Introduction
Corneal endothelial cells do not sufficiently
proliferate to enable endothelial regeneration; therefore, diseases
of the corneal endothelium, including pseudophakic bullous
keratopathy and Fuchs' endothelial dystrophy, require treatment by
transplantation of cadaveric donor corneal endothelial cells
(1). The two major goals of any
corneal transplant procedure are to restore vision and to promote
longevity of the donor cornea (1).
The surgical treatment for endothelial disease has evolved over
time toward endothelial keratoplasty or selective tissue
transplantation and away from full-thickness penetrating
keratoplasty (PK) (1). Endothelial
keratoplasty is associated with less astigmatism, more predictable
refractive outcomes, faster visual rehabilitation, a
biomechanically more stable globe, as well as reduced frequency of
suture infections and rejection (2).
Descemet stripping automated endothelial
keratoplasty (DSAEK) has become a common alternative to PK and is
becoming the procedure of choice for treating endothelial
dysfunction (1-4).
In fact, it is already reported to be more popular and adopted more
frequently than PK in the US (5),
Europe (6), Australia (7) and Asia (8) for the surgical management of corneal
endothelial diseases. The primary complication after DSAEK is donor
material detachment and dislocation (2). A newer surgical approach is Descemet
membrane endothelial keratoplasty (DMEK), which allows for
transplantation of less tissue, resulting in improved visual
acuity, faster visual rehabilitation and a lower rate of rejection
compared with DSAEK and PK (2,3).
However, DMEK is the more technically challenging procedure when
compared with PK and DSAEK (2).
Several studies have compared visual outcomes,
including uncorrected visual acuity, best spectacle-corrected
visual acuity (BSCVA), intraoperative and postoperative
complications, corneal biomechanical properties, corneal resistance
factors and graft survival, following PK and DSAEK (3,4,9-20).
The objective of the present study was to compare the outcomes of
graft survival, endothelial cell loss and vision improvement
between PK and DSAEK in the treatment of corneal endothelial
disease.
Materials and methods
Search strategy
The study was performed in accordance with the
Preferred Reporting Items for Systematic Reviews and Meta-analyses
guidelines (21). The PubMed,
CENTRAL (Cochrane) and Embase databases were searched for entries
added until September 20, 2019. The following keywords were used:
(penetrating keratoplasty) AND (Descemet stripping automated
endothelial keratoplasty). The search filters applied were as
follows: Abstract available; humans. Only two-arm prospective and
retrospective studies whose patients underwent PK or DSAEK were
considered for inclusion. Studies were required to quantitatively
report the outcomes of graft survival, endothelial cell density or
visual acuity at baseline and during follow-up. One-arm studies,
reviews, letters, comments, editorials, case reports, proceedings
and personal communications were excluded.
Initially, the titles and abstracts of the
identified studies were screened for eligibility and studies not
meeting the inclusion criteria were discarded. The remaining
studies underwent full-text review to further determine
eligibility. A total of two independent reviewers (KY and YZ)
performed the review and a third reviewer (YM) was consulted to
resolve any uncertainties. In addition, the reference lists of the
relevant studies were hand-searched to identify further studies
that met the inclusion criteria.
Data extraction
The following data were extracted from the included
studies: First author's name, study design, study period,
indication/diagnosis, incidence of prospective glaucoma, type of
keratoplasty, number of eyes or procedures, patient age and gender
and length of follow-up. The outcomes of endothelial cell density,
graft survival and BSCVA were also extracted. BSCVA was presented
in logMAR with lower values representing better visual acuity.
Endothelial cell density was reported as the mean ± standard
deviation (SD) (cells/mm2) and as % loss at each
time-point of evaluation during follow-up. Data were extracted by
two independent reviewers (KY and YZ) and a third author (YM) was
consulted, if necessary, to resolve any disagreements.
Quality assessment
The studies included were assessed with the Cochrane
Risk of Bias Assessment Tool for Non-Randomized Studies of
Interventions (22). The assessment
tool includes seven domains: Bias due to confounding, bias in
selection of participants into the study, bias in measurement of
interventions, bias due to departures from intended interventions,
bias due to missing data, bias in measurement of outcomes and bias
in selection of the reported result.
Statistical analysis
Patients' baseline characteristics and adverse
events are summarized descriptively using the mean ± SD, mean or
median of age with range (min-max). The summary effects of BSCVA
and endothelial cell density were calculated as the difference
(diff.) in means of change from baseline with 95% CI between the
two groups.
For the categorical outcome of graft survival rate,
an effect size of odds ratios (ORs) with 95% CIs were calculated to
compare the rates of graft survival between the two groups. The
heterogeneity test among studies was determined using a
χ2-based Cochran's Q statistic and I2. For
the Q statistic, P-values <0.10 were considered to indicate
statistically significant heterogeneity. For the I2
statistic, heterogeneity was assessed as follows: No heterogeneity
(I2=0-25%), moderate heterogeneity
(I2=25-50%), high heterogeneity (I2=50-75%)
and very high heterogeneity (I2=75-100%). If the
I2 statistic was >50% or the Q statistic value of the
P-value was <0.05, a random-effects model was used for the
meta-analysis. Otherwise, a fixed-effects model was employed. A
two-sided P-value of <0.05 was considered to indicate
statistical significance. A sensitivity analysis was performed
using a leave-one-out approach. Publication bias was not assessed,
as only ≤10 studies were included in any given meta-analysis
(23). All statistical analyses were
performed using the statistical software Comprehensive
Meta-Analysis, version 2.0 (Biostat).
Results
Search results and study
characteristics
The initial search identified 351 unique studies,
303 of which were removed after the screening of the abstract and
title for not meeting the inclusion criteria. Of the 48 studies
that underwent full-text review, 38 studies were excluded for not
reporting outcomes of interest, being single-arm studies, reviews
or having irrelevant study objectives (Fig. 1).
A total of 10 studies were included in the present
review and meta-analysis, comprising a total of 2,634 patients,
with 910 eyes treated with DSAEK and 1,804 with PK (9-18).
Of the 10 studies, 4 had a prospective design and the others were
retrospective studies (Table I). The
number of patients who received DSAEK surgery across the studies
ranged from 12 to 828 and those who were treated with PK ranged
from 11 to 1,101. The proportion of patients who received DSAEK
across studies ranged from 13.6 to 61%, whereas that of patients
treated by PK ranged from 39 to 86.4%. The patients' mean age was
>60 years. The indication/diagnosis varied across the studies.
The length of follow-up ranged from 1 to 5 years among the studies
that reported graft survival and endothelial cell density, and
ranged from 3 months to 5 years in the studies that reported on
visual acuity outcomes (Table
II).
 | Table ICharacteristics and diagnostic data of
studies included in the meta-analysis. |
Table I
Characteristics and diagnostic data of
studies included in the meta-analysis.
| 1st author
(year) | Study design | Study period |
Indication/diagnosis | Preoperative
glaucoma | Type of
keratoplasty | Patients | Eyes or
procedures | Age (years) | Male sex | Length of
follow-up | (Refs.) |
|---|
| Kim (2016) | Retrospective | Mar 2009-Sep
2012 | BK | n/a | DSAEK | 15 (57.7) | 15 (57.7) | 60.48±10 | 11(73) | 2 years | (15) |
| | | | | | PK | 11 (42.3) | 11 (42.3) | 60.17±13 | 8(73) | | |
| Ishiyama (2016) | Retrospective | DSAEK: Aug. 2007-Jul.
2015 PK: Apr. 1998-Apr. 2014 | Corneal endothelial
dysfunction (BK) | n/a | DSAEK | 78 (44.6) | 85(44) | 75.8±9.0 | 20 (23.5) | 5 years | (14) |
| | | | | | PK | 97 (55.4) | 108(56) | 71.5±10.8 | 30 (27.7) | | |
| Ang (2016) | Prospective | 1991-2001 | BK, (68.1); | (3.48) | DSAEK | 828(100) | 423 (51.1) | 67.8±9.8 | 415 (50.1) | 5 years | (10) |
| | | | FED, (31.9) | (3.51) | PK | | 405 (48.9) | | | | |
| Pedersen
(2015) | Retrospective | 2006-2013 | PBK, 57(73);
Previous eye trauma, 9(12) Herpes simplex-induced uveitis, 4(5);
Juvenile glaucoma, 3(4); Chronic uveitis, 2(3); Idiopathic bullous
keratopathy, 1(1);Corneal edema after choroidal tumor, 1(1);
Corneal birth trauma, 1(1) | ;20(26) | DSAEK | 78(100) | n/a | 71.1±12.0 | 43(55) | Median, 2.1 (range,
1.0-3.9) years | (18) |
| | | 1998-2013 | PBK, 72(90);
Previous eye trauma, 3(4); Iridocorneal endothelial syndrome, 1(1);
Juvenile glaucoma, 1(1); Chronic uveitis, 1(1); Idiopathic bullous
keratopathy, 1(1); Corneal edema due to previous ulcus cornea,
1(1) | 19(24) | PK | 80(100) | n/a | 71.6±12.1 | 41(51) | Median, 2.4 (range,
1.1-4.0) years | |
| Price (2013) | Prospective | Jun 2006-Sep
2007 | FED, (85);
pseudophakic or aphakic corneal edema, (13); FED (64); pseudophakic
or aphakic corneal edema, (32) | n/a | DSAEK PK | 173 (13.6) 1101
(86.4) | 173 (13.6) 1101
(86.4) | 72±11 70±8 | 510(40) | 3 years | (16) |
| Ang (2012) | Prospective | Apr 2006-Apr
2008 | FED, 41 (34.5);
PBK, 78 (65.5); | n/a | DSAEK | n/a | 119 (57.8) | 65.5±11.3 | 58 (48.7) | 3 years | (9) |
| | | | FED, 24 (27.6);
PBK, 63 (72.4) | | PK | n/a | 87 (42.2) | 65.2±11.2 | 41 (47.1) | | |
| Uchino (2011) | Retrospective | Nov 2007-Jan
2008 | PBK, 6 eyes; BK
after argon laser iridotomy, 4 eyes; FED, 5 eyes; herpetic
endothelial keratitis, 1 eye; iridocorneal endothelial syndrome, 2
eyes | n/a | DSAEK | n/a | 18(60) | 71.42± 9.91 | 7 (38.9) | 3 months | (17) |
| | | | Decompensation
after PK, 4 eyes; keratoconus, 3 eyes; PBK, 2 eyes; corneal
opacity, 2 eyes; decompensation after deep lamellar endothelial
keratoplasty, 1 eye | | PK | n/a | 12(40) | 60.58± 16.58 | 8 (66.7) | | |
| Bahar (2009) | Retrospective | n/a | FED and PBK, 11;
PKB, 1 | n/a | DSAEK | 12(100) | 12(50) | 72.5± 10.13 | 6(50) | 7.8±5.0 months | (11) |
| | | | | | PK | | 12(50) | | | 40.4±26.3
months | |
| Hjortdal
(2009) | Retrospective | Sep 2003-Aug
2006 | FED | n/a | DSAEK | n/a | 20(50) | Mean, 72.7 (range,
54-86) | 10(50) | 12 months | (13) |
| | | | | | PK | n/a | 20(50) | Mean, 70.3 (range,
51-82) | 8(40) | | |
| Bahar (2008) | Prospective | Feb 2003-2008 | PBK, (33.3); FED,
(35.4); ABK, (6.3); ICE syndrome, (2.1); Failed graft, (22.9); | 11 (22.9) | PK | Total patients,
161(100) | 48 (51.6) | 73.7±9.6 | 28 (58.3) | 15.8±11.1
months | (12) |
| | | | PBK, (26.6) FED,
(62.2) ABK, -; ICE syndrome, (4.4); Failed graft, (6.7) | 3 (6.7) | DSAEK | | 45 (48.4) | 70.2±10.9 | 20 (44.4) | 9.8±4.3 months | |
 | Table IISummary of outcomes for each
study. |
Table II
Summary of outcomes for each
study.
| 1st author
(year) | Type of
keratoplasty | Patients |
Eyes/procedures | ECD
(cells/mm2) | Change in ECD
(%) | Graft survival, %
(range) | BSCVA (logMAR) | (Refs.) |
|---|
| Kim (2016) | DSAEK | 15 (57.7) | 15 (57.7) | Baseline:
2,570±462 | 1 yr: -45.33 | 2 yr: 93.3 | Pre: 1.89±0.48 | (15) |
| | | | | 1yr: 1,405±475 | 2 yr: -39.77 | | Post:
0.69±0.51 | |
| | | | | 2yr: 1,548±456 | | | | |
| | PK | 11 (42.3) | 11 (42.3) | Baseline:
2,720±448 | 1 yr: -58.38 | 2 yr: 81.8 | Pre: 1.95±0.63 | |
| | | | | 1 yr:
1,132±661 | 2 yr: -61.32 | | Post:
0.88±0.48 | |
| | | | | 2 yr:
1,052±567 | | | | |
| Ishiyama
(2016) | DSAEK | 78 (44.6) | 85(44) | Baseline:
2,779±350 | 1 yr: -37.21 | n/a | Pre: 1.21±0.56 | (14) |
| | | | | 1 yr:
1,744.8±838.226 | 2 yr: -32.66 | | 1 yr:
0.178±0.306 | |
| | | | | 2 yr:
1,871.5±953.470 | 3 yr: -56.35 | | 2 yr:
0.132±0.334 | |
| | | | | 3 yr:
1,212.9±1,105.875 | 4 yr: -63.49 | | 3 yr:
0.126±0.376 | |
| | | | | 4 yr:
1,014.6±1,364.587 | 5 yr: -72.77 | | 4 yr:
0.134±0.456 | |
| | | | | 5 yr:
756.8±1,638.821 | | | 5 yr:
0.175±0.536 | |
| | PK | 97 (55.4) | 108(56) | Baseline:
2,838±276 | 1 yr: -32.05 | n/a | Pre: 1.78±0.50 | |
| | | | | 1 yr:
1,928.4±863.197 | 2 yr: -50.27 | | 1 yr:
0.342±0.313 | |
| | | | | 2 yr:
1,411.3±951.214 | 3 yr: -62.03 | | 2 yr:
0.327±0.345 | |
| | | | | 3 yr:
1,077.6±991.511 | 4 yr: -71.02 | | 3 yr:
0.351±0.350 | |
| | | | | 4 yr:
822.5±1,159.060 | 5 yr: -77.57 | | 4 yr:
0.319±0.398 | |
| | | | | 5 yr:
636.5±1,258.741 | | | 5 yr:
0.319±0.419 | |
| Ang (2016) | DSAEK | 828(100) | 423 (51.1) | Baseline:
2,853±234 | 1 yr: -23.40 | 1 yr: 95.5 | n/a | (10) |
| | | | | 1 yr:
2,185.488±233.74 | 3 yr: -35.48 | 2 yr: 90.7 | | |
| | | | | 3 yr:
1,840.719±102.262 | 5 yr: -46.75 | 3 yr: 86.9 | | |
| | | | | 5 yr:
1,519.323±204.5243 | | 4 yr: 82.9 | | |
| | | | | | | 5 yr: 79.4 | | |
| | PK | | 405 (48.9) | Baseline: 2,764
±278 | 1 yr: -23.68 | 1 yr: 91.4 | n/a | |
| | | | | 1 yr:
2,109.522±356.4565 | 3 yr: -52.43 | 2 yr: 82.2 | | |
| | | | | 3 yr:
1,314.799±350.6131 | 5 yr: -60.89 | 3 yr: 76.3 | | |
| | | | | 5 yr:
1,081.057±321.3953 | | 4 yr: 71.5 | | |
| | | | | | | 5 yr: 66.5 | | |
| Pedersen
(2015) | DSAEK | 78 (49.4) | | n/a | n/a | 4 yr: 45
(17-42) | n/a | (18) |
| | PK | 80 (50.6) | | n/a | n/a | 4 yr: 75
(58-85) | n/a | |
| Price (2013) | DSAEK | 173 (13.6) | 173 (13.6) | Baseline:
2,754±67.167 | 1 yr: -36.13 | 94 FED: 96 other:
86 | n/a | (16) |
| | | | | 1 yr:
1,759±176.50 | 2 yr: -35.98 | | | |
| | | | | 2 yr:
1,763±173.667 | 3 yr: -47.46 | | | |
| | | | | 3 yr:
1,447±294.50 | | | | |
| | PK | 1,101 (86.4) | 1,101 (86.4) | Baseline:
2,731±63.667 | 1 yr: -17.87 | FED: 96 Other:
84 | n/a | |
| | | | | 1 yr:
2,243±152.333 | 2 yr: -40.10 | | | |
| | | | | 2 yr:
1,636±205.50 | 3 yr: -54.67 | | | |
| | | | | 3 yr:
1,238±188.833 | | | | |
| Ang (2012) | DSAEK | - | 119 (57.8) | Baseline:
2,887±225 | 1 yr: -29.65 | 1 yr: 94.1 | Pre: 1.28±0.79 | (9) |
| | | | | 1 yr:
2,031±652 | 2 yr: -35.85 | 2 yr: 88.2 | Post: n/a | |
| | | | | 2 yr:
1,852±662 | 3 yr: -38.97 | 3 yr: 86.5 | | |
| | | | | 3 yr:
1,762±746 | | | | |
| | PK | - | 87 (42.2) | Baseline:
2,888±219 | 1 yr: -36.84 | 1 yr: 89.7 | Pre: 1.3±0.86 | |
| | | | | 1 yr:
1,824±736 | 2 yr: -44.18 | 2 yr: 85.0 | Post: n/a | |
| | | | | 2 yr:
1,612±826 | 3 yr: -48.23 | 3 yr: 85.0 | | |
| | | | | 3 yr:
1,495±965 | | | | |
| Uchino (2011) | DSAEK | - | 18(60) | n/a | n/a | n/a | Pre: 1.06±0.46 | (17) |
| | | | | | | | 3-mon:
0.25±0.27 | |
| | PK | - | 12(40) | n/a | n/a | n/a | Pre: 1.20±1.19 | |
| | | | | | | | 3-mon:
0.43±0.37 | |
| Bahar (2009) | DSAEK | 12(100) | 12(50) | n/a | n/a | n/a | Pre: 0.65±0.18 | (11) |
| | | | | | | | Post:
0.22±0.18 | |
| | PK | | 12(50) | n/a | n/a | n/a | Pre: 0.72±0.23 | |
| | | | | | | | Post:
0.33±0.19 | |
| Hjortdal
(2009) | DSAEK | - | 20(50) | n/a | n/a | n/a | Pre: 0.59 | (13) |
| | | | | | | | Post: 0.25 | |
| | PK | - | 20(50) | n/a | n/a | n/a | Pre: 0.6 | |
| | | | | | | | Post: 0.48 | |
| Bahar (2008) | DSAEK | 161(100) | 48 (51.6) | n/a | n/a | n/a | Pre: 0.9±0.5 | (12) |
| | | | | | | | Post:
0.34±0.17 | |
| | PK | | 45 (48.4) | n/a | n/a | n/a | Pre: 1.27±0.75 | |
| | | | | | | | Post:
0.42±0.14 | |
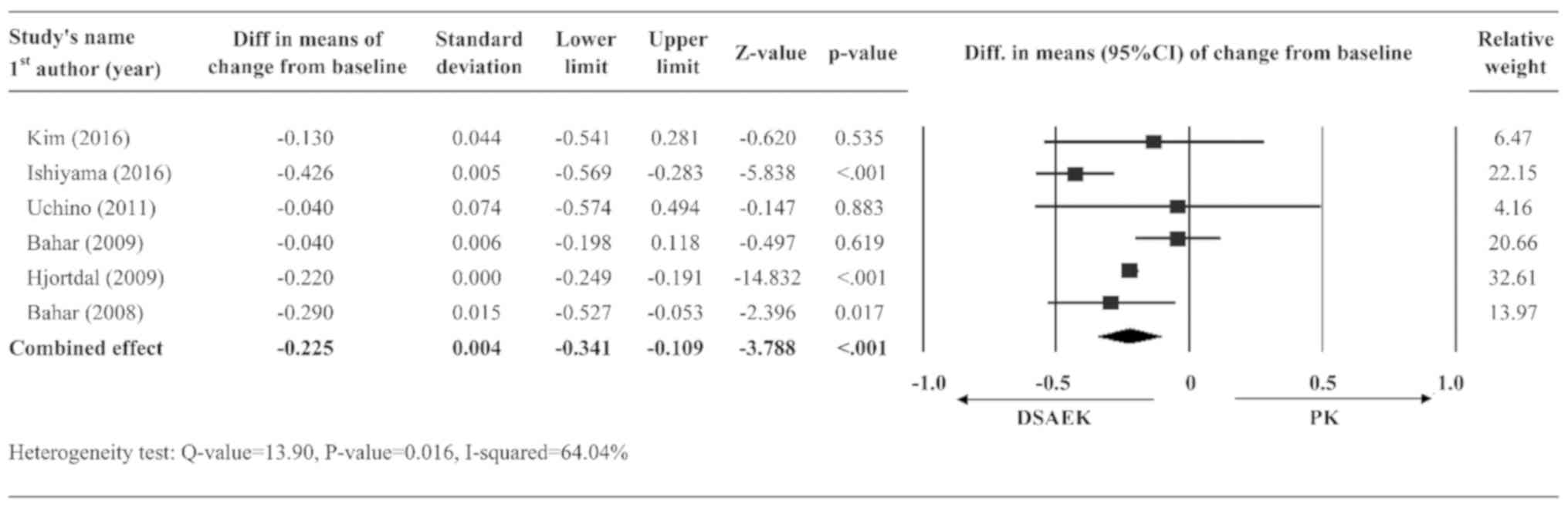
Change of BSCVA from baseline
A total of six studies (11-15,17)
had complete data on BSCVA and were included in the pooled
analysis. A random-effects model was used for BSCVA, as significant
heterogeneity was observed in the data (Q-value=13.90, P=0.016,
I2=64.04%). The summary effect indicated that the DSAEK
group had a greater BSCVA improvement from baseline as compared
with the PK group (diff. in means of change from baseline=-0.225,
95% CI=-0.341 to -0.109, P<0.001; Fig. 2).
Graft survival rate
A total of four studies (9,10,15,18)
had complete data on the rate of graft survival and were included
in the analysis. A random-effects model was applied due to the high
degree of heterogeneity in the data (Q-value=27.37, P<0.001,
I2=89.04%). The summary effect indicated that the rate
of graft survival was similar between the PK and DSAEK groups
(OR=1.005, 95% CI=0.329-3.071, P=0.993; Fig. 3).
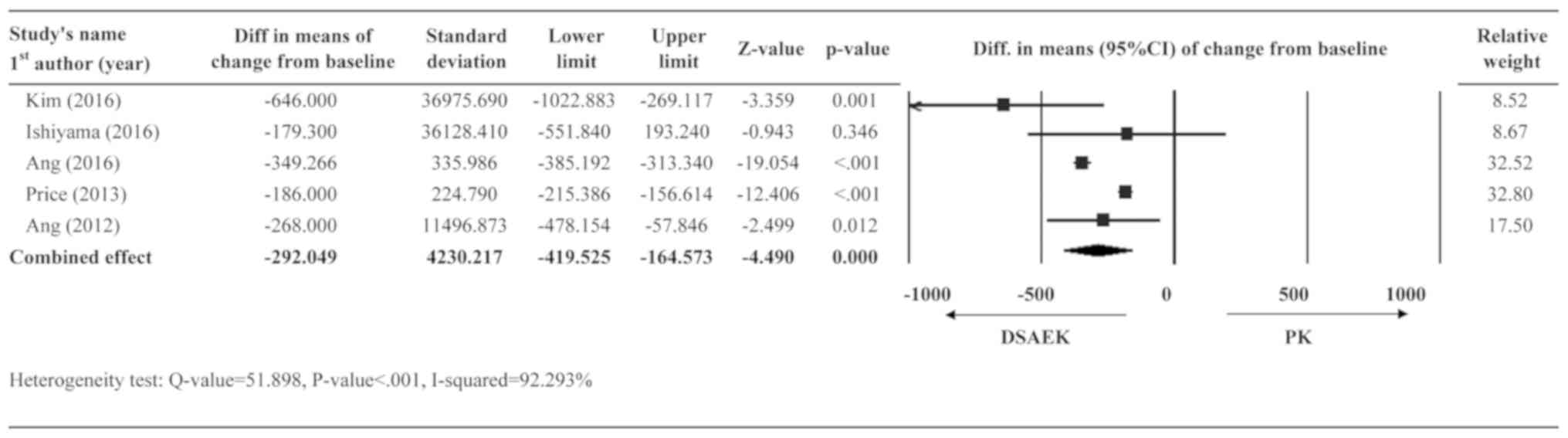
Changes in endothelial cell
density
A total of five studies (9,10,14-16)
had post-operative data regarding endothelial cell density and were
included in the analysis. A random-effects model was applied due to
very high heterogeneity in the data (Q-value=51.90; P<0.001;
I2=92.29%). The summary effect revealed that the DSAEK
group had less loss of endothelial cell density than the PK group
(diff. in means =-292.05 cells/mm2, 95% CI=-419.53 to
-146.57 cells/mm2, P<0.001; Fig. 4).
Adverse events
Table III presents
the adverse events in patients who received DSAEK and PK, including
intraocular pressure elevation (13.3-17.2 vs. 9.1-36.8%), graft
detachment/dislocation (0-13.3 vs. 6.3%), anterior synechiae (0 vs.
1.1%), wound dehiscence (0 vs. 2.7-4.6%) and secondary glaucoma
(6.7 vs. 6.3%), respectively. However, quantitative data synthesis
was not performed due to the insufficient data reported (Table III).
 | Table IIISummary of adverse events among
studies. |
Table III
Summary of adverse events among
studies.
| 1st author
(year) | Type of
keratoplasty | IOP elevation | Graft
detachment/dislocation | Anterior
synechiae | Wound
dehiscence | Secondary
glaucoma | (Refs.) |
|---|
| Kim (2016) | DSAEK | 2 (13.3) | 2 (13.3) | n/a | n/a | n/a | (15) |
| | PK | 1 (9.1) | n/a | n/a | n/a | n/a | |
| Ishiyama
(2016) | DSAEK | n/a | 5 (5.9) | n/a | n/a | n/a | (14) |
| | PK | n/a | n/a | n/a | 3 (2.7) | n/a | |
| Ang (2012) | DSAEK | 20 (17.2) | 3 (2.6) | 0 (0) | 0 (0) | n/a | (9) |
| | PK | 32 (36.8) | n/a | 1 (1.1) | 4 (4.6) | n/a | |
| Bahar (2008) | DSAEK | n/a | 0 (0) | n/a | n/a | 3 (6.7) | (12) |
| | PK | n/a | 3 (6.3) | n/a | n/a | 3 (6.3) | |
Sensitivity analysis
Sensitivity analysis was performed using the
leave-one-out approach, in which the meta-analyses were performed
after each study was removed in turn (Fig. S1). The direction and magnitude of
combined estimates did not vary markedly with the removal of any
single study, indicating that the results were robust and no single
study overly influenced the results. However, the removal of
Pedersen et al (2015) (18)
produced a significant result regarding graft survival rate.
Quality assessment
The results of the quality assessment of the
included studies are presented in Fig.
5. Overall, the studies were of fair quality and most studies
had a low risk of bias in the selection of participants into the
study, bias in measurement of interventions, bias in measurement of
outcomes, bias due to departures from intended interventions and
bias in selection of the reported result. However, with regard to
bias due to confounding, one study had high risk of bias and about
half of the included studies had intermediate risk of bias. The
risk of bias of most concern was bias due to missing data, with
high risk determined in four studies (Fig. 5).
Discussion
Diseases of the corneal endothelium require
treatment by transplantation of corneal endothelial cells (2). The present review and meta-analysis was
designed to compare outcomes following PK and DSAEK surgeries. The
results indicated that DSAEK was associated with a greater
improvement from baseline in BSCVA and a reduced loss of
endothelial cell density during follow-up compared with PK
(P<0.001). The rates of graft survival were similar between the
two procedures. However, the exclusion of the study by Pedersen
et al (18) led to a
significant benefit from DSAEK over PK.
A prior meta-analysis performed by Akanda et
al (20) in 2015 compared the
major surgical outcomes following PK and lamellar procedures. The
analysis included 22 studies, three of which were randomized
controlled trials and the others were cohort studies. Lamellar
procedures included deep anterior lamellar keratoplasty and
pre-Descemet anterior lamellar keratoplasty, and were referred to
as ‘anterior lamellar procedures’. They also included Descemet
stripping endothelial keratoplasty and DMEK and were referred to as
‘posterior lamellar keratoplasty’. Akanda et al (20) determined that PK was associated with
a greater risk of rejection (OR=3.56; 95% CI=1.76-7.20) and graft
failure (OR=2.85; 95% CI=0.84-9.66) than anterior lamellar
procedures. Compared with posterior lamellar procedures, PK also
had a greater likelihood for rejection (OR=1.52; 95% CI=1.00-2.32)
and outright failure (OR=2.09; 95% CI=0.57-7.59). In addition, PK
resulted in a longer follow-up time for full transplants than the
lamellar procedures.
The pooled graft survival rate in the present review
did not show significant difference between PK and DSAEK, which was
inconsistent with the result in the meta-analysis by Akanda et
al (20). However, this was
mostly due to the study by Pedersen et al (18). In the present review, of the four
studies included in the meta-analysis for graft survival, only the
findings of Pedersen et al (18) favored the PK group, possibly
reflecting the fact that the investigators focused on secondary
endothelial dysfunction. Removal of Pedersen et al (18) from the pooled analysis resulted in a
reduction in the heterogeneity of the data (Q-value=1.73, P=0.421,
I2=0%) and the pooled analysis indicated that DSAEK
resulted in higher graft survival compared with PK (OR=1.817,
95%CI=1.360-2.426, P<0.001), which was comparable to the result
of Akanda et al (20). These
results suggest that PK and DSAEK treatments may have different
effects on primary and secondary endothelial dysfunction.
The present systematic review and meta-analysis had
several limitations that should be considered when interpreting the
results. The meta-analyses included only a small number of studies
and none of the included studies was a randomized controlled trial.
The underlying disease/cause for the requirement of corneal
transplant varied across studies, which may have confounded the
results, and in addition, the meta-analysis did not assess any
potential differences in the incidence of adverse events between
the surgical approaches. In addition, the range of surgical
indications was broad (e.g., Fuchs' endothelial disease and bullous
keratopathy, post-keratoplasty endothelial failure, iridocorneal
endothelial syndrome and herpetic keratitis), and each of them has
a different prognosis, leading to high complexity in this
comparison. Furthermore, the outcomes between the two procedures
were not compared, e.g., by categorizing the patients into
different surgical indications. In addition, graft survival and
endothelial density changes from baseline to the last follow-up
were assessed in the present analysis, which were not uniform
across the studies included. However, it is known that these
outcomes are time-sensitive and it is possible that the results may
have differed depending upon the time-points chosen. For instance,
in the study by Ishiyama et al (14) from 2016, corneal endothelial cell
density was lower for DSAEK at 6 months post-surgery but was
significantly higher at 2 years. However, at three years, the
endothelial cell density was similar between the groups. Another
study by Price et al (2016) (24) indicated that the rate of cell loss
over time differed between DSAEK and PK; specifically, at 3-5
years, cell loss was less with DSAEK but at the 10-year follow-up,
no significant differences were identified. Accordingly,
well-designed studies with clear time frames for evaluating these
outcomes are warranted. Quantitative data synthesis on adverse
events was not performed due to the insufficient data reported,
precluding definite conclusions regarding adverse events.
In summary, the present review and meta-analysis
suggested that DSAEK results in a significantly greater improvement
in BSCVA compared with PK. Overall, the results suggested that
DSAEK may be a better surgical treatment option than PK in terms of
visual acuity outcome for corneal endothelial dysfunction.
Supplementary Material
Sensitivity analysis with the
leave-one-out method of studies for (A) best spectacle-corrected
visual acuity, (B) graft survival rate and (C) endothelial cell
density compared between the PK and DSAEK groups. Diff, difference;
PK, penetrating keratoplasty; DSAEK, Descemet stripping automated
endothelial keratoplasty.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KY, YZ, HSL and YXZ conceived the review. KY and YJ
conducted the literature search. Titles, abstracts and articles
were screened by KY, YZ, HSL and YXZ. Data extraction was performed
by KY, YZ and YM. JXH performed the statistical analysis. KY, YZ,
HSL and YXZ drafted the manuscript. YJ critically revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Patel SV: Graft survival and endothelial
outcomes in the new era of endothelial keratoplasty. Exp Eye Res.
95:40–47. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ple-Plakon PA and Shtein RM: Trends in
corneal transplantation: Indications and techniques. Curr Opin
Ophthalmol. 25:300–305. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Price MO and Price FW Jr: Endothelial
keratoplasty-a review. Clin Exp Ophthalmol. 38:128–140.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lee WB, Jacobs DS, Musch DC, Kaufman SC,
Reinhart WJ and Shtein RM: Descemet's stripping endothelial
keratoplasty: Safety and outcomes: A report by the American Academy
of Ophthalmology. Ophthalmology. 116:1818–1830. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Park CY, Lee JK, Gore PK, Lim CY and Chuck
RS: Keratoplasty in the United States: A 10-year review from 2005
through 2014. Opthalmology. 122:2432–2442. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dickman MM, Peeters JM, vonden Biggelaur
FJ, Ambergen TA, van Dongen MC, Kruit PJ and Nuijts RM: Changing
practice patterns and long-term outcomes of endothelialversus
penetrating keratoplasty: A prospective dutch registry study. Am J
Opthalmol. 170:133–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Williams KKM, Galettis R, Jones V, Mills R
and Coster D: The Australian Corneal Graft Resitry-2015 Report.
Available at: https://dspace.flinders.edu.au/scmhai/handle/2328/35402.
Published 2015. Accessed Feb 16, 2020.
|
|
8
|
Tan D, Ang M, Arundhati A and Khor WB:
Development of selectivelamellar keratoplasty within an Asian
corneal transplant program: The Singapore Corneal Transplant Study.
Trans Am Opthalmol Soc. 113(T10)2015.PubMed/NCBI
|
|
9
|
Ang M, Mehta JS, Lim F, Bose S, Htoon HM
and Tan D: Endothelial cell loss and graft survival after
Descemet's stripping automated endothelial keratoplasty and
penetrating keratoplasty. Ophthalmology. 119:2239–2244.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ang M, Soh Y, Htoon HM, Mehta JS and Tan
D: Five-year graft survival comparing descemet stripping automated
endothelial keratoplasty and penetrating keratoplasty.
Ophthalmology. 123:1646–1652. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bahar I, Kaiserman I, Levinger E,
Sansanayudh W, Slomovic AR and Rootman DS: Retrospective
contralateral study comparing descemet stripping automated
endothelial keratoplasty with penetrating keratoplasty. Cornea.
28:485–488. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bahar I, Kaiserman I, McAllum P, Slomovic
A and Rootman D: Comparison of posterior lamellar keratoplasty
techniques to penetrating keratoplasty. Ophthalmology.
115:1525–1533. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hjortdal J and Ehlers N: Descemet's
stripping automated endothelial keratoplasty and penetrating
keratoplasty for Fuchs' endothelial dystrophy. Acta Ophthalmol.
87:310–314. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ishiyama S, Mori Y, Nejima R, Tokudome T,
Shimmura S, Miyata K and Amano S: Comparison of long-term outcomes
of visual function and endothelial cell survival after descemet
stripping automated endothelial keratoplasty and penetrating
keratoplasty using mixed-effects models. Cornea. 35:1526–1532.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim SE, Lim SA, Byun YS and Joo CK:
Comparison of long-term clinical outcomes between Descemet's
stripping automated endothelial keratoplasty and penetrating
keratoplasty in patients with bullous keratopathy. Korean J
Ophthalmol. 30:443–450. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Price MO, Gorovoy M, Price FW Jr, Benetz
BA, Menegay HJ and Lass JH: Descemet's stripping automated
endothelial keratoplasty: Three-year graft and endothelial cell
survival compared with penetrating keratoplasty. Ophthalmology.
120:246–251. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Uchino Y, Shimmura S, Yamaguchi T,
Kawakita T, Matsumoto Y, Negishi K and Tsubota K: Comparison of
corneal thickness and haze in DSAEK and penetrating keratoplasty.
Cornea. 30:287–290. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pedersen IB, Ivarsen A and Hjortdal J:
Graft rejection and failure following endothelial keratoplasty
(DSAEK) and penetrating keratoplasty for secondary endothelial
failure. Acta Ophthalmol. 93:172–177. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Nanavaty MA, Wang X and Shortt AJ:
Endothelial keratoplasty versus penetrating keratoplasty for Fuchs
endothelial dystrophy. Cochrane Database Syst Rev: Cd008420,
2014.
|
|
20
|
Akanda ZZ, Naeem A, Russell E, Belrose J,
Si FF and Hodge WG: Graft Rejection rate and graft failure rate of
penetrating keratoplasty (PKP) vs lamellar procedures: A systematic
review. PLoS One. 10(e0119934)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moher D, Liberati A, Tetzlaff J, Altman DG
and Group P: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sterne JA, Hernán MA, Reeves BC, Savović
J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT,
Boutron I, et al: ROBINS-I: A tool for assessing risk of bias in
non-randomized studies of interventions. BMJ.
355(i4919)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sterne JA, Sutton AJ, Ioannidis JP, Terrin
N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH,
et al: Recommendations for examining and interpreting funnel plot
asymmetry in meta-analyses of randomised controlled trials. BMJ.
343(d4002)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Price MO, Calhoun P, Kollman C, Price FW
Jr and Lass JH: Descemet stripping endothelial keratoplasty:
Ten-year endothelial cell loss compared with penetrating
keratoplasty. Opthalmology. 123:1421–1427. 2016.PubMed/NCBI View Article : Google Scholar
|