Introduction
Appropriate control of body fluids is extremely
important while managing postoperative cardiac surgery patients.
Any cardiac procedure involving or not involving cardiopulmonary
bypass can cause a significant increase in total body water due to
high fluid load (1). The surgical
stress of the procedure and the use of extra-corporeal circulation
also contribute to increased body water levels (2). Management of such patients has been
primarily dependent on the use of loop diuretics such as
furosemide, which is the drug of choice. However, continual use of
loop diuretics is associated with several adverse effects including
electrolyte disturbances, deterioration of kidney function and
diuretic resistance (3). Furosemide
acts on the thick ascending loop of Henle where it inhibits sodium
reabsorption thereby leading to hyponatremia. Hyponatremia, in
turn, reduces drug efficacy and an increased dose of furosemide is
required for maintaining diuresis. Higher doses further aggravate
hyponatremia, leading to an endless cycle (4). To overcome these limitations of loop
diuretics, newer therapeutic agents have been developed.
Tolvaptan is an orally administered diuretic that
selectively antagonizes vasopressin V2 receptors. The unique
property of the drug is that it causes electrolyte-free diuresis
(5). In a randomized
placebo-controlled crossover trial of heart failure patients,
Costello-Boerrigter et al (5)
demonstrated the similar diuretic efficacy of tolvaptan and
furosemide. However, unlike furosemide, tolvaptan was not
associated with electrolyte imbalance and did not affect renal
function. Due to its beneficial pharmacological properties,
tolvaptan has been approved for managing heart failure patients
with numerous studies establishing its efficacy and safety profile
(6). Over the last decade,
researchers have also evaluated the efficacy of tolvaptan for
postoperative fluid management in cardiac surgery (1,7). In one
of the earliest studies, Nishi et al (1) compared a prospective cohort of 64 heart
valve patients managed using tolvaptan with a historic control
group of 55 patients managed with conventional diuretics. The
authors of that study found tolvaptan to be an effective diuretic
without causing electrolyte disbalance or worsening of renal
function. Other published retrospective observational studies have
also reported similar results (7-9).
However, a significant limitation of observational studies is the
high risk of bias. The best possible evidence to guide clinical
practice has always been in the form of a meta-analysis of
randomized controlled trials (RCTs). Therefore, the aim of the
present study was to systematically search the literature and
analyze evidence from RCTs comparing tolvaptan with conventional
diuretics for postoperative fluid management in cardiac surgery
patients.
Materials and methods
Study selection and search
strategy
We conducted this systematic review and
meta-analysis following the guidelines of the PRISMA statement
(Preferred Reporting Items for Systematic Reviews and
Meta-analyses) (10) and the
Cochrane Handbook for Systematic Reviews of Intervention (11). We followed the common medicine PICO
(Population, Intervention, Comparison, Outcome) framework for
selecting studies. This review included only randomized controlled
trials (RCTs) conducted on adult patients undergoing any type of
cardiac surgery (Population), and compared the efficacy
(Outcomes) of tolvaptan (Intervention) with
conventional diuretics (Comparison). Studies evaluating the
use of tolvaptan without any cardiac surgical intervention, on the
pediatric population and patients with kidney diseases were
excluded. We also excluded non-randomized studies, retrospective
observational studies, and case series.
An electronic literature search without any language
or time restriction was carried out on PubMed, Scopus, BioMed
Central, CENTRAL (Cochrane Central Register of Controlled Trials)
and Google scholar databases up to 1st December 2019. Keywords
‘Tolvaptan’, ‘Vasopressin receptor antagonist’, ‘surgery’, ‘cardiac
surgery’, ‘diuretics’, and ‘randomised controlled trials’ were used
in different combinations by two independent reviewers. Reference
lists of eligible studies and pertinent review articles were
hand-searched for the identification of any other studies.
Data extraction and outcomes
Search results were first screened by careful
evaluation of titles and abstracts. Full-texts of selected trials
were then obtained for further evaluation. Any discrepancies
between the two reviewers were resolved by discussion. Using a
standardized data collection sheet, data were extracted from the
included trials by the two reviewers independently. We extracted
names of study authors, year of the study, sample size,
inclusion/exclusion criteria, demographic details of the sample,
tolvaptan protocol, use of other diuretics and study outcomes.
Corresponding authors were contacted for any missing data via
emails.
The primary outcomes of interest were postoperative
urine output and the number of days for the return to pre-operative
body weight. Other secondary outcomes were serum levels of sodium
and potassium, post-operative kidney function, length of ICU
(intensive care unit) stay and new postoperative arrhythmias.
Risk of bias
Since the review included only RCTs, we utilized the
Cochrane Collaboration risk assessment tool for quality assessment
of the included trials (12). We
rated studies for risk of bias on the following items: random
sequence generation, allocation concealment, blinding of
participants and personnel, blinding of outcome assessment,
incomplete outcome data, selective reporting, and other biases. The
risk of bias was presented as a summary chart with a green circle
denoting low risk of bias, yellow circle denoting unclear risk of
bias and red circle denoting a high risk of bias for the particular
item.
Statistical analysis
Mean ± standard deviation (SD) values were extracted
for continuous variables and the number of events for categorical
variables. Studies in which continuous data were presented only in
graphical format, Engauge Digitizer Version 12.1 was used to
extract numerical values from study graphs by two independent
reviewers. Considering the heterogeneity among studies, we used the
random-effects model to pool data. Continuous variables were pooled
using mean difference (MD) and 95% confidence interval (CI) while
categorical data were summarised using the Mantel-Haenszel odds
ratio (OR) and 95% CI. Meta-analysis was conducted only if at least
three studies reported data on the same scale. We assessed
inter-study heterogeneity using the I2 statistic. Values
of 25-50% represented low, 50-75% medium and >75% represented
substantial heterogeneity. The software Review Manager (RevMan,
version 5.3; Nordic Cochrane Centre [Cochrane Collaboration],
Copenhagen, Denmark; 2014) was used for conducting the statistical
analysis. Since the number of studies was less than 10, publication
bias was not assessed.
Results
The results of the literature search and selection
process are denoted in Fig. 1. A
total of 11 studies were screened by their full text. Five were
excluded as they were not RCTs (1,7,9,13,14). One
study was conducted on pediatric patients (15), and another on patients with chronic
kidney disease (8). Finally, four
studies were included in this systematic review and meta-analysis
(2,16-18).
Details of the included studies are presented in Table I. All four trials were conducted in
Japan and published between January 2016 and February 2018.
Patients with chronic renal disease were excluded from all the
studies. The number of patients randomized to the tolvaptan group
varied from 19 patients in one study to 147 patients in another
trial. The mean age of included patients was more than 65 years in
all the studies. The dose of tolvaptan was standard at 7.5 mg
across trials but the duration of therapy varied. Tolvaptan was
given as needed after 3 days, for a total of 5 days or until the
body weight returned to pre-operative levels. Conventional
diuretics were administered in both tolvaptan and control groups in
all the studies. One trial studied tolvaptan in two separate groups
of high dose (15 mg) and low dose (7.5 mg) (18). Data from the low dose group were
extracted for this review.
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| | Sample size | Age in years (mean
± SD) | Male gender
(n) | |
|---|
| Author (Ref) | Study type | Study | Control | Study | Control | Study | Control | Surgery | Tolvaptan
protocol | Other diuretics
administered | Study
conclusions |
|---|
| Kato et al
(17) | RCT | 27 | 30 | 69±10.7 | 70.1±7.5 | 23 | 20 | Off pump coronary
artery bypass | Drug given on POD
1&2 with a dose of 7.5 mg Drug given as needed from POD 3 | Conventional loop
diuretics | Tolvaptan increases
urine output, minimally affects serum electrolytes and promotes
elimination of artery bypass |
| Matsuyama et
al (16) | RCT | 25 | 25 | 71.4±8.2 | 68.6±10 | 17 | 20 | Elective open heart
surgery | Drug started on POD
1 with a dose of 7.5 mg up to 5 days | Furosemide and
spironolactone | Tolvaptan increases
urine output without renal dysfunction |
| Suehiro et
al (18) | RCT | 19 | 20 | 66±15 | 71.6±8.6 | 11 | 11 | Open heart or
aortic surgery using CBP | Drug started on POD
1 with a dose of 7.5 mg Drug stopped when body weight returned to
normal | Furosemide and
spironolactone | Tolvaptan increases
urine output without adverse effects on serum electrolytes and
renal dysfunction |
| Kishimoto et
al (2) | RCT | 147 | 133 | 70.8±11.4 | 69.5±12.2 | 91 | 74 | Open heart surgery
using CBP | Drug started on POD
1 with a dose of 7.5 mg Drug stopped when body weight returned to
normal or till POD 5 | Furosemide | Tolvaptan helps
maintain urine output without affecting renal function |
Primary outcomes
Three trials reported data on urine output (2,17,18) and
number of days to return to pre-operative body weight (2,16,18) as
mean ± SD. Mean post-operative urine output was significantly
greater in patients receiving tolvaptan as compared to controls
(MD=0.39; 95% CI: 0.17 to 0.61; P=0.0006, I2=48%)
(Fig. 2). Similarly, the body weight
of patients on tolvaptan returned to pre-operative levels
significantly earlier as compared to control group (MD=-1.57; 95%
CI: -2.48 to -0.66; P=0.0007, I2=50%) (Fig. 3).
Secondary outcomes
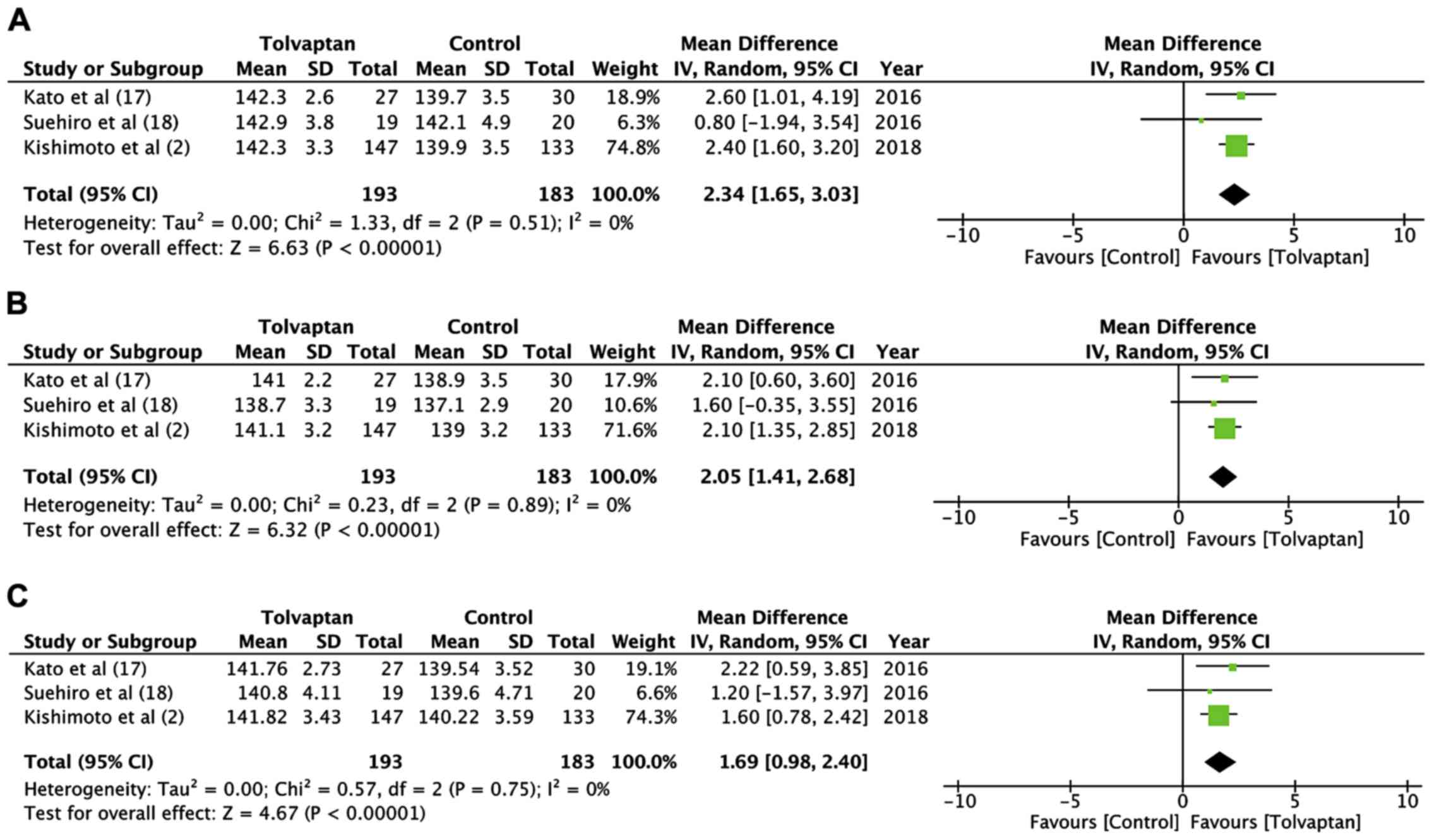
Data on highest, lowest and mean serum sodium levels
were available from three studies and were pooled separately
(2,17,18).
There was a statistically significant difference in the highest
postoperative serum sodium levels (MD=2.34; 95% CI:-1.65 to 3.03;
p<0.00001, I2=0%) (Fig.
4A) and lowest post-operative serum sodium levels (MD=2.05; 95%
CI: 1.41 to 2.68; p<0.00001, I2=0%) (Fig. 4B) between the tolvaptan and the
control groups. Similarly, the mean postoperative serum sodium
levels were significantly higher in the tolvaptan group as compared
to the controls (MD=1.69; 95% CI: 0.98 to 2.40; p<0.00001,
I2=0%) (Fig. 4C). The
study not included in the meta-analysis also reported significantly
higher levels of serum sodium from postoperative day 3 to 6 in
patients receiving tolvaptan (16).
For serum potassium levels, data only on the lowest
serum potassium level were available for a meta-analysis. Lowest
serum potassium was significantly higher with tolvaptan as compared
to control (MD=0.10; 95% CI: 0.01 to 0.18; P=0.03,
I2=19%) (Fig. 5).
Descriptive analysis of results from three studies indicated no
significant difference in mean serum potassium levels between the
two study groups (2,16,17).
There was no difference in the length of ICU stay
(MD=-0.09; 95% CI: -0.44 to 0.26; P=43, I2=60%)
(Fig. 6) and the number of new
post-operative arrhythmias between the two groups (OR=0.92; 95% CI:
0.43 to 1.97; P=0.84, I2=0%) (Fig. 7).
Kidney function
In the absence of coherent data for a meta-analysis,
kidney function outcomes from included studies are presented
descriptively. Kato et al (17) measured blood urea nitrogen (BUN) and
fractional excretion of urea nitrogen (FEUN) in both groups up to
postoperative day 7. There was no statistical significant
difference in BUN between the two groups; however, FEUN was
significantly higher on postoperative day 2 and 5 in the tolvaptan
group. In the study of Matsuyama et al (16), no significant difference was evident
in serum creatinine and BUN levels between the two groups except on
postoperative day 3 when the levels were significantly lower in the
tolvaptan group. Suehiro et al (18) found no significant difference in the
highest serum creatinine levels between the two study groups. No
other kidney function data was recorded in their trial. Kishimoto
et al (2) evaluated the
incidence of worsening of renal function which was defined as an
increase in the serum creatinine concentration of ≥0.3 mg/dl
compared to the preoperative value at any given day until
postoperative day 5. The incidence of worsening renal function was
significantly less in the tolvaptan group as compared to the
control group.
Risk of bias analysis
Fig. 8 presents the
authors' judgment of the risk of bias summary of included studies.
The randomization technique was clearly described by only one study
(18). None of the trials were
blinded. Selective reporting was observed in all except one trial
(2).
Discussion
Patients undergoing cardiac surgery experience
significant operative stress which leads to increased secretion of
arginine vasopressin (AVP) (19).
High levels of AVP act on the renal collecting duct causing
excessive water reabsorption and subsequent water imbalance in the
early postoperative period. Findings have shown that post-operative
cardiac patients tend to have a higher level of serum AVP
concentration as compared to non-cardiac surgery patients (20). Consequently, in view of increased
water imbalance in this cohort, the appropriate and timely use of
diuretics is extremely crucial to prevent morbidity and mortality.
The purpose of this study was therefore to investigate the efficacy
of tolvaptan, an aquaretic diuretic, which acts by selectively
inhibiting AVP from binding to V2 receptors in the kidney and its
role in cardiac surgery patients.
After a thorough literature search, a total of four
RCTs were found to compare tolvaptan with loop diuretics for
cardiac surgery patients. Importantly, patients randomized to
tolvaptan were also given low doses of loop diuretics and therefore
the results of this meta-analysis do not represent outcomes of
singular tolvaptan administration. The primary outcome of our
analysis was a significantly higher urine output and a lower number
of days required to return to pre-operative body weight with
tolvaptan. Our results concur with findings of studies on
congestive heart failure (CHF) patients which also demonstrated
higher urine output and a decrease in body weight when tolvaptan
was added to standard diuretic therapy (21-23).
However, although body weight tends to return to pre-operative
levels after the 5th postoperative day (24), excessive fluid retention in the early
period can cause a worsening of respiratory function due to
pulmonary congestion. This, in turn, increases the duration of
oxygenation and bed rest (2). It is
postulated that higher urine output and faster return to
pre-operative body weight with tolvaptan may contribute to faster
patient ambulation as well as reduced ICU and hospital stay
(2,25). However, in our analysis, we did not
find any difference in ICU stay between the two study groups.
Length of ICU stay could have been influenced by several other
factors such as the type of surgical procedure, duration of
surgery, and patient response to treatment.
An important secondary outcome of our study was
postoperative electrolyte levels. Loop diuretics, not only cause
hyponatremia, but they also increase sodium transport to the distal
tubule which stimulates the aldosterone-sensitive sodium pump,
leading to potassium excretion and consequent hypokalaemia
(4). Tolvaptan, in contrast to loop
diuretics, is expected to maintain electrolyte levels with a
water-only diuresis. Data from the included studies did not permit
a per-day analysis of sodium and potassium levels and meta-analysis
only for highest, lowest and mean sodium and lowest potassium
levels were carried out in this study. Our results indicate that
the highest, lowest, as well as mean sodium levels, were
significantly higher in the tolvaptan group as compared to patients
receiving only loop diuretics. The lower dose of loop diuretics and
the use of tolvaptan probably resulted in higher sodium levels in
the study group. Although hyponatremia is an independent predictor
of postoperative complications after cardiac surgery (26), it is important to note that the
lowest sodium levels in the control group were not below 135 mEq/l.
Secondly, electrolyte-free diuresis with tolvaptan can lead to
hypernatremia, which is a major side-effect (27). However, none of the four studies
reported extreme hypernatremia with tolvaptan. The exception was
Kishimoto et al (2) who
reported sodium levels of >147 mEq/l in 5 patients with
tolvaptan therapy, which returned to normal after discontinuation
of the drug.
As with sodium, maintenance of serum potassium
levels is also crucial in cardiac surgery patients. Significant
hypokalaemia can lead to supraventricular and ventricular
arrhythmias, which can increase morbidity, prolong hospital stay,
and escalate healthcare expenses for a cardiac patient (28). Our results indicated that the lowest
potassium levels were significantly higher with tolvaptan as
compared to the control group, albeit with small effect size and
the lower end of the CI close to zero (95% CI: 0.01-0.18). In
addition, we found no difference in the incidence of arrhythmias
between the two cohorts. The descriptive analysis revealed there
was no difference in the mean potassium levels between the
tolvaptan and control groups in any of the included studies.
Absence of any effect of tolvaptan on potassium excretion and use
of potassium-sparing diuretics along with furosemide may have
prevented significant hypokalaemia in both groups.
An aggressive diuretic treatment has been associated
with deterioration of renal function. Loop diuretics are known to
inhibit sodium-chloride transport in the ascending Henle loop and
increase flow in the distal nephron. This triggers
tubulo-glumerular feedback causing constriction of the afferent
arteriole and reduction in glomerular filtration rate (GFR)
(29). On the other hand, tolvaptan
has no influence on renal blood flow or GFR even in individuals
with chronic kidney disease (30).
One of the studies excluded from this review has demonstrated
tolvaptan to be safe in chronic renal patients undergoing cardiac
surgery (8). Similarly, studies on
CHF patients have shown that tolvaptan prevents acute renal failure
and reduces the amount of loop diuretic required for the management
of such patients (31). The absence
of coherent study variables and lack of data precluded a
meta-analysis of kidney function in the current study. Three of the
four included trials did not report any significant difference in
serum creatinine and BUN levels between the two groups while only
one trial (2) reported increased
incidence of worsening of renal function in the control group. In
the absence of sufficient high-quality studies, further trials are
required to establish the reno-protective effect of tolvaptan in
cardiac surgery patients.
The results of the present study should be
interpreted with the following limitations. Firstly, only four RCTs
were available for analysis for this review. The majority of the
included RCTs had a small sample size. Secondly, the quality of the
included trials was not high, which potentially downgrades the
level of evidence from our results. Thirdly, there was
methodological heterogeneity in the included studies for the
surgical procedure, duration of tolvaptan therapy, and
perioperative fluid management. Lastly, a meta-analysis on renal
function outcomes could not be conducted due to a lack of data.
Nevertheless, to the best of our knowledge, our
study is the first meta-analysis of only RCTs evaluating the
efficacy of tolvaptan for cardiac surgery patients. The last
meta-analysis on this topic was a pooled analysis of RCTs as well
as observational studies (25). It
omitted one of the four RCTs included in this review while
including observational studies on pediatric patients and renal
disease patients. Therefore, we believe the results of the present
study provide the most optimal level 1 evidence on the role of
tolvaptan in cardiac surgery patients. Within the limitations of
the current study, the results indicate that co-administration of
tolvaptan with low-dose of conventional diuretics significantly
increases urine output while maintaining serum sodium and potassium
levels in postoperative cardiac surgery patients. Faster return of
body-weight to pre-operative levels is seen with tolvaptan. Further
high-quality RCTs are required to provide stronger evidence on this
topic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HLC and WJ designed the paper. XL, ZM, HMC, JL, JW
and XZ were involved in literature search and data interpreted.
HLC, XL and ZM were responsible for the data analysis. HMC prepared
the manuscript. WJ edited the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nishi H, Toda K, Miyagawa S, Yoshikawa Y,
Fukushima S, Kawamura M, Yoshioka D, Saito T, Ueno T, Kuratani T,
et al: Effects of tolvaptan in the early postoperative stage after
heart valve surgery: Results of the STAR (Study of Tolvaptan for
fluid retention AfteR valve surgery) trial. Surg Today.
45:1542–1551. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kishimoto Y, Nakamura Y, Harada S, Onohara
T, Kishimoto S, Kurashiki T, Fujiwara Y and Nishimura M: Can
Tolvaptan Protect Renal Function in the Early Postoperative Period
of Cardiac Surgery? - Results of a Single-Center Randomized
Controlled Study. Circ J. 82:999–1007. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Felker GM: Loop diuretics in heart
failure. Heart Fail Rev. 17:305–311. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Haller C, Salbach P, Katus H and Kübler W:
Refractory oedema in congestive heart failure: A contributory role
of loop diuretics? J Intern Med. 237:211–214. 1995.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Costello-Boerrigter LC, Smith WB,
Boerrigter G, Ouyang J, Zimmer CA, Orlandi C and Burnett JC Jr:
Vasopressin-2-receptor antagonism augments water excretion without
changes in renal hemodynamics or sodium and potassium excretion in
human heart failure. Am J Physiol Renal Physiol. 290:F273–F278.
2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sen J, Chung E and McGill D: Tolvaptan for
Heart Failure in Chronic Kidney Disease Patients: A Systematic
Review and Meta-Analysis. Heart Lung Circ. 27:928–939.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ito H, Mizumoto T, Tempaku H, Fujinaga K,
Sawada Y and Shimpo H: Efficacy of Tolvaptan on Fluid Management
After Cardiovascular Surgery Using Cardiopulmonary Bypass. J
Cardiothorac Vasc Anesth. 30:1471–1478. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamada M, Nishi H, Sekiya N, Horikawa K,
Takahashi T and Sawa Y: The efficacy of tolvaptan in the
perioperative management of chronic kidney disease patients
undergoing open-heart surgery. Surg Today. 47:498–505.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kido T, Nishi H, Toda K, Ueno T, Kuratani
T, Sakaki M, Takahashi T and Sawa Y: Predictive factors for
responders to tolvaptan in fluid management after cardiovascular
surgery. Gen Thorac Cardiovasc Surg. 65:110–116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Higgins J and Green S (eds): Cochrane
Handbook for Systemic Reviews of Interventions Version 5.1.0. The
Cochrane Collaboration, 2011. http://www.handbook.cochrane.orgsimplewww.handbook.cochrane.org.
Updated March, 2011.
|
|
12
|
Higgins J, Altman D and Sterne J: Cochrane
Statistical Methods Group and the Cochrane Bias Methods Group.
Chapter 8: Assessing risk of bias in included studies. In: Cochrane
Handbook for Systemic Reviews of Interventions, Version 5.1.0. The
Cochrane Collaboration, 2011. http://www.handbook.cochrane.orgsimplewww.handbook.cochrane.org.
Accessed May 8, 2014.
|
|
13
|
Noguchi K, Tanaka M, Katayama I, Yamabe T,
Yuji D, Oosiro N and Sirouzu M: Efficacy of Tolvaptan in Patients
with Volume Overload after Cardiac Surgery. Heart Surg Forum.
18:E232–E236. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Kato TS, Ono S, Kajimoto K, Kuwaki K,
Yamamoto T and Amano A: Early introduction of tolvaptan after
cardiac surgery: A renal sparing strategy in the light of the renal
resistive index measured by ultrasound. J Cardiothorac Surg.
10(143)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Katayama Y, Ozawa T, Shiono N, Masuhara H,
Fujii T and Watanabe Y: Safety and effectiveness of tolvaptan for
fluid management after pediatric cardiovascular surgery. Gen Thorac
Cardiovasc Surg. 65:622–626. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Matsuyama K, Koizumi N, Nishibe T, Iwasaki
T, Iwahasi T, Toguchi K, Takahashi S, Iwahori A, Maruno K and Ogino
H: Effects of short-term administration of tolvaptan after open
heart surgery. Int J Cardiol. 220:192–195. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kato TS, Nakamura H, Murata M, Kuroda K,
Suzuki H, Yokoyama Y, Shimada A, Matsushita S, Yamamoto T and Amano
A: The effect of tolvaptan on renal excretion of electrolytes and
urea nitrogen in patients undergoing coronary artery bypass
surgery. BMC Cardiovasc Disord. 16(181)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Suehiro Y, Hosono M, Shibata T, Sasaki Y,
Hirai H, Nakahira A, Kubota Y, Kaku D and Suehiro S: Efficacy and
Safety Evaluation of Tolvaptan on Management of Fluid Balance after
Cardiovascular Surgery Using Cardiopulmonary Bypass. Osaka City Med
J. 62:111–119. 2016.PubMed/NCBI
|
|
19
|
Woods WG, Forsling ML and Le Quesne LP:
Plasma arginine vasopressin levels and arterial pressure during
open heart surgery. Br J Surg. 76:29–32. 1989.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jochberger S, Mayr VD, Luckner G, Wenzel
V, Ulmer H, Schmid S, Knotzer H, Pajk W, Hasibeder W, Friesenecker
B, et al: Serum vasopressin concentrations in critically ill
patients. Crit Care Med. 34:293–299. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Miyazaki T, Fujiki H, Yamamura Y, Nakamura
S and Mori T: Tolvaptan, an orally active vasopressin V(2)-receptor
antagonist - pharmacology and clinical trials. Cardiovasc Drug Rev.
25:1–13. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gheorghiade M, Gattis WA, O'Connor CM,
Adams KF Jr, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew
FA, Klapholz M, et al: Acute and Chronic Therapeutic Impact of a
Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF)
Investigators: Effects of tolvaptan, a vasopressin antagonist, in
patients hospitalized with worsening heart failure: A randomized
controlled trial. JAMA. 291:1963–1971. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gheorghiade M, Konstam MA, Burnett JC Jr,
Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T,
Ouyang J, et al: Efficacy of Vasopressin Antagonism in Heart
Failure Outcome Study With Tolvaptan (EVEREST) Investigators:
Short-term clinical effects of tolvaptan, an oral vasopressin
antagonist, in patients hospitalized for heart failure: The EVEREST
Clinical Status Trials. JAMA. 297:1332–1343. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Slight RD, Demosthenous N, Nzewi OC,
Soliman AR, McClelland DBL and Mankad PS: The effect of gain in
total body water on haemoglobin concentration and body weight
following cardiac surgery. Heart Lung Circ. 15:256–260.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bellos I, Iliopoulos DC and Perrea DN: The
Role of Tolvaptan Administration After Cardiac Surgery: A
Meta-Analysis. J Cardiothorac Vasc Anesth. 33:2170–2179.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Crestanello JA, Phillips G, Firstenberg
MS, Sai-Sudhakar C, Sirak J, Higgins R and Abraham WT:
Postoperative hyponatremia predicts an increase in mortality and
in-hospital complications after cardiac surgery. J Am Coll Surg.
216:1135–43, 1143.e1. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Matsuzaki M, Hori M, Izumi T and Fukunami
M: Tolvaptan Investigators. Efficacy and safety of tolvaptan in
heart failure patients with volume overload despite the standard
treatment with conventional diuretics: A phase III, randomized,
double-blind, placebo-controlled study (QUEST study). Cardiovasc
Drugs Ther. 25 (Suppl 1):S33–S45. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Peretto G, Durante A, Limite LR and
Cianflone D: Postoperative arrhythmias after cardiac surgery:
Incidence, risk factors, and therapeutic management. Cardiol Res
Pract. 2014(615987)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lazzarini V, Bettari L, Bugatti S,
Carubelli V, Lombardi C, Metra M and Dei Cas L: Can we prevent or
treat renal dysfunction in acute heart failure? Heart Fail Rev.
17:291–303. 2012.
|
|
30
|
Tominaga N, Kida K, Matsumoto N, Akashi
YJ, Miyake F, Kimura K and Shibagaki Y: Safety of add-on tolvaptan
in patients with furosemide-resistant congestive heart failure
complicated by advanced chronic kidney disease: A sub-analysis of a
pharmacokinetics/ pharmacodynamics study. Clin Nephrol. 84:29–38.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Shirakabe A, Hata N, Yamamoto M, Kobayashi
N, Shinada T, Tomita K, Tsurumi M, Matsushita M, Okazaki H,
Yamamoto Y, et al: Immediate administration of tolvaptan prevents
the exacerbation of acute kidney injury and improves the mid-term
prognosis of patients with severely decompensated acute heart
failure. Circ J. 78:911–921. 2014.PubMed/NCBI View Article : Google Scholar
|