Introduction
Acute kidney injury (AKI) is a serious postoperative
complication of cardiopulmonary bypass (CPB) and may affect up to
50% of patients (1). AKI after
cardiac surgery is the second most common form of AKI that is
treated within the intensive care unit (ICU), second only to septic
shock related-AKI (2). AKI after
cardiac surgery is associated with increased mortality and
morbidity; however, there is a great variation in the AKI
severities. AKI which requires continuous renal replacement
treatment, only constitutes ~5% of total patients with AKI
(3-5).
It has been reported that a small increase in serum creatinine
after cardiac surgery is also associated with an increase in the
30-day mortality rate (6). Mild
increases of the serum creatinine levels, manifested as AKI stage
I, was the main pattern observed among patients who underwent
cardiac surgery. The incidence of mild AKI; however, shows a wide
variation in the reported literature. It is reported that patients
with persistent renal dysfunction have a higher long-term mortality
risk compared with patients who display renal function recovery to
baseline levels (7). The mechanisms
of action behind AKI after cardiac surgery may be related to
hemodynamic changes, ischemia/reperfusion injury, inflammatory
factors, microembolization and toxins. AKI stage I requires
commonly conservative treatment (if CRRT is not required), which is
cost-effective, but maintains a high morbidity. If patients receive
insufficient attention, a poor prognosis may result in increased
mortality compared to other forms of AKI. The effect of AKI stage I
on mortality needs further investigation to ensure that suitable
clinical treatment is available for postoperative patients of
cardiac surgery.
To this end, the objective of the present study was
to define the association between AKI stage I and the mortality
among patients undergoing cardiovascular surgery with CPB.
Subjects and methods
Study population
This prospective observational study was conducted
at the Department of Anesthesiology of Beijing Anzhen Hospital,
Capital Medical University, Beijing, China. The protocol was
approved by the Ethics Committee of Beijing Anzhen Hospital,
Capital Medical University. Patients or their family members were
fully informed of the study details and signed informed consent
forms. All of the eligible patients (male: 1,788 and female: 1,080;
age, >18 years old) were selected from among inpatients who
underwent cardiac surgery with CPB between July 1, 2013, and May
31, 2014.
Exclusion criteria
For each patient, information was collected on the
demographics, surgical procedure, transfusion and complications
from the internal hospital anesthetic database and clinical
information system using. The following exclusion criteria were
used: i) No serum creatinine indicated before the operation; ii) no
serum creatinine after the operation; iii) mortality within the
course of surgery; iv) cardiac transplantation; v) aortic surgery
with kidney arteries involved, or vi) patients with AKI stage
II/III.
Definitions
AKI was defined according to the Kidney Disease
Improving Global Outcomes criteria (8). Stage I corresponds to serum creatinine
levels >26.5 µmol/l (0.3 mg/dl) or levels 1.5-1.9 times greater
than baseline levels; stage II corresponds to serum creatinine
levels 2.0-2.9 times greater than baseline levels; stage III
corresponds to serum creatinine levels >353.6 µmol/l (4.0
mg/dl), or levels greater than 3.0 times higher than baseline
levels, or patients who had begun renal replacement therapy. Urine
output criteria were not used in the present study. In the present
study, patients were dichotomized into non-AKI group and AKI stage
I group. Patients with AKI stage I were included in the recovery
group if the serum creatinine levels at discharge decreased,
otherwise patients were included in the non-recovery group.
All-cause mortality was defined as death at a final follow-up
(December 17, 2014), which was obtained by telephone consultation.
The primary endpoint of this present study was to examine the
association of AKI stage I with all-cause mortality. The second
endpoint of the present study was to examine 30-day mortality,
length of hospital stay after surgery, length of stay in the ICU,
mechanical ventilation time and the post-operation morbidity.
Statistical analysis
Data are presented as the mean ± standard deviation
when normally distributed, as median and interquartile range when
data are skewed, and as frequencies and percentages for categorical
variables. Patient demographics, intraoperative variables and
measures of renal function were compared between the non-AKI
patients group and the AKI stage I group using Student t-test for
normally distributed data, Kruskal-Wallis test or χ2
test when appropriate. Kaplan-Meier survival analysis was used to
draw survival curves, which were analyzed using log-rank test. A
multivariate Cox proportional hazards survival model was used to
estimate hazard ratios (HRs) with 95% confidence intervals (CIs).
Two-sided P values were used, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline characteristics
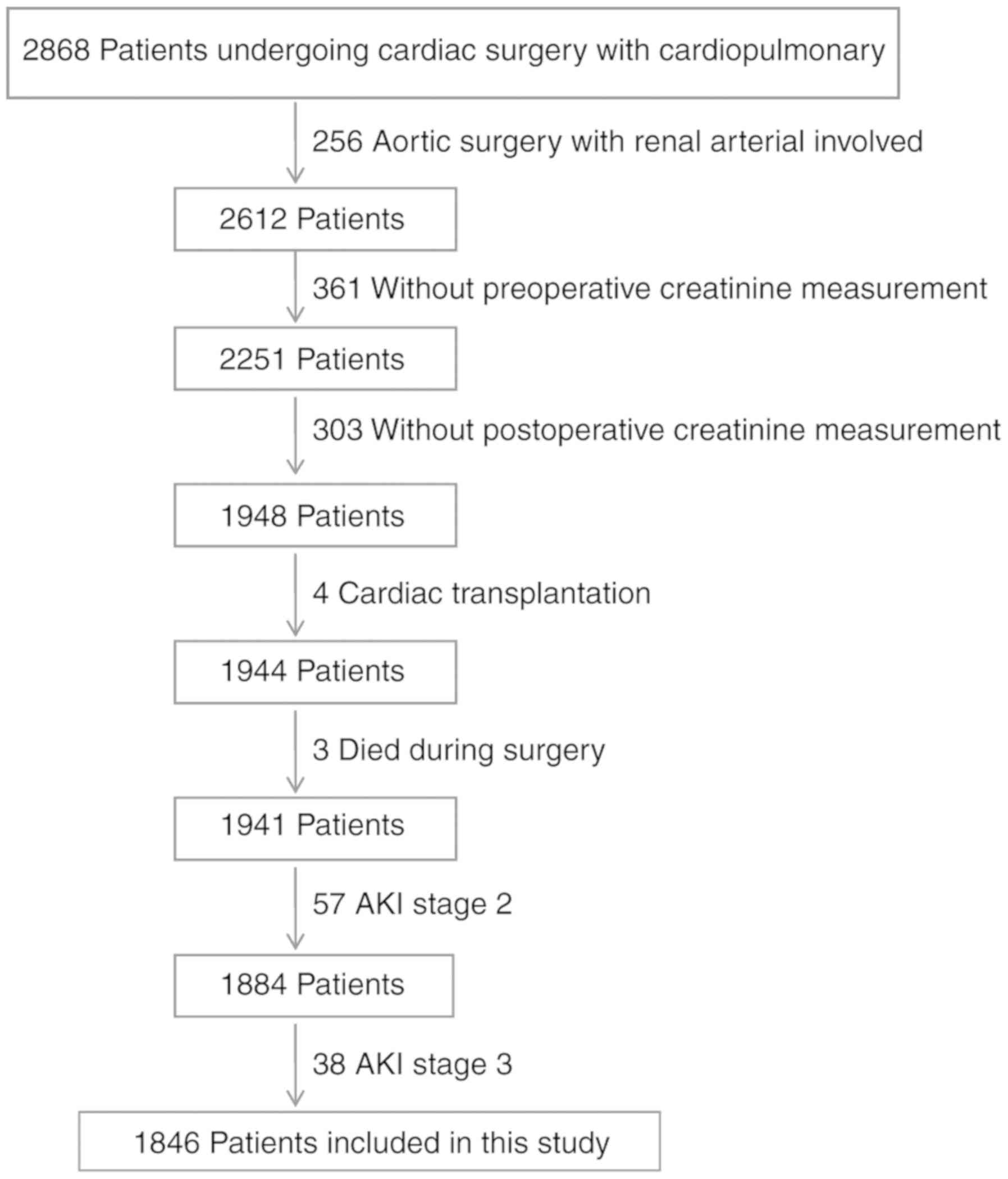
A total of 2,868 patients were screened following
cardiac surgery with CPB between July 1, 2013, and May 31, 2014.
Fig. 1 shows the flowchart of this
study, including the included and excluded patients. In total,
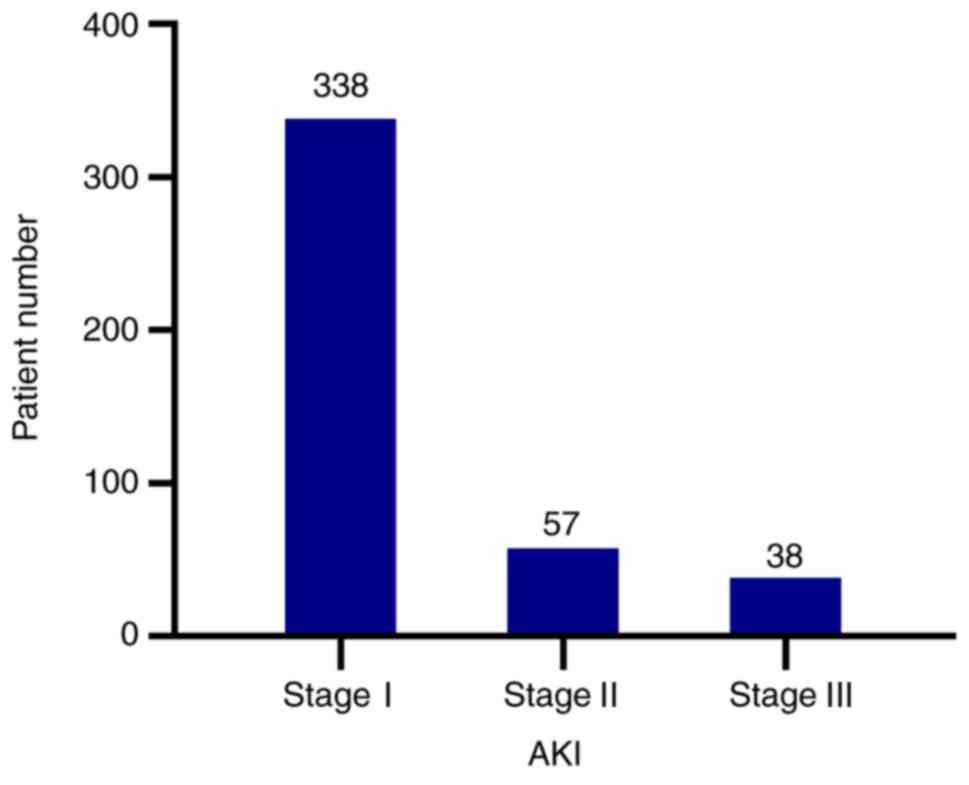
1,941 patients were included, with 433 (22.31%) AKI patients. Of
those AKI patients, 338 (78.06%) were AKI stage I patients
(Fig. 2). Finally, 1,846 patients
constituted the study population. Of the study patients, the mean
age was 51.76±13.56 years and 856 (46.37%) were men. Valve surgery
accounted for 63.81% of surgical types, and coronary artery bypass
graft (CABG) + valve surgery was the second predominantly used
surgical type (9.15%). CABG + vascular surgery had the highest
incidence of AKI stage I, followed by CABG + valve surgery
(Table I). Among the 1,846 patients
included in this study, 1,508 patients did not have AKI and 338 had
AKI stage I. There were older patients in AKI stage I group than
the non-AKI group. The AKI stage I patients demonstrated a greater
proportion of comorbidities of coronary heart disease, hypertension
and/or diabetes, compared with the non-AKI group (Table II).
 | Table ISurgery types and patients with AKI
stage I. |
Table I
Surgery types and patients with AKI
stage I.
| Surgery | All cases, n (%) | Percentage among
patients with AKI stage I, n (%) |
|---|
| Valve surgery | 1178 (63.81) | 227 (19.27) |
| CABG | 26 (1.41) | 5 (19.23) |
| CABG + valve
surgery | 169 (9.15) | 58 (33.58) |
| Vascular surgery | 166 (8.99) | 22 (13.25) |
| Congenital disease
surgery | 173 (9.37) | 5 (2.89) |
| Valve + vascular
surgery | 31 (1.68) | 8 (25.8) |
| CABG + congenital
disease surgery | 6 (0.33) | 2 (33.33) |
| Cardiac tumor
surgery | 48 (2.60) | 5 (1.42) |
| Congenital disease +
valve surgery | 38 (2.06) | 0 (0.00) |
| CABG + vascular
surgery | 11 (0.59) | 6 (54.55) |
 | Table IICharacteristics in the non-AKI group
and the AKI stage I group. |
Table II
Characteristics in the non-AKI group
and the AKI stage I group.
| Variables | non-AKI group
(n=1,508) | AKI stage I group
(n=338) | P-value |
|---|
| Sex, male | 733 | 169 | 0.074 |
| Age, year | 50.37±13.57 | 57.99±11.68 | <0.0001 |
| Eject fraction,
% | 60.85±8.4 | 58.34±10.3 | <0.0001 |
| sCR before surgery,
mmol/l | 72.57±18.35 | 76.33±40.53 | <0.0001 |
| CPB time, mins | 109.34±50.04 | 138.29±59.74 | <0.0001 |
| Aortic cross-clamp,
mins | 75.23±35.22 | 92.19±43.45 | <0.0001 |
| RBC transfusion,
u | 4.72±10.86 | 4.88±8.1 | 0.056 |
| Blood plasma
transfusion, ml | 421.10±483.19 | 614.23±713.92 | <0.0001 |
| Fluid balance during
surgery, ml | 675.78±942.43 | 870.05±1015.75 | 0.001 |
| minimally invasive
surgery, n | 62 | 5 | 0.02 |
| Coronary artery
disease, n | 136 | 65 | <0.0001 |
| Hypertension, n | 174 | 164 | <0.0001 |
| Pulmonary artery
hypertension, n | 230 | 43 | 0.24 |
| DM, n | 93 | 133 | 0.001 |
| Peripheral vascular
disease, n | 24 | 17 | 0.236 |
AKI stage I and mortality
Mortality data of all patients were available. The
mean follow-up period among survivors was 9.95±3.45 months (5.5
days to 15.5 months). Kaplan-Meier survival curves examining the
variation between the two groups are shown in Fig. 3. The data showed that patients with
AKI stage I were at a higher risk of mortality compared with
non-AKI patients (P<0.0001).
A univariate Cox analysis was performed first
(Table III). All the meaningful
factors which were significantly associated with AKI stage I were
all included in the multivariate Cox analysis. The following
factors were included; Age, blood plasma transfusion, fluid balance
during surgery, coronary artery disease, diabetes mellitus, and
mechanical ventilation time. In multivariate Cox regression
analysis, AKI stage I remained independently associated with
reduced survival [(HR, 2.412; 95% CI, 1.40 to 4.15; P
=0.001)(Table IV)]. Patients with
AKI stage I had a higher 30-day mortality rate than patients
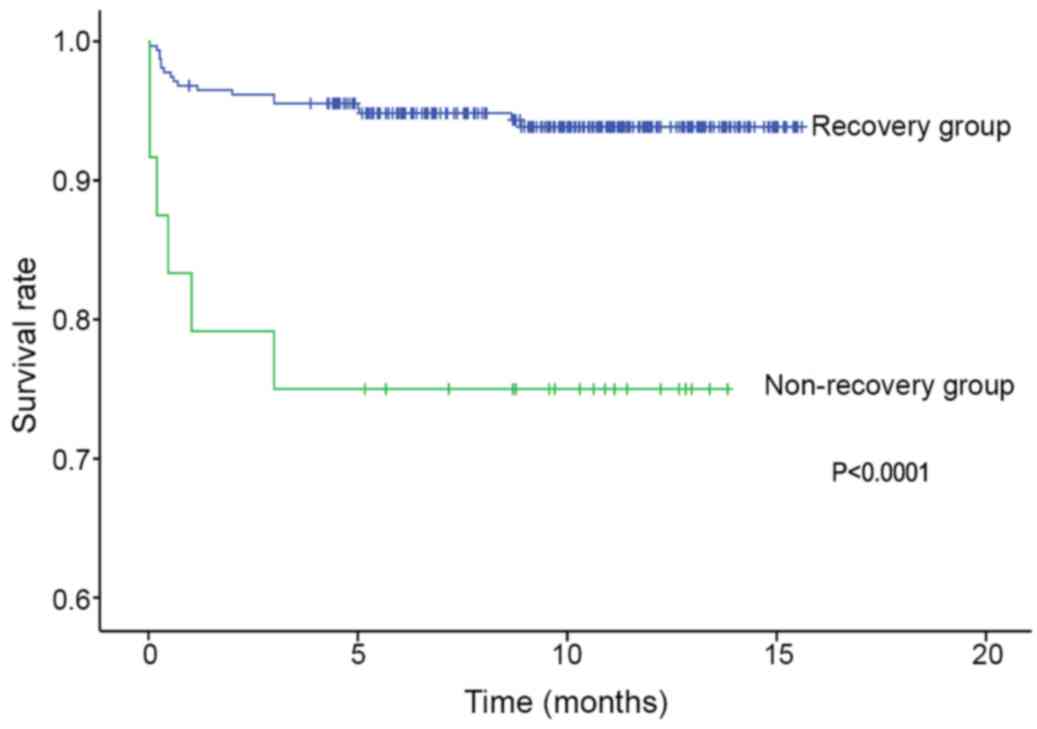
without AKI (3.25 vs. 0.025%, P<0.05; Table V). In a subgroup Kaplan-Meier
survival curve analysis, patients with non-recovery AKI had a
higher mortality rate than patients with recovery AKI (log-rank
test, P<0.0001; Fig. 4).
Mechanical ventilation time was longer in the AKI stage I group
than that in the non-AKI group (P<0.0001). Hospital stay after
surgery non-AKI group were also longer compared with the non-AKI
group (Table V).
 | Table IIIFactors associated with mortality in
univariate analysis. |
Table III
Factors associated with mortality in
univariate analysis.
| Variables | Hazard ratio (95%
CI) | P-value |
|---|
| Age | 1.253
(1.121-1.405) | <0.00 |
| Body mass index | 1.110
(1.067-1.157) | <0.001 |
| Hypertension | 1.560
(1.203-2.076) | 0.002 |
| Chronic kidney
disease | 5.70 (1
1.199-8.062) | 0.031 |
| Coronary artery
disease | 1.903
(1.071-3.385) | 0.021 |
| Pulmonary artery
hypertension | 1.116
(1.027-1.210) | 0.013 |
| Peripheral vascular
disease | 0.983
(0.971-1.017) | 0.041 |
| sCR before surgery,
mmol/l | 3.267
(1.932-5.383) | <0.001 |
| Peripheral vascular
disease | 1.050
(0.927-1.032) | 0.394 |
| Ejection fraction,
% | 0.986
(0.972-1.000) | 0.053 |
| RBC transfusion | 1.103
(0.999-1.050) | 0.057 |
| AKI stage I | 2.432
(1.41-4.19) | 0.001 |
| Blood plasma
transfusion, ml | 1.127
(1.022-1.226) | 0.015 |
| Fluid balance during
surgery, ml | 0837
(0.671-1.224) | 0.542 |
| MAP intraoperation,
mmHg | 0.983
(0.973-0.982) | 0.001 |
| CPB | 1.003
(1.001-1.006) | 0.033 |
| DM | 1.040
(1.02-1.06) | 0.008 |
| MV time, day | 1.014
(1.009-1.020) | <0.001 |
 | Table IVFactors associated with mortality in
multivariate analysis. |
Table IV
Factors associated with mortality in
multivariate analysis.
| Variables | Hazard ratio (95%
CI) | P-value |
|---|
| AKI stage I | 2.432
(1.41-4.19) | 0.001 |
| CPB | 1.003
(1.001-1.006) | 0.033 |
| DM | 1.040
(1.02-1.06) | 0.008 |
 | Table VPostoperative characteristics in the
non-AKI group and the AKI stage I group. |
Table V
Postoperative characteristics in the
non-AKI group and the AKI stage I group.
| Variables | non-AKI group | AKI stage I
group | P-value |
|---|
| ICU stay, h | 21.92±28.88 | 40.54±66.45 | <0.0001 |
| Mechanical
ventilation time, hours | 21.28±27.78 | 38.85±64.88 | <0.0001 |
| Hospital stay after
surgery, days | 8.37±4.88 | 10.5±6.85 | <0.0001 |
| Respiratory
dysfunction, n | 155 | 106 | <0.0001 |
| Cardiac events,
n | 9 | 15 | <0.0001 |
| 30-day mortality,
n | 6 | 11 | <0.0001 |
Discussion
In the present study, it was observed that AKI was a
common complication of cardiac surgery, and AKI stage I made up to
78% of all AKI cases. Patients with AKI stage I had a higher 30-day
mortality rate compared to patients without AKI. Furthermore, AKI
stage I was an independent determinant for all-cause long-term
mortality among patients who underwent cardiac surgery.
Additionally, patients with AKI stage I had a longer length of
hospital stay and required longer treatment periods than non-AKI
patients. Finally, the present study showed that patients with
recovery AKI stage I had a better prognosis than those with
non-recovery AKI.
The present investigation observed that the main
stage of AKI after cardiac surgery with CPB is AKI stage I. AKI is
a frequent postoperative clinical complication for patients,
especially during cardiac surgery. AKI may affect up to 30% of
patients following cardiac surgery (9). The present study showed that 22.31% of
patients who underwent cardiac surgery developed AKI. The
difference between the previously published incidence of AKI and
the observation in the present study cannot exclude the improvement
of recognition and treatment in recent years. However, the severity
of AKI is vastly different between postoperative AKI and septic
AKI. In septic patients, AKI stage III was previously reported to
be the main stage (10). In the
current investigation, stage I AKI, was the most common stage. It
has been previously reported that the incidence of stage I AKI was
more than 70% in patients who underwent cardiac surgery procedures
with CPB, which was in agreement with the results of the present
study (11). It has been shown that,
in patients who had undergone coronary artery bypass grafting, ~50%
AKI cases were at stage I (12).
In the present study, patients with AKI stage I had
more than a two-fold increase in long-term mortality during a mean
follow-up of approximately 10 months, compared with non-AKI
patients. In a recent study, Liotta et al (12) reported the AKI stage I patients
exhibited a 1.33 increased risk of mortality after 6 years. The
present study also found that even minimal increases in the
postoperative serum creatinine levels of <0.3 mg/dl (26 mmol/l)
were related to a significant increase in mortality in patients who
had undergone coronary artery bypass graft surgery (11). It was further observed that patients
with AKI stage I had a two-fold higher 30-day mortality rate when
compared to patients without AKI using propensity matching scores
(10).
The present study showed also that patients with
stage I AKI were prone to worse postoperative condition than
non-AKI patients. ICU stay, mechanical ventilation time and
hospital stay were significantly higher longer among patients with
AKI stage I than patients without AKI. There were more cases of
respiratory dysfunction and adverse cardiac events in patients with
AKI stage I compared with patients without AKI. Previous studies
also reported similar results (13-15).
Furthermore, the present study showed that patients with recovery
AKI had a better long-term outcome than those with non-recovery
AKI. It has also been reported that the risk of late death (after
discharge from hospital) was related to the recovery of AKI and the
remaining renal function at discharge (16). Recovery from AKI and the correlation
with the long-term outcome of the patient are important issues,
which have now recognized as being of key importance (17). Determining the mechanisms by which
AKI is associated with mortality is critical, as this may gain a
greater understanding of how to provide more effective preventative
interventions. However, the occurrence of AKI stage I is likely to
be a strong indicator of a poor physiological condition for
postoperative patients, as well as having a direct causative
influence on mortality. Therefore, it is difficult to determine the
modifiable, causative influence of AKI on risk of death. The
results of the present study are useful for addressing this issue,
as recovery stage from AKI stage I was also related to mortality.
The results highlight the significance of prompting treatment for
AKI stage I.
The mechanisms underlying the role of AKI stage I in
the postoperative course are unclear. Possible pathophysiological
mechanisms linking AKI stage I and its clinical effects may include
the activation of inflammatory pathways and fluid resuscitation.
Activation of inflammatory pathways is another important mechanism
behind the development of AKI (18).
However, the degree of inflammation may vary from that observed in
septic AKI. The level of inflammation may partially explain the
difference in the AKI stage between cardiac surgery patients and
septic patients (4). Inadequate
circulation is common in AKI patients; however, volume overload is
a more frequent side-effect of AKI currently (18). This could in turn require patients to
extend the time of mechanical ventilation, ICU stay and hospital
stay.
It is important to pay more attention to AKI stage I
among patients who have undergone cardiac surgery with CPB.
Compared with the more severe levels of AKI, there are more
patients who develop AKI stage I. In addition, the treatment of AKI
stage I may be less invasive and more cost-effective than treating
more sever levels of AKI. Therefore, more meticulous diagnosis and
treatment might be needed for patients with AKI stage I to ensure a
better prognosis.
In conclusion, the present study showed that the AKI
stage I was the predominant form of AKI, making up 78% of all AKI
cases. AKI stage I was an independent determinant of all-cause
long-term mortality among cardiac surgery patients. In addition,
the present study showed that patients with recovery AKI stage I
had a better prognosis than those with non-recovery AKI. As there
are more patients who develop AKI stage I compared with the other
stages, AKI stage I was indicated to lead to as many late deaths
associated with AKI compared with patients who develop the more
severe stages of AKI. As such, a more meticulous treatment protocol
is needed for patients with AKI stage I.
Acknowledgements
Not applicable.
Funding
The present study was funded by grants received from
Beijing Municipal Natural Science Foundation (grant no. 7192052);
the National Key R&D Program of China: Perioperative evaluation
of acute kidney injury and protection (grant no. 018YFC2001900);
and Beijing Municipal Administration of Hospitals Clinical Medicine
Development of Special Funding Support (grant no. ZYLX201810).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request
Authors' contributions
YYL and JM led the conception and design of this
study. YYL and JM were responsible for the data collection and
analysis. YYL were in charge of drafting the manuscript. YYL made
revision from critical perspective for important intel¬lectual
content. The final version was read and approved by all the
authors
Ethics approval and consent to
participate
The protocol was approved by the Ethics Committee of
Beijing Anzhen Hospital, Capital Medical University. Patients or
their family members were fully informed of the study details and
provided their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Meersch M, Schmidt C, Hoffmeier A, Van
Aken H, Wempe C, Gerss J and Zarbock A: Prevention of cardiac
surgery-associated AKI by implementing the KDIGO guidelines in high
risk patients identified by biomarkers: The PrevAKI randomized
controlled trial. Intensive Care Med. 43:1551–1561. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Uchino S, Kellum JA, Bellomo R, Doig GS,
Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al:
Beginning and ending supportive therapy for the kidney (BEST
Kidney) investigators: Acute renal failure in critically ill
patients: A multinational, multicenter study. JAMA. 294:813–818.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ranucci M, Aloisio T, Cazzaniga A, Di
Dedda U, Gallazzi C and Pistuddi V: Validation of renal-risk models
for the prediction of non-renal replacement therapy cardiac
surgery-associated acute kidney injury. Int J Cardiol. 272:49–53.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mehta RH, Grab JD, O'Brien SM, Bridges CR,
Gammie JS, Haan CK, Ferguson TB and Peterson ED: Society of
Thoracic Surgeons National Cardiac Surgery Database Investigators.
Bedside tool for predicting the risk of postoperative dialysis in
patients undergoing cardiac surgery. Circulation. 114:2208–2216;
quiz 2208. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thakar CV, Arrigain S, Worley S, Yared JP
and Paganini EP: A clinical score to predict acute renal failure
after cardiac surgery. J Am Soc Nephrol. 16:162–168.
2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lassnigg A, Schmidlin D, Mouhieddine M,
Bachmann LM, Druml W, Bauer P and Hiesmayr M: Minimal changes of
serum creatinine predict prognosis in patients after cardiothoracic
surgery: A prospective cohort study. J Am Soc Nephrol.
15:1597–1605. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Corredor C, Thomson R and Al-Subaie N:
Long-term consequences of acute kidney injury after cardiac
surgery: A systematic review and meta-analysis. J Cardiothorac Vasc
Anesth. 30:69–75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kidney International. KDIGO Clinical
Practice Guideline for Acute Kidney Injury. Kidney Int Suppl.
2:1–138. 2012.
|
|
9
|
Thanavaro J, Taylor J, Vitt L and Guignon
MS: Predictors and outcomes of acute kidney injury after cardiac
surgery. Nephrol Nurs J. 46:31–40. 2019.PubMed/NCBI
|
|
10
|
Kellum JA, Wen X, de Caestecker MP and
Hukriede NA: Sepsis-associated acute kidney injury: A problem
deserving of new solutions. Nephron. 143:174–178. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Elmistekawy E, McDonald B, Hudson C, Ruel
M, Mesana T, Chan V and Boodhwani M: Clinical impact of mild acute
kidney injury after cardiac surgery. Ann Thorac Surg. 98:815–822.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liotta M, Olsson D, Sartipy U and Holzmann
MJ: Minimal changes in postoperative creatinine values and early
and late mortality and cardiovascular events after coronary artery
bypass grafting. Am J Cardiol. 113:70–75. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hobson CE, Yavas S, Segal MS, Schold JD,
Tribble CG, Layon AJ and Bihorac A: Acute kidney injury is
associated with increased long-term mortality after cardiothoracic
surgery. Circulation. 119:2444–2453. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gangadharan S, Sundaram KR, Vasudevan S,
Ananthakrishnan B, Balachandran R, Cherian A, Varma PK, Gracia LB,
Murukan K, Madaiker A, et al: Predictors of acute kidney injury in
patients undergoing adult cardiac surgery. Ann Card Anaesth.
21:448–454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nadim MK, Forni LG, Bihorac A, et al:
Cardiac and Vascular Surgery-Associated Acute Kidney Injury: The
20th international Consensus Conference of the ADQI (Acute Disease
Quality Initiative) Group. J Am Heart Assoc.
7(11)(e008834)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Engoren M, Habib RH, Arslanian-Engoren C,
Kheterpal S and Schwann TA: The effect of acute kidney injury and
discharge creatinine level on mortality following cardiac surgery.
Crit Care Med. 42:2069–2074. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Prowle JR and Kirwan CJ: Acute kidney
injury after cardiac surgery: The injury that keeps on hurting?
Crit Care Med. 42:2142–2143. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Song N, Thaiss F and Guo L: NFκB and
kidney injury. Front Immunol. 10(815)2019.PubMed/NCBI View Article : Google Scholar
|