Introduction
Osteoarthritis (OA) is the most common form of
arthritis and the main cause of disability in people ≤65 years old
(1). While OA is affected by
multiple factors, age is considered the single greatest risk factor
for its development in susceptible joints (2). Chondrocytes are regarded as key players
in OA pathology, since they are the only cell type present in
articular cartilage. The degeneration of autophagy caused by aging
results in the accumulation of senescent chondrocytes in articular
cartilage (3). Senescent
chondrocytes were observed near the osteoarthritic lesions, but not
in intact cartilage from patients with OA or in normal donors
(4). Senescent chondrocytes exhibit
a senescence-associated secretory phenotype (SASP), characterized
by the secretion of several inflammatory cytokines, growth factors
and other soluble and insoluble factors (5). These senescent cells are generally
detected using the senescence-associated indicator acidic
senescence-associated β-galactosidase (SA-β-gal), which is a
distinct marker of senescence for OA (6). Chondrocyte SASP is known to include the
production of matrix-degrading proteases, including the matrix
metalloproteinase (MMP)-3 and -13 enzymes (5). MMP-13 is believed to be central to the
irreversible degradation of the cartilage type II collagen, which
is the protein that constitutes the majority of the extracellular
matrix (5). Recent studies have
suggested that transplanted senescent cells induce an OA-like state
in mice, whereas local removal of senescent cells can reduce the
development of post-traumatic OA and create an environment that
promotes regeneration (7,8). These results suggest an important role
for chondrocyte senescence in the development and progression of
OA. However, to the best of the authors' knowledge, the specific
mechanisms underlying chondrocyte senescence have not been
described to date.
The Wnt/β-catenin signaling pathway serves an
important role in joint embryogenesis and adult skeletal
homeostasis (9,10). Previous studies demonstrated
increased levels of β-catenin in degenerative or osteoarthritic
cartilage (11,12). In chondrocytes, overexpression of
β-catenin induced expression of matrix degradation enzymes
(12). Moreover, activation of the
Wnt/β-catenin signaling pathway induced rapid gene expression of
MMPs and other proteases, which resulted in the degradation of
proteoglycan matrix (13). In
β-catenin(ex3)Col2CreER mice, β-catenin degradation is
selectively inhibited, which results in the increased expression
levels of this protein in articular chondrocytes. This ablation
resulted in an OA-like phenotype, including progressive loss of
articular cartilage and osteophyte formation (14). Altogether, these studies demonstrated
that Wnt/β-catenin signaling serves a significant role in the
pathogenesis of OA. However, the effect of Wnt/β-catenin signaling
in chondrocyte senescence has not been previously investigated, to
the best of the author's knowledge.
β-catenin is a key protein in the Wnt/β-catenin
signaling pathway. In the absence of Wnt proteins, β-catenin is
phosphorylated by a degradation complex, including glycogen
synthase kinase-3β (GSK-3β), adenomatous polyposis coli protein and
Axin. The tagged β-catenin is ubiquitinated and subsequently
degraded by the 26S proteasome. The Wnt protein binds to the
co-receptor complex on the cell surface, leading to the activation
of Dishevelled, a downstream protein of the receptor complex. This
process inhibits the activity of GSK-3β resulting in the
accumulation of β-catenin. The accumulated β-catenin is
subsequently transferred to the nucleus, where it binds to
transcription factors and alters the expression of several target
genes (9,11). Most of these target genes, such as
cyclin D1, are important in cell cycle regulation (15), which means that they may play an
important role in regulating cell senescence, because the prominent
feature of cell senescence is cell cycle arrest. Therefore, it may
be hypothesized that Wnt/β-catenin signaling could regulate
chondrocyte senescence.
In the present study, the activity of the
Wnt/β-catenin signaling pathway was experimentally modulated in
order to assess its effect on chondrocyte senescence in vivo
and in vitro. Furthermore, the possible mechanisms
underlying chondrocyte senescence were investigated.
Materials and methods
Animals
Rabbits and rats were obtained from Animal Center of
Zhejiang University. A total of 12 male, 1-year old, New Zealand
rabbits, weighing 2.0-2.5 kg, were used for the in vivo
study. Rabbits were raised in a single cage with food and bottled
water, at room temperature (24-26˚C), with 40-60% humidity and a 12
h light/dark cycle. Cultured chondrocytes from 4 male,
four-week-old rabbits and 2 male, two-week-old rats under the same
housing condition as aforementioned were used for the in
vitro study. The Institutional Animal Care and Use Committee of
The Second Affiliated Hospital of Medical College, Zhejiang
University approved the present study.
Reagents
Wnt-1, LiCl, recombinant human interleukin (IL)-1β
and collagenase II were purchased from Sigma-Aldrich; Merck KGaA.
Recombinant human Dickkopf (DKK1) was purchased from R&D
Systems, Inc. Anti-β-catenin was purchased from EMD Millipore.
Anti-MMP-13, anti-p16, anti-p53, anti-GAPDH, anti-acetylated p53
and anti-SIRT-1 were obtained from Abcam. Fetal bovine serum (FBS),
Dulbecco's modified Eagle's medium (DMEM), penicillin, streptomycin
and 0.25% trypsin were purchased from Gibco; Thermo Fisher
Scientific, Inc.
Culture of rabbit/rat articular
chondrocytes
Articular cartilage was isolated from the knee joint
of both adult rabbits and rats under sterile conditions.
Subsequently, 0.1% collagenase II was used to digest the cartilage
at 37.8˚C for 4 h in order to cause detachment of the chondrocytes.
Chondrocytes were transferred to 75 cm2 culture flasks at
a density of 1x105 cells/cm2 in DMEM medium
with 10% FBS and antibiotics (100 U/ml penicillin, 100 µg/ml
streptomycin). The temperature of the incubator used for
chondrocyte culture was set at 37.8˚C and the carbon dioxide
content was 5%. The chondrocytes were passaged at a ratio of 1:3.
Cell culture passages of the third or fourth generation were used
for all in vitro experiments.
Treatment of rabbit chondrocytes with
DKK1 and LiCl
The rabbit chondrocytes were seeded in six-well
plates at a density of 2x105 cells/well and
serum-starved overnight, then treated with 10 ng/ml IL-1β for 23 h
in serum-free medium at 37.8˚C in a 5% CO2 incubator.
Before adding IL-1β, chondrocytes were pre-treated with DKK1 (100
ng/ml) (16) or LiCl (20 mM)
(17) for 1 h at 37.8˚C.
Chondrocytes treated with PBS were used as controls. The cells were
then harvested for reverse-transcription-quantitative (RT-qPCR)
analysis and western blotting.
Treatment of rat chondrocytes with
Wnt-1 and IL-1β
Rat chondrocytes were seeded at a density of
2x105 cells/well in six-well plates and serum-starved
overnight. Subsequently, they were treated with 10 ng/ml
Wnt-1(18) in serum-free medium for
72 h at 37.8˚C in a 5% CO2 incubator. Chondrocytes
treated with PBS were used as controls. A part of the chondrocyte
culture was used for SA-β-gal staining, whereas the remaining cells
were harvested for western blotting. A second group of chondrocytes
were treated with 10 ng/ml Wnt-1 for 72 h in the absence of serum
and subsequently treated with 10 ng/ml IL-1β for 24 h at 37.8˚C in
a 5% CO2 incubator. Chondrocytes treated with Wnt-1 for
72 h were then treated with PBS for 24 h at 37.8˚C in a 5%
CO2 incubator were used as controls. The cells were
finally used for RT-qPCR analysis.
SA-β-gal staining
Cells were stained with senescence-associated
β-galactosidase staining kit (cat. no. 9860; CST Biological
Reagents Co., Ltd.), according to manufacturer's protocol. The
percentage of senescent cells was the total number of senescent
cells divided by the total number of cells counted per microscope
view, using a light microscope, magnification, x200.
β-catenin plasmid cell
transfection
β-catenin plasmid construction was performed based
on a previous study (19). The
β-catenin gene was ligated into the pTagRFP eukaryotic expression
vector (Evrogen JSC). 10 µg of DNA was used to transfect 70-80%
confluent cells. Lipofectamine® 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to
transfect the plasmid into the chondrocytes. The expression levels
of β-catenin were assessed via fluorescence microscopy and western
blot analysis. An empty vector was used as the control. 48 h after
transfection, cells were collected or used for subsequent
experimentation.
RT-qPCR
The cells harvested for RT-qPCR analysis were
mentioned before. The rabbit cartilage was collected from the left
femur (n=4) and frozen in-196˚C liquid nitrogen prior to use. The
cartilage samples were then transferred to a 2 ml pre-filled bead
mill tube (cat. no. 15-340-153; Thermo Fisher scientific, Inc.)
containing 1 ml TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and then grinded using a Bead Mill 24
homogenizer (cat. no. 15-340-163; Thermo Fisher Scientific, Inc.).
Total RNA extraction was performed using the TRIzol®
reagent. The SuperScript III reverse transcriptase cDNA synthesis
kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used for
first-strand cDNA synthesis. The RT reaction contained 1 µl Oligo
(dT)18 primer, 2 µg total RNA, 25 units of RNase
inhibitor, 4 µl SuperScript III, 5X reaction buffer and 2 µl dNTPs
(10 mmol/l). Sterile distilled water was added to create a total
reaction volume of 20 µl. The RT reaction was incubated at 50˚C for
60 min. The iCycler apparatus system (Bio-Rad Laboratories, Inc.)
and the SYBR-Green I (Invitrogen; Thermo Fisher Scientific, Inc.)
technology were used for RT-qPCR. The thermocycling conditions were
as follows: i) Denaturation: 95˚C, 5 min on initial cycle; 30 sec
on rest; ii) annealing: 55˚C, 30 sec; iii) extension: 72˚C, 60 sec;
5 min on the last cycle. A total of 40 PCR cycles were perfumed.
The rabbit and rat primer sequences are shown in Table I and 18s rRNA was used as an internal
control. The qPCR data were obtained using the DCq method. The
formulas were as follows: N=100x2-(ΔCq targeted gene-ΔCq 18s
rRNA) (20).
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Targeted genes | Sequence, 5' →
3' | Amplicon length,
bp | Genbank
accession |
|---|
| Rabbit MMP-13 | F:
CAGATGGGCATATCCCTCTAAGAA | 88 | NM_001082037 |
| | R:
CCATGACCAAATCTACAGTCCTCAC | | |
| Rabbit p53 | F:
GCCCATCCTCACCATCATCACACT | 82 | NM_001082404.1 |
| | R:
GCACACACTCGCACCTCAAAGC | | |
| Rabbit 18s
rRNA | F:
GACGGACCAGAGCGAAAGC | 119 | EU236696 |
| | R:
CGCCAGTCGGCATCGTTTATG | | |
| Rat MMP-3 | F:
CTGGGCTATCCGAGGTCATG | 77 | NM_133523 |
| | R:
TGGACGGTTTCAGGGAGGC | | |
| Rat MMP-13 | F:
CAACCCTGTTTACCTACCCACTTAT | 85 | NM_133530 |
| | R:
CTATGTCTGCCTTAGCTCCTGTC | | |
| Rat GAPDH | F:
GAAGGTCGGTGTGAACGGATTTG | 127 | NM_017008.4 |
| | R:
CATGTAGACCATGTAGTTGAGGTCA | | |
Western blotting
Chondrocytes were initially washed with cold PBS and
subsequently lysed in the RIPA lysis buffer (Thermo Fisher
Scientific, Inc.) containing proteinase inhibitor for 30 min at
4˚C. The Bradford assay was used for detection of the protein
concentration. 30 µg protein samples were loaded on 8-12% SDS-PAGE
for protein separation. The proteins were transferred from the gels
to PVDF membranes that were blocked for non-specific binding using
5% bovine serum albumin (Sigma Aldrich; Merck KGaA) for 1 h at room
temperature. The membranes were initially incubated overnight at
4˚C with the following antibodies: β-catenin (cat. no. 06-734; EMD
Millipore; 1:1,000), MMP-13 (cat. no. ab39012; Abcam; 1:1,000), p16
(cat. no. ab51243; Abcam; 1:1,000), p53 (cat. no. ab131442; Abcam;
1:1,000), acetylated p53 (cat. no. 183544; Abcam; 1:1,000), SIRT-1
(cat. no. 189494; Abcam; 1:1,000), GAPDH (cat. no. 245355; Abcam;
1:1,000) and β-actin (cat. no. A5441; Sigma Aldrich; Merck KGaA;
1:1,000). On the following day, the membranes were incubated for 1
h with horseradish peroxidase-linked secondary antibodies (cat. no.
G-21234; Thermo Fisher Scientific, Inc.; 1:2,000) at room
temperature. The SuperSignal® West Dura Extended
Duration Substrate (cat. no. 34075; Thermo Fisher Scientific, Inc.)
was used for visualization. The intensity of the protein bands was
quantified using Image Lab 3.0 software (Bio-Rad Laboratories,
Inc.).
OA induction and rabbit treatment
Rabbits were divided into three groups (n=4 in each
group). Anterior cruciate ligament transaction of bilateral knee
joints was performed in each rabbit according to a previous study
(19). The rabbits were housed under
standard conditions for four weeks following the operation, and 0.3
ml DKK1, LiCl and vehicle (PBS) were injected respectively into the
knee joint once every week. After 6 weeks of weekly intra-articular
injections, the rabbits were anesthetized by intravenous injection
of 3% sodium pentobarbital (30 mg/kg), then sacrificed by air
embolism experimental specimen collection.
Histological evaluation
The femoral condyle of the right knee joint (n=4)
was selected for tissue sectioning and was subsequently used for
histological evaluation. The specimens were fixed with 10% neutral
buffered formalin for 48 h at room temperature. Following removal
of the excess tissue, the inner and outer femoral condyles were
separated. Subsequently, the specimens were decalcified in 10%
EDTA, which was replaced weekly for 2 months. The specimens were
dehydrated using an ethanol gradient treatment, namely: 70%
ethanol, 15 min; 90% ethanol, 15 min; 100% ethanol, 15 min; 100%
ethanol, 15 min; 100% ethanol, 30 min; 100% ethanol, 45 min and
infiltrated with xylene for, 20 min, twice and then 45 min, before
being embedded in paraffin, all at room temperature. Paraffin
specimens were cut into 5-µm thick sections for subsequent use. The
sections were then stained with Safranin O and fast green for 5 min
each at room temperature. The specimens were photographed using a
light microscope at a magnification of x200. The Mankin score
system was used to assess the severity of arthritis (21).
Immunohistochemistry
β-catenin protein expression in cartilage specimens
was assessed by immunohistochemistry. The incubator temperature was
set at 50-55˚C and 5-µm thick paraffin sections were fixed on
slides coated with Aminopropyltriethoxysilane. The slides were
removed from paraffin using xylol. Specimen rehydration was
completed using descending graded alcohols and distilled water. The
endogenous peroxidase activity was blocked by incubating the
sections for 15 min in a methanol solution containing 1%
H2O2. Heat-induced antigen retrieval was
obtained by pressure cooking in a 0.01 M citrate buffer (pH 6) at
95˚C for 2 min and cooled. The slides were subsequently washed 3
times, for 5 min each, with PBST. Non-specific antibody-binding was
blocked by incubating the samples for 10 min with 10% normal goat
serum (Thermo Fisher Scientific, Inc.) at room temperature. The
slides were incubated overnight at 4˚C with primary anti-β-catenin
antibody (cat. no. 06-734; EMD Millipore; 1:200) and washed with
PBST again 3 times for 5 min each. The slides were incubated with
EnVision secondary antibody (cat. no. LSAB2; Agilent Technologies,
Inc.; 1:200) for 30 min at room temperature and subsequently
incubated with horseradish peroxidase-linked complex (cat. no.
LSAB2; Agilent Technologies, Inc.) for 30 min at room temperature.
Chromogen (DAB) and 1% copper sulphate were used to enhance the
color. Finally, the slides were counterstained in hematoxylin of
Mayer for 10 min at room temperature and ascending graded alcohol
was used for dehydration. Xylol was used for cleaning and the
samples were placed on coverslips with Entellan. The slides were
photographed using a light microscope at a x200 magnification.
Statistical analysis
All experiments were repeated three times. Data are
presented as the mean ± SD. All data were analyzed using SPSS
version 16 (SPSS, Inc.). The significance between two groups was
evaluated using unpaired Student's t-test. ANOVAs with the post hoc
Tukey-Kramer honest significance tests were used for multi-group
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of Wnt/β-catenin signaling on
the expression levels of MMP-13, p53, acetylated p53 and SIRT-1 in
chondrocytes
The expression levels of β-catenin in chondrocytes
increased following treatment with LiCl, an activator of the Wnt
pathway. Conversely, β-catenin expression decreased after treatment
with DKK1, an inhibitor of the Wnt pathway. Moreover, the levels of
MMP-13 and acetylated p53 were increased in LiCl-treated
chondrocytes and decreased in DKK1-treated chondrocytes. There was
no significant change in p53 protein level. The relative protein
expression ratio of acetylated p53/total p53 in chondrocytes
treated with LiCl was much higher. The activation of Wnt/β-catenin
signaling also decreased SIRT-1 expression (Fig. 1A). Fluorescence microscopy and
western blotting confirmed that the expression of β-catenin in
chondrocytes was increased 28 h following transfection of the cells
with a β-catenin overexpression plasmids, compared with an empty
vector control. In transfected cells, the expression levels of
MMP-13 and acetylated p53 were significantly increased, whereas
SIRT-1 levels were decreased by transfection of β-catenin into
chondrocytes (Fig. 1B).
Wnt/β-catenin signaling induces
chondrocyte senescence in OA
SA-β-gal staining indicated that treatment of
chondrocytes with Wnt-1 resulted in a significantly higher
senescence rate, compared with the control group (15.9 and 9.1%,
respectively; Fig. 2A and B). The protein levels of p53 and p16 were
increased following 72-h treatment with Wnt-1 (Fig. 2C). The addition of IL-1β following
treatment with Wnt-1 for 72 h resulted in higher mRNA levels of
MMP-3 and MMP-13, compared with the control group (Fig. 2D).
Gene expression, histological
evaluation and immunohistochemical analysis of cartilage
tissues
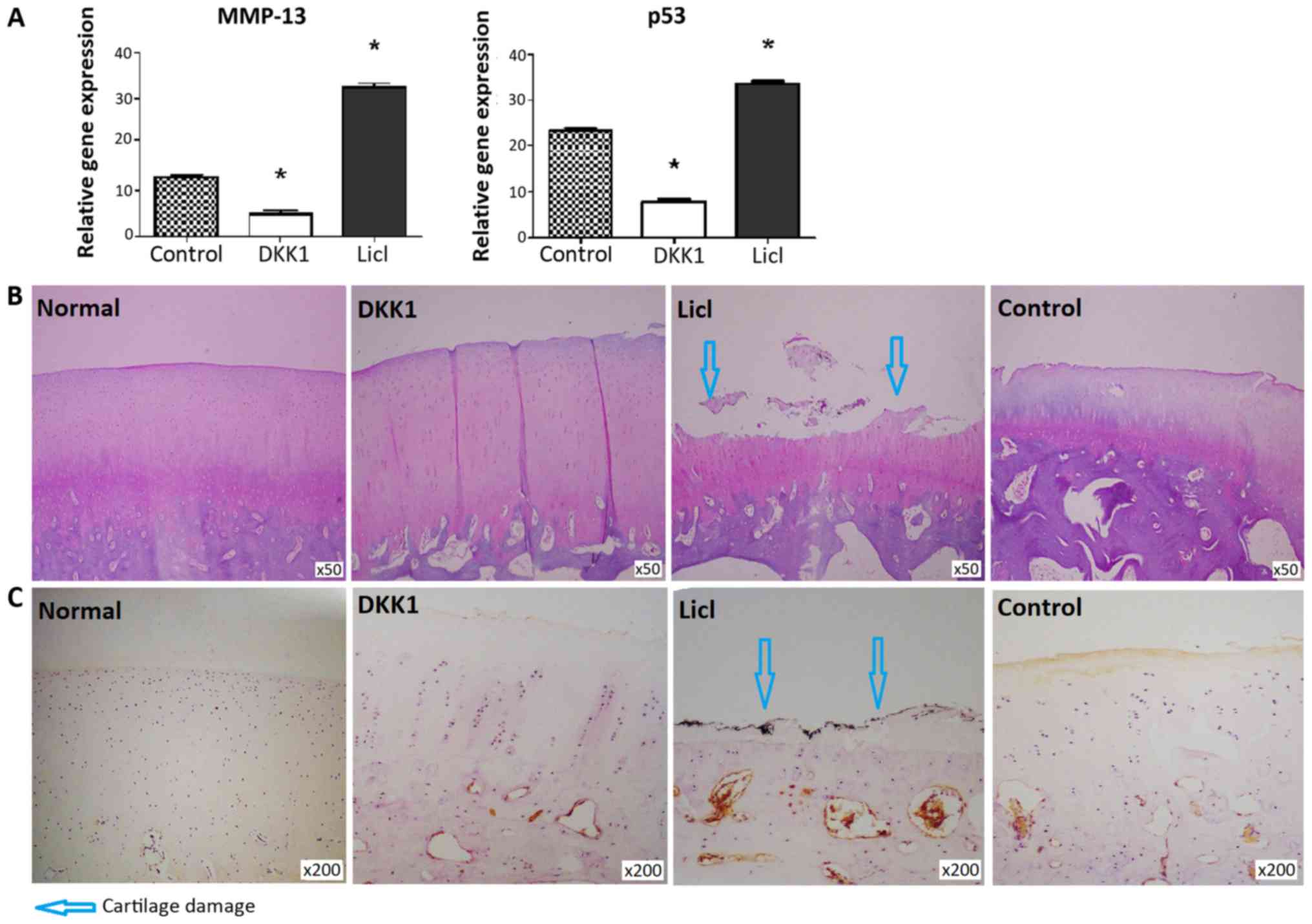
MMP-13 and p53 expression levels were decreased in
cartilage from DKK1-treated knees and increased in the LiCl-treated
group (Fig. 3A). DKK1-treated
cartilage did not exhibit structural changes, while apparent
cartilage damage was noted in cartilage injected with LiCl
(Fig. 3B). Consistent with these
findings, the DKK1-treated group exhibited a lower modified Mankin
score than the LiCl-treated group (P<0.05; Table II). β-catenin expression was not
detected in normal cartilage. β-catenin expression was much higher
in cartilage from rabbits injected with LiCl, compared with
cartilage tissues injected with DKK1 (Fig. 3C).
 | Table IIHistological score of articular
cartilage. |
Table II
Histological score of articular
cartilage.
| Parameter | DKK1 | LiCl | Control |
|---|
| Structural
changes |
0.43±0.53a |
2.67±0.50a | 1.0±0.50 |
| Cellular
changes |
0.56±0.53a |
1.67±0.50a | 1.11±0.33 |
| Safranin
staining |
0.71±0.49a |
1.56±0.73a | 1.11±0.60 |
| Total score |
1.86±1.07a |
5.89±1.17a | 3.22±1.30 |
Discussion
IL-1β is a pivotal catabolic factor involved in the
joint destruction observed during OA. IL-1β induces the expression
of inflammatory mediators and MMPs in arthritis, leading to
disruption of the balance between biosynthesis and degradation of
the extracellular matrix (ECM) (22). Therefore, IL-1β has been widely used
to mimic the OA microenvironment in vitro (23). The degradation of cartilage in OA is
primarily mediated by MMPs, and the collagenase MMP-13 specifically
targets collagen type II, which is the main component of the ECM
(24). Therefore, in the present
study, expression levels of MMP-13 were evaluated in order to
assess the extent of OA.
The present findings confirmed the role of the
Wnt/β-catenin signaling pathway in the development of OA.
Activation of Wnt/β-catenin signaling increased the expression
levels of MMP-13 in LiCl-treated chondrocytes and chondrocytes
transfected with β-catenin. Rabbit cartilage tissues that were
treated with LiCl indicated higher MMP-13 expression levels and a
higher modified Mankin score.
Previous studies indicated that Wnt/β-catenin
signaling may be an antagonist of senescence. Firstly, Wnt
signaling promoted proliferation by inhibiting senescence of
epithelial cells and fibroblasts (25), while senescence cell impacted
proliferation of the same cell populations (26). Secondly, elevated Wnt signaling is
associated with the development of several types of cancer
(27), whereas senescence acts as an
important mechanism of tumor suppression (25). However, the present study supported
the opposite conclusion. Indeed, activation of Wnt/β-catenin
signaling resulted in an increase in the expression of the
senescence markers SA-β-gal, p53 and p16. Furthermore, in
vivo experiments demonstrated that the expression levels of p53
were decreased in cartilage from DKK1-treated knees, and increased
in LiCl-treated cartilage tissues, suggesting that the Wnt pathway
promoted chondrocyte senescence both in vitro and in
vivo. This result accords with a study by Liu et al
(28), which suggested that
continuous Wnt exposure triggered accelerated cellular senescence
in vitro and in vivo. A previous study on nucleus
pulposus cells suggested that activation of Wnt/β-catenin signaling
promoted cellular senescence (17).
The present results suggested that the Wnt pathway
promoted chondrocyte senescence. During senescence, p53 is
activated and in certain cell types, induced expression of the p53
protein results in senescence (29-31).
In addition, the histone deacetylase SIRT-1 is particularly
important in this respect and its expression in senescent cells is
downregulated (32). SIRT-1 which
regulates p53 activity via deacetylation and inhibits the induction
of senescence (33,34). The results of the present study
indicated that the activation of the Wnt/β-catenin signaling
pathway increased the expression and acetylation of p53 in
chondrocytes. In LiCl-induced senescent chondrocytes, the
expression levels of acetylated p53 were increased, whereas the
opposite pattern of expression was noted in DKK1-treated
chondrocytes. Transfection of β-catenin in chondrocytes increased
the expression levels of acetylated p53. Furthermore, activation of
the Wnt/β-catenin signaling following LiCl treatment or with
β-catenin transfection decreased the expression levels of
SIRT-1.
In conclusion, the present study demonstrated that
activation of Wnt/β-catenin signaling promoted chondrocyte
senescence in vivo. These effects were confirmed using an
animal model of OA. The underlying mechanisms may involve
downregulation of SIRT-1 and increased p53 acetylation levels. The
findings suggest an important role for Wnt/β-catenin signaling in
the regulation of chondrocyte senescence in OA and may provide new
insight into the potential treatment of OA using targeted
therapies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301584).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW made substantial contributions to conception and
design. WL was mainly responsible for cell and animal experiments.
YX was a major contributor for the data analysis. WC contributed to
the animal experiments as well as helping with the drafting and
revisions to the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee of
The Second Affiliated Hospital of Medical College, Zhejiang
University approved the present study (approval no. 2018-015).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garstang SV and Stitik TP: Osteoarthritis:
Epidemiology, risk factors, and pathophysiology. Am J Phys Med
Rehabil. 85 (11 Suppl):S2–S11; quiz S12-S14. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Arden N and Nevitt MC: Osteoarthritis:
Epidemiology. Best Pract Res Clin Rheumatol. 20:3–25.
2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vinatier C, Domínguez E, Guicheux J and
Caramés B: Role of the inflammation-autophagy-senescence
integrative network in osteoarthritis. Front Physiol.
25(706)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Erusalimsky JD and Kurz DJ: Cellular
senescence in vivo: Its relevance in ageing and cardiovascular
disease. Exp Gerontol. 40:634–642. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McCulloch K, Litherland GJ and Rai TS:
Cellular senescence in osteoarthritis pathology. Aging Cell.
16:210–218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Price JS, Waters JG, Darrah C, Pennington
C, Edwards DR, Donell ST and Clark IM: The role of chondrocyte
senescence in osteoarthritis. Aging Cell. 1:57–65. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xu M, Bradley EW, Weivoda MM, Hwang SM,
Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson
KO, et al: Transplanted senescent cells induce an
osteoarthritis-like condition in mice. J Gerontol A Biol Sci Med
Sci. 72:780–785. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jeon OH, Kim C, Laberge RM, Demaria M,
Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, et al:
Local clearance of senescent cells attenuates the development of
post-traumatic osteoarthritis and creates a pro-regenerative
environment. Nat Med. 23:775–781. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Johnson ML and Kamel MA: The wnt signaling
pathway and bone metabolism. Curr Opin Rheumatol. 19:376–382.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Schett G, Zwerina J and David JP: The role
of wnt proteins in arthritis. Nat Clin Pract Rheumatol. 4:473–480.
2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Corr M: Wnt-beta-catenin signaling in the
pathogenesis of osteoarthritis. Nat Clin Pract Rheumatol.
4:550–556. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yuasa T, Otani T, Koike T, Iwamoto M and
Enomoto-Iwamoto M: Wnt/beta-catenin signaling stimulates matrix
catabolic genes and activity in articular chondrocytes: Its
possible role in joint degeneration. Lab Invest. 88:264–274.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tamamura Y, Otani T, Kanatani N, Koyama E,
Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici
M, et al: Developmental regulation of Wnt/beta-catenin signals is
required for growth plate assembly, cartilage integrity, and
endochondral ossification. J Biol Chem. 13:19185–19195.
2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C,
Rosier RN, O'Keefe RJ, Zuscik M and Chen D: Activation of
beta-catenin signaling in articular chondrocytes leads to
osteoarthritis-like phenotype in adult beta-catenin conditional
activation mice. J Bone Miner Res. 24:12–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 3:469–480. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gregory CA, Singh H, Perry AS and Prockop
DJ: The Wnt signaling inhibitor dickkopf-1 is required for reentry
into the cell cycle of human adult stem cells from bone marrow. J
Biol Chem. 278:28067–28078. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hiyama A, Sakai D, Risbud MV, Tanaka M,
Arai F, Abe K and Mochida J: Enhancement of intervertebral disc
cell senescence by WNT/β-catenin signaling-induced matrix
metalloproteinase expression. Arthritis Rheum. 62:3036–3047.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song P, Zheng JX, Liu JZ, Xu J, Wu LY, Liu
C, Zhu Q and Wang Y: Effect of the Wnt1/β-catenin signalling
pathway on human embryonic pulmonary fibroblasts. Mol Med Rep.
10:1030–1036. 2014.
|
|
19
|
Li WJ, Tang LP, Xiong Y, Chen WP, Zhou XD,
Ding QH and Wu LD: A possible mechanism in DHEA-mediated protection
against osteoarthritis. Steroids. 89:20–26. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II. Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg
Am. 53:523–537. 1971.PubMed/NCBI
|
|
22
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Heinecke LF, Grzanna MW, Au AY, Mochal CA,
Rashmir-Raven A and Frondoza CG: Inhibition of cyclooxygenase-2
expression and prostaglandin E2 production in chondrocytes by
avocado soybean unsaponifiables and epigallocatechin gallate.
Osteoarthritis Cartilage. 18:220–227. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Mitchell PG, Magna HA, Reeves LM,
Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF and Hambor
JE: Cloning, expression, and type II collagenolytic activity of
matrix metalloproteinase-13 from human osteoarthritic cartilage. J
Clin Invest. 1:761–768. 1996.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye X, Zerlanko B, Kennedy A, Banumathy G,
Zhang R and Adams PD: Downregulation of wnt signaling is a trigger
for formation of facultative heterochromatin and onset of cell
senescence in primary human cells. Mol Cell. 27:183–196.
2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Campisi J: Senescent cells, tumor
suppression, and organismal aging: Good citizens, bad neighbors.
Cell. 120:513–522. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 14:843–850. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu H, Fergusson MM, Castilho RM, Liu J,
Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, et al:
Augmented Wnt signaling in a mammalian model of accelerated aging.
Science. 10:803–806. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ferbeyre G, de Stanchina E, Lin AW,
Querido E, McCurrach ME, Hannon GJ and Lowe SW: Oncogenic ras and
p53 cooperate to induce cellular senescence. Mol Cell Biol.
22:3497–3508. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee SW, Fang L, Igarashi M, Ouchi T, Lu KP
and Aaronson SA: Sustained activation of Ras/Raf/mitogen-activated
protein kinase cascade by the tumor suppressor p53. Proc Natl Acad
Sci USA. 18:8302–8305. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Narita M, Nũnez S, Heard E, Narita M, Lin
AW, Hearn SA, Spector DL, Hannon GJ and Lowe SW: Rb-mediated
heterochromatin formation and silencing of E2F target genes during
cellular senescence. Cell. 13:703–716. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sasaki T, Maier B, Bartke A and Scrable H:
Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell.
5:413–4122. 2006.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Langley E, Pearson M, Faretta M, Bauer UM,
Frye RA, Minucci S, Pelicci PG and Kouzarides T: Human SIR2
deacetylates p53 and antagonizes PML/p53-induced cellular
senescence. EMBO J. 15:2383–2396. 2002.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Vigneron A and Vousden KH: p53, ROS and
senescence in the control of aging. Aging (Albany NY). 2:471–474.
2010.PubMed/NCBI View Article : Google Scholar
|