and then dialyzed
against PBS at 4˚C to remove free HOCl for 24 h. Native BSA was
dissolved in PBS alone as the control. The AOPP content was
measured at a wavelength of 340 nm to obtain the absorbance under
acidic conditions and calibrated using chloramine-T in the presence
of potassium iodide.
HK-2 cell culture and treatment
HK-2 cells were purchased from the American Type
Culture Collection and cultured in DMEM/nutrient mixture F-12
(DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS and maintained at 37˚C in a humidified incubator containing
a 5% CO2 atmosphere. Cells were incubated in BSA (200
µg/ml), AOPP (200 µg/ml), rhHGF (343 pg/ml) conditions until they
reached 70-80% confluence for 48 h at 37˚C. In subsequent
experiments, cells were pretreated with 10 µM Ly294002, tivantinib,
or 10 ng/ml IGF-1 for 1 h and then incubated with or without AOPP
or co-cultured with hUC-MSCs for 48 h at 37˚C until the end of the
experiments.
hUC-MSC isolation and co-culture with
HK-2 cells
An adherent tissue method was used to isolate
hUC-MSCs. A umbilical cord sample was obtained from the Department
of Gynecology and Obstetrics, Zhujiang Hospital of Southern Medical
University (Guangzhou, China). The sample was harvested with the
mother's written informed consent. In the current study, hUC-MSCs
were extracted from a newborn whose mother was 35 years old,
hospitalized in March 2018. A large amount of hUC-MSCs could be
extracted from a single individual, and the cells were frozen when
the first generation reached 70-80% confluence and later thawed for
use. Briefly, a 10 cm hUC from a full-term healthy newborn was
cleaned with a PBS solution (containing 1% penicillin-streptomycin
double-resistant solution). The hUC was subsequently cut into small
pieces, the umbilical vein and artery were dislodged and stripped
from the Wharton's jelly tissue. Wharton's jelly was then cut into
1x1x1 mm fragments at room temperature and cultured in DMEM/F12
medium containing 5% FBS at 37˚C; generations 3-6 were identified
using flow cytometry, as previously described (22), and selected for follow-up
experiments. When the hUC-MSC were co-cultured with HK-2 cells at
37˚C for 48 h, a co-culture chamber, including 6 wells, were used
to block off the immediate contact between the hUC-MSC and HK-2
cells in order to explore the paracrine action of hUC-MSC. A total
of 5x104 HK-2 cells were seeded into the lower chamber
compartment and 4x103 hUC-MSC into the upper chamber
compartment. Cells were co-cultured in DMEM/F12 medium containing
5% FBS at 37˚C for 48 h.
Western blotting
Total HK-2 cell protein was extracted from cells
using pre-cooled radioimmunoprecipitation assay lysis buffer
containing cocktail protease inhibitors (Biotool; Stratech
Scientific, Ltd.). Protein concentrations were determined using a
Micro Bicinchoninic Acid Assay kit (CoWin Biosciences), according
to the manufacturer's protocol. According to the expression
abundance and molecular weight of the proteins, 50 µg of LC3B and
p62 were separated using 12% SDS-PAGE and 20 µg of the remaining
proteins were separated using 10% SDS-PAGE and then transferred to
PVDF membranes. Subsequently, the membranes were blocked in 5%
non-fat milk powder at room temperature for 2 h, followed by
primary antibody incubation at 4˚C overnight. The following primary
antibodies were used: Anti-LC3B, beclin 1, p62, p-mTOR, mTOR,
p-AKT, AKT, PI3K (dilution, 1:1,000) and anti-GAPDH (dilution,
1:5,000). After incubation with the horseradish peroxidase
(HRP)-conjugated secondary antibodies at room temperature for 2 h,
immunoreactive proteins were detected using an enhanced
chemiluminescence system. Semiquantitative analysis was performed
using the ImageJ system (National Institutes of Health). GAPDH was
used as the internal control.
Immunofluorescence staining
A total of 103 HK-2 cells plated in
96-well plates were fixed with 4% paraformaldehyde for 10 min at
room temperature, permeabilized with 0.5% Triton X-100 for 10 min
and incubated in 5% BSA for 1 h at room temperature, followed by
incubation with LC3B antibodies (dilution, 1:50) overnight at 4˚C.
Fluorescently-labeled secondary antibodies (Alexa Fluor®
488; dilution, 1:400) were applied for 1 h at room temperature
while the samples were protected from light, followed by an
incubation with 0.1% DAPI for 10 min at room temperature. The cells
were observed and recorded using an inverted fluorescence
microscope (magnification, x40).
CCK-8 assay
A total of 103 HK-2 cells were cultured
under tivantinib treatment for 48 h. The growth medium was removed
and the wells were washed twice with PBS. All the wells were filled
with fresh medium containing 90 µl DMEM/F12 and 10 µl CCK-8
solution. After incubation for 30 min at 37˚C, cell viability was
assessed via optical density (OD) detection at a wavelength of 450
nm with a microplate reader. The cell viability and IC50
were calculated using the OD values, according to the
manufacturer's instructions.
ELISA
HGF ELISA kits were used to measure the expression
of HGF (cat. no. 70-EK1H011) according to the manufacturer's
protocol. HK-2 cells without rhHGF served as the normal control
group. The supernatant of HK-2 cells was collected after
centrifugation at a speed of 1,000 rpm for 5 min at room
temperature and 100 µl standards or samples were added to the
microplates in triplicate, followed by addition of 50 µl diluted
detection antibody and incubation at room temperature for 2 h. A
total of 100 µl diluted streptavidin-HRP was added and incubated
for 45 min at room temperature. Finally, 100 µl of substrate
solution protected from light was applied at room temperature for
20 min. The absorbance was read at 450 and 630 nm using a
microplate reader.
Statistical analysis
All experiments were conducted in triplicate. The
results are presented as the mean ± SD. Differences among the
groups were determined using one-way ANOVA. The Least Significant
Difference method or Bonferroni's test was used to compare two
groups when the assumption of equal variances was met. Otherwise,
the Dunnett T3 method was used. P<0.05 was considered to
indicate a statistically significant difference. Analysis was
performed using SPSS software (version 20.0; IBM Corp.).
Results
hUC-MSCs enhance autophagy in
AOPP-treated HK-2 cells
The present study investigated whether hUC-MSCs
enhanced autophagy in AOPP-treated HK-2 cells. The expression
levels of the autophagy-related proteins LC3II/LC3I, beclin 1 and
p62 were determined via western blotting. As indicated in Fig. 1A and B, AOPP significantly decreased the protein
levels of LC3II/LC3I and beclin 1 and increased the protein level
of p62. When AOPP-treated HK-2 cells were co-cultured with
hUC-MSCs, this effect was partially reversed. Similarly,
immunofluorescence staining revealed that LC3BII-positive staining
was markedly increased in the hUC-MSC and HK-2 co-culture system
compared with the HK-2 only group (Fig.
1C). These results indicated that hUC-MSCs may increase HK-2
cell autophagy in the presence of AOPP.
hUC-MSCs inhibit the PI3K/AKT/mTOR
signaling pathway in AOPP-treated HK-2 cells
HK-2 cells, alone or in co-culture with hUC-MSC,
were treated with AOPP and PI3K, AKT and mTOR levels were measured
via western blotting. In the present study, hUC-MSCs decreased the
PI3K and the phosphorylation of, AKT and mTOR in the AOPP-treated
hUC-MSC and HK-2 cell co-culture system compared with HK-2 cells
alone (Fig. 2A and B). However, IGF-1, an inducer of the
PI3K/AKT/mTOR signaling pathway (23), partially abrogated this effect in the
AOPP-treated hUC-MSC and HK-2 cell co-culture system (Fig. 2C and D). Therefore, hUC-MSCs inhibited the
PI3K/AKT/mTOR signaling pathway in AOPP-treated HK-2 cells.
HGF enhances HK-2 cell autophagy
ELISA was used to detect the levels of HGF in
rhHGF-treated HK-2 cells to investigate the role of HGF in HK-2
cell autophagy. It was revealed that the HGF level was increased in
the rhHGF treatment group compared with the normal control group
(Fig. 3A). Furthermore, western
blotting revealed that rhHGF increased the protein expression
levels of LC3II/LC3I and beclin 1 and decreased the p62 protein
level, compared with the normal control group (Fig. 3B and C). In conclusion, these data suggest that
HGF promotes HK-2 cell autophagy.
hUC-MSCs enhance AOPP-inhibited
autophagy in HK-2 cells via the secretion of HGF via the
PI3K/AKT/mTOR signaling pathway
To further confirm whether hUC-MSC-enhanced
autophagy was mediated by the secretion of HGF, the effect of
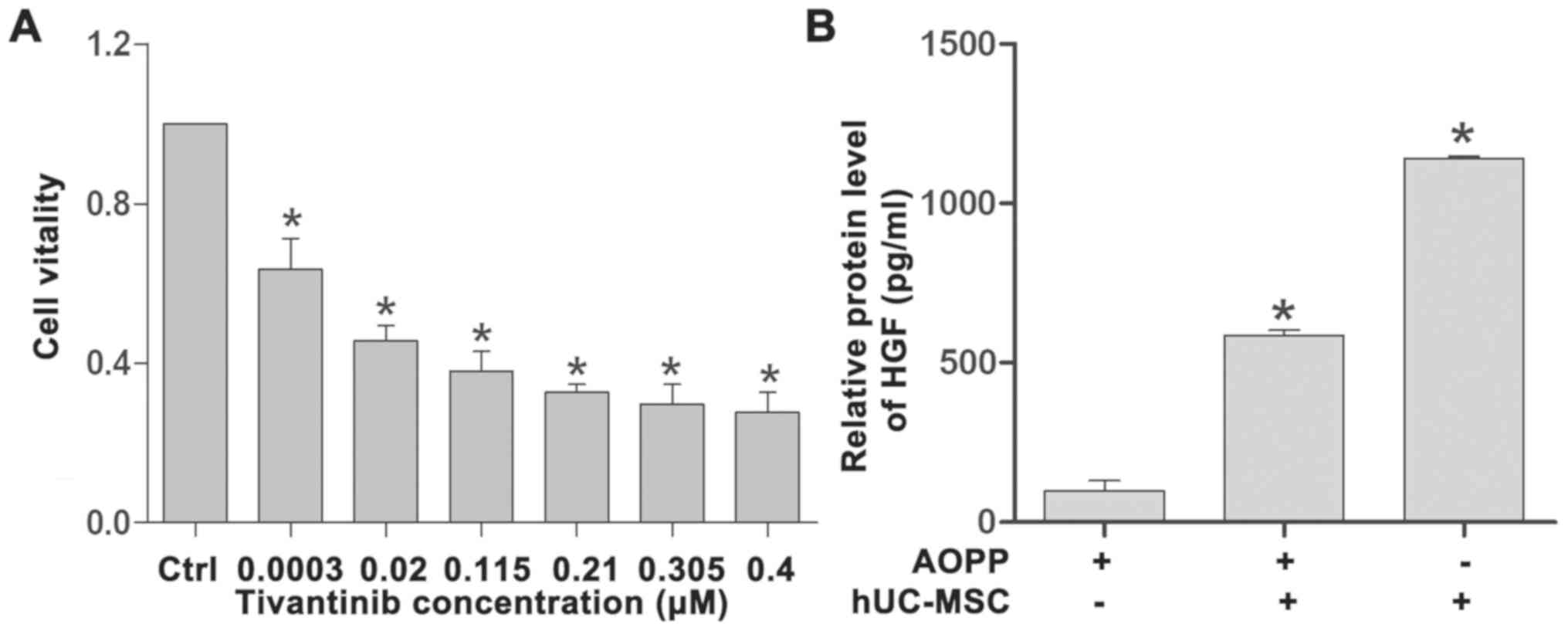
tivantinib, a competitive inhibitor of HGF (24), in HK-2 cells was investigated. HK-2
cells were cultured with 0.0003, 0.0200, 0.1150, 0.2100, 0.3050 and
0.4000 µM tivantinib and cell viability was measured via the CCK-8
assay. The results indicated that cell viability was gradually
decreased with increasing concentrations of tivantinib (Fig. 4A) and that the IC50 value
was 0.006 µM (data not shown). Tivantinib was added to block the
effect of hUC-MSCs on HK-2 cells. ELISA analysis revealed that the
level of HGF was increased in both the hUC-MSC co-culture and
hUC-MSC and AOPP co-culture groups compared with the AOPP only
group (Fig. 4B). In addition, the
HGF level in the hUC-MSC co-culture group was increased compared
with the hUC-MSC and AOPP co-culture group, which indicated that
AOPP may affect HGF expression.
In the AOPP-treated hUC-MSC and HK-2 cell co-culture
system, tivantinib downregulated LC3II/LC3I and beclin 1,
upregulated p62 and activated the PI3K and the phosphorylation of
AKT and mTOR (Fig. 5A-C). In
addition, immunofluorescence staining revealed that LC3BII-positive
staining was decreased in the AOPP-treated hUC-MSC and HK-2 cell
co-culture group incubated with tivantinib compared with the
AOPP-treated hUC-MSC and HK-2 cell co-culture group alone (Fig. 5D). However, with the further addition
of Ly294002, the PI3K and the phosphorylation of, AKT and mTOR was
partially inhibited (Fig. 5E and
F).
Discussion
The present study demonstrated the significant role
of hUC-MSCs in HK-2 cell autophagy in CKD. hUC-MSCs promoted cell
autophagy via inhibition of the PI3K/AKT/mTOR signaling pathway in
AOPP-treated HK-2 cells. Moreover, hUC-MSCs increased autophagy by
secreting HGF, an antifibrotic factor. Collectively, the results
suggested that hUC-MSCs may serve as a promising therapeutic
strategy in CKD through their paracrine action.
Our previous study reported that AOPP inhibited RTEC
autophagy and that autophagy inhibition induced RTEC injury
(5). The present study revealed that
hUC-MSCs enhanced AOPP-inhibited autophagy in HK-2 cells. A
previous study revealed that MSCs increased autophagy, thereby
protecting nerve cells in an Alzheimer's disease model (8). Furthermore, MSCs were demonstrated to
increase α-synuclein removal in Parkinson's disease by increasing
autophagy (25). In addition, Li
et al (26) demonstrated that
early intervention with MSCs prevented renal injury by ameliorating
the inflammatory microenvironment in diabetic rats and Tang et
al (27) reported that MSCs
alleviate acute renal injury by suppressing the C5a/C5a
anaphylatoxin chemotactic receptor-NF-κB signaling pathway. The
results of the present study suggested that hUC-MSCs enhanced HK-2
cell autophagy and inhibited the PI3K/AKT/mTOR signaling pathway,
which demonstrated a protective role of hUC-MSC in the
aforementioned studies. Liu et al (28) demonstrated that MSC-derived exosomes
inhibited H9C2 cell apoptosis by regulating autophagy via the
PI3K/Akt/mTOR signaling pathway. Moreover, Zhu et al
(29) revealed that MSCs affected
autophagy via the PI3K/AKT/mTOR signaling pathway in the treatment
of erectile dysfunction. To the best of our knowledge, the present
study was the first to demonstrate that hUC-MSCs enhanced autophagy
via inhibition of the PI3K/AKT/mTOR signaling pathway in HK-2
cells.
MSCs possess paracrine and endocrine functions and
serve anti-inflammatory, antiapoptotic, antioxidative,
proangiogenic, immunoregulatory and antifibrotic roles (30). Kennelly et al (31) reported that human MSC-derived HGF
exerted an antiapoptotic effect in chronic obstructive pulmonary
disease. Chang et al (32)
determined that several angiogenic cytokines, including HGF,
protected endothelial cells against radiation-induced apoptosis and
accelerated the recovery of irradiated mice. To determine whether
HGF enhanced autophagy, the present study investigated HK-2 cells
treated with rhHGF and the results suggested that rhHGF increased
HK-2 cell autophagy. It was previously reported that HGF activated
autophagy in colorectal cancer cells (33). Furthermore, another study indicated
that HGF protected cardiomyocytes from hypoxia-induced apoptosis by
upregulating cell autophagy (34).
To the best of our knowledge, the present study was the first to
demonstrate that HGF enhanced autophagy in renal cells, indicating
that HGF might exhibit therapeutic potential for renal diseases.
Furthermore, the present study revealed that hUC-MSCs enhanced
AOPP-inhibited HK-2 cell autophagy via the secretion of HGF.
Previous studies have demonstrated that MSCs
prevented renal injury and promoted renal recovery in renal
transplantation (35) and acute
kidney injury (36). Additionally,
clinical trials assessing the safety, feasibility and efficacy of
MSC-based therapy in various kidney diseases have been registered
with ClinicalTrials.Gov. However, the
majority of these clinical trials are still in phase I or II,
indicating the importance of exploring the mechanism of MSCs in
kidney protection. The present study revealed that HGF protein
levels were increased in the hUC-MSC and HK-2 cell co-culture
system, indicating that hUC-MSCs secreted HGF, which had an effect
on HK-2 cells. As hUC-MSCs and HGF enhanced HK-2 cell autophagy,
tivantinib was added to the AOPP-treated hUC-MSC and HK-2
co-culture system to block the effect of HGF. Tivantinib inhibited
hUC-MSC-upregulated autophagy via activating the PI3K/AKT/mTOR
signaling pathway. Lee et al (37) revealed that HGF is more abundantly
expressed in human embryonic stem cell-derived mesenchymal stem
cells than in adult bone marrow-derived MSCs (hBM-MSCs). However,
HGF-treated hBM-MSCs exhibited significantly improved therapeutic
efficacy by promoting telomere lengthening and inducing
mitochondrial DNA replication and function. Zhao et al
(34) reported that MSCs
overexpressing HGF were associated with decreased cardiomyocyte
apoptosis, enhanced angiogenesis and increased proliferation of
cardiomyocytes in myocardial infarction. These studies indicate
that MSCs serve a favorable role via HGF upregulation. Similarly,
the results of the present study demonstrated that hUC-MSCs
increased HGF levels and inhibited the PI3K/AKT/mTOR signaling
pathway and that this signaling pathway was re-activated by the HGF
inhibitor, tivantinib. Therefore, HGF may regulate the
PI3K/AKT/mTOR signaling pathway in HK-2 cells. Furthermore,
following the addition of Ly294002, an inhibitor of the
PI3K/AKT/mTOR signaling pathway, the PI3K/AKT/mTOR signaling
pathway was significantly inhibited. These results indicated that
hUC-MSCs enhanced HK-2 cell autophagy and inhibited the
PI3K/AKT/mTOR signaling pathway by secreting HGF. Furthermore,
hUC-MSCs enhanced HK-2 cell autophagy by secreting HGF, which
provided a novel mechanism for the role of hUC-MSCs in the
treatment of kidney diseases.
The present study did not explore how the
hUC-MSC-secreted HGF was transported to HK-2 cells to enhance cell
autophagy. Future in-depth studies will aim to explore the effect
of hUC-MSCs on HK-2 cells. In a future study, the expression of HGF
should be knocked out in hUC-MSCs prior to co-culture with HK-2
cells to confirm that hUC-MSC-secreted HGF affects HK-2 cells. In
addition, further in vivo studies are necessary to validate the
significance of hUC-MSCs and the secreted HGF in autophagy
enhancement in HK-2 cells and in renal diseases.
In conclusion, the present study revealed that
hUC-MSCs enhanced HK-2 cell autophagy by secreting HGF. MSCs may
serve a therapeutic role in regenerative medicine and understanding
the mechanisms of MSCs in renal protection may aid the development
of novel therapeutic strategies in CKD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Guangdong Province (grant no.
2018A030313557), the Health and Family Planning Bureau Scientific
Research Project of Foshan (grant no. 20170214) and the Project of
Guangdong Medical Science and Technology Research Foundation (grant
no. A2017157).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ML designed the current study and involved in
drafting the manuscript. TJ conducted the majority of the
experiments, assisted in designing the present study and revising
the manuscript. WZ assisted in isolating hUC-MSCs, co-culturing
with HK-2 cells and western blotting. WX conducted
immunofluorescence staining, CCK-8 assays and ELISA experiments. TG
performed data analysis. XT took participation in the majority
design of the work, in drafting the general content of the
manuscript and revising it critically for important intellectual
content, JZ made substantial contributions to conception, design,
analysis and interpretation of data, and revised and submitted the
manuscript. Moreover, XT and JZ both gave approval for the final
manuscript and agreed to be accountable for any queries related to
the accuracy or integrity of the current study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medial Ethics
Committee of Zhujiang Hospital, Southern Medical University
(Guangzhou, China). Samples were harvested with the mother's
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Woo KT, Choong HL, Wong KS, Tan HB and
Chan CM: The contribution of chronic kidney disease to the global
burden of major noncommunicable diseases. Kidney Int. 81:1044–1045.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ding Y, Kim S, Lee SY, Koo JK, Wang Z and
Choi ME: Autophagy regulates TGF-β expression and suppresses kidney
fibrosis induced by unilateral ureteral obstruction. J Am Soc
Nephrol. 25:2835–2846. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nijholt DA, de Graaf TR, van Haastert ES,
Oliveira AO, Berkers CR, Zwart R, Ovaa H, Baas F, Hoozemans JJ and
Scheper W: Endoplasmic reticulum stress activates autophagy but not
the proteasome in neuronal cells: Implications for Alzheimer's
disease. Cell Death Differ. 18:1071–1081. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Majmundar AJ, Wong WJ and Simon MC:
Hypoxia-inducible factors and the response to hypoxic stress. Mol
Cell. 40:294–309. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang J, Xiang X, Shu S, Zhang C, Liang Y,
Jiang T, Zhang W, Guo T, Liang X and Tang X: Advanced oxidation
protein products inhibit the autophagy of renal tubular epithelial
cells. Exp Ther Med. 15:3908–3916. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Detamore MS: Human umbilical cord
mesenchymal stromal cells in regenerative medicine. Stem Cell Res
Ther. 4(142)2013.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Morigi M and Benigni A: Mesenchymal stem
cells and kidney repair. Nephrol Dial Transplant. 28:788–793.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shin JY, Park HJ, Kim HN, Oh SH, Bae JS,
Ha HJ and Lee PH: Mesenchymal stem cells enhance autophagy and
increase β-amyloid clearance in Alzheimer disease models.
Autophagy. 10:32–44. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Amiri F, Molaei S, Bahadori M, Nasiri F,
Deyhim MR, Jalili MA, Nourani MR and Habibi Roudkenar M:
Autophagy-modulated human bone marrow-derived mesenchymal stem
cells accelerate liver restoration in mouse models of acute liver
failure. Iran Biomed J. 20:135–144. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Luo D, Hu S, Tang C and Liu G: Mesenchymal
stem cells promote cell invasion and migration and
autophagy-induced epithelial-mesenchymal transition in A549 lung
adenocarcinoma cells. Cell Biochem Funct. 36:88–94. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Zhao K, Hao H, Liu J, Tong C, Cheng Y, Xie
Z, Zang L, Mu Y and Han W: Bone marrow-derived mesenchymal stem
cells ameliorate chronic high glucose-induced β-cell injury through
modulation of autophagy. Cell Death Dis. 6(e1885)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ionescu L, Byrne RN, van Haaften T,
Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F
and Thébaud B: Stem cell conditioned medium improves acute lung
injury in mice: In vivo evidence for stem cell paracrine action. Am
J Physiol Lung Cell Mol Physiol. 303:L967–L977. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Matsuno Y, Iwata H, Umeda Y, Takagi H,
Mori Y, Kosugi A, Matsumoto K, Nakamura T and Hirose H: Hepatocyte
growth factor gene transfer into the liver via the portal vein
using electroporation attenuates rat liver cirrhosis. Gene Ther.
10:1559–1566. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lv W, Booz GW, Wang Y, Fan F and Roman RJ:
Inflammation and renal fibrosis: Recent developments on key
signaling molecules as potential therapeutic targets. Eur J
Pharmacol. 820:65–76. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu XS, Li JF, Wang SS, Wang YT, Zhang YZ,
Yin HL, Geng S, Gong HC, Han B and Wang YL: Human umbilical cord
mesenchymal stem cells infected with adenovirus expressing HGF
promote regeneration of damaged neuron cells in a Parkinson's
disease model. BioMed Res Int. 2014(909657)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lan YW, Theng SM, Huang TT, Choo KB, Chen
CM, Kuo HP and Chong KY: Oncostatin M-preconditioned mesenchymal
stem cells alleviate bleomycin-induced pulmonary fibrosis through
paracrine effects of the hepatocyte growth factor. Stem Cells
Transl Med. 6:1006–1017. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Eom YW, Oh JE, Lee JI, Baik SK, Rhee KJ,
Shin HC, Kim YM, Ahn CM, Kong JH, Kim HS, et al: The role of growth
factors in maintenance of stemness in bone marrow-derived
mesenchymal stem cells. Biochem Biophys Res Commun. 445:16–22.
2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bjørkøy G, Lamark T, Pankiv S, Øvervatn A,
Brech A and Johansen T: Monitoring autophagic degradation of
p62/SQSTM1. Methods Enzymol. 452:181–197. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Varshney P and Saini N: PI3K/AKT/mTOR
activation and autophagy inhibition plays a key role in increased
cholesterol during IL-17A mediated inflammatory response in
psoriasis. Biochim Biophys Acta Mol Basis Dis. 1864 (5 Pt
A):1795–1803. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tang X, Rong G, Bu Y, Zhang S, Zhang M,
Zhang J and Liang X: Advanced oxidation protein products induce
hypertrophy and epithelial-to-mesenchymal transition in human
proximal tubular cells through induction of endoplasmic reticulum
stress. Cell Physiol Biochem. 35:816–828. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xiang J, Jiang T, Zhang W, Xie W, Tang X
and Zhang J: Human umbilical cord-derived mesenchymal stem cells
enhanced HK-2 cell autophagy through MicroRNA-145 by inhibiting the
PI3K/AKT/mTOR signaling pathway. Exp Cell Res. 15:198–205.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rong L, Li Z, Leng X, Li H, Ma Y, Chen Y
and Song F: Salidroside induces apoptosis and protective autophagy
in human gastric cancer AGS cells through the PI3K/Akt/mTOR
pathway. Biomed Pharmacother. 122(109726)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ghanaatgar-Kasbi S, Khorrami S, Avan A,
Aledavoud SA and Ferns GA: Targeting the C-MET/HGF Signaling
Pathway in Pancreatic Ductal Adenocarcinoma. Curr Pharm Des.
24:4619–4625. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Park HJ, Shin JY, Kim HN, Oh SH and Lee
PH: Neuroprotective effects of mesenchymal stem cells through
autophagy modulation in a parkinsonian model. Neurobiol Aging.
35:1920–1928. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Y, Liu J, Liao G, Zhang J, Chen Y, Li
L, Li L, Liu F, Chen B, Guo G, et al: Early intervention with
mesenchymal stem cells prevents nephropathy in diabetic rats by
ameliorating the inflammatory microenvironment. Int J Mol Med.
41:2629–2639. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang M, Zhang K, Li Y, He QH, Li GQ, Zheng
QY and Zhang KQ: Mesenchymal stem cells alleviate acute kidney
injury by down-regulating C5a/C5aR pathway activation. Int Urol
Nephrol. 50:1545–1553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu H, Sun X, Gong X and Wang G: Human
umbilical cord mesenchymal stem cells derived exosomes exert
antiapoptosis effect via activating PI3K/Akt/mTOR pathway on H9C2
cells. Cell Biochem. 120:14455–14464. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhu GQ, Jeon SH, Bae WJ, Choi SW, Jeong
HC, Kim KS, Kim SJ, Cho HJ, Ha US, Hong SH, et al: Efficient
promotion of autophagy and angiogenesis using mesenchymal stem cell
therapy enhanced by the low-energy shock waves in the treatment of
erectile dysfunction. Stem Cells Int. 2018(1302672)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Khubutiya MS, Vagabov AV, Temnov AA and
Sklifas AN: Paracrine mechanisms of proliferative, anti-apoptotic
and anti-inflammatory effects of mesenchymal stromal cells in
models of acute organ injury. Cytotherapy. 16:579–585.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kennelly H, Mahon BP and English K: Human
mesenchymal stromal cells exert HGF dependent cytoprotective
effects in a human relevant pre-clinical model of COPD. Sci Rep.
6(38207)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chang PY, Zhang BY, Cui S, Qu C, Shao LH,
Xu TK, Qu YQ, Dong LH and Wang J: MSC-derived cytokines repair
radiation-induced intra-villi microvascular injury. Oncotarget.
8:87821–87836. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mira A, Morello V, Céspedes MV, Perera T,
Comoglio PM, Mangues R and Michieli P: Stroma-derived HGF drives
metabolic adaptation of colorectal cancer to angiogenesis
inhibitors. Oncotarget. 8:38193–38213. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhao L, Liu X, Zhang Y, Liang X, Ding Y,
Xu Y, Fang Z and Zhang F: Enhanced cell survival and paracrine
effects of mesenchymal stem cells overexpressing hepatocyte growth
factor promote cardioprotection in myocardial infarction. Exp Cell
Res. 344:30–39. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Casiraghi F, Perico N, Cortinovis M and
Remuzzi G: Mesenchymal stromal cells in renal transplantation:
Opportunities and challenges. Nat Rev Nephrol. 12:241–253.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Morigi M and De Coppi P: Cell therapy for
kidney injury: Different options and mechanisms - mesenchymal and
amniotic fluid stem cells. Nephron, Exp Nephrol. 126:59–63.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lee EJ, Hwang I, Lee JY, Park JN, Kim KC,
Kim GH, Kang CM, Kim I, Lee SY and Kim HS: Hepatocyte growth factor
improves the therapeutic efficacy of human bone marrow mesenchymal
stem cells via RAD51. Mol Ther. 26:845–859. 2018.PubMed/NCBI View Article : Google Scholar
|