Introduction
Lower back pain, which is one of the most common
condition, not only causes considerable disability and compromised
quality of life but also places a burden on the family of the
patient affected (1,2). Lumbar disc degeneration (LDD), as the
pathogenic cause of discogenic pain, has previously been correlated
with lower back pain (3). The
proportion of LDD still remains high in the asymptomatic population
(4,5). The development of LDD has previously
been described as a progressive process from mild to severe.
Considering the essential prevention and evaluation of LDD, it is
increasingly important to investigate the potential risk factors of
LDD in asymptomatic individuals.
A variety of patient-specific internal and external
factors contribute to the initiation and progression of LDD,
including biomechanics, heritability, environmental factors,
systemic diseases and smoking (3,6-10).
At present, as a possible causative mechanism, biomechanical
factors and sagittal alignment are used to influence intervertebral
disc degeneration (11). Lumbosacral
morphology has also been considered to serve a potential role in
the degree of LDD and herniation (12). However, these aforementioned studies
are aimed at symptomatic individuals with lower back pain or
incapacitating symptoms. Whether biomechanical factors serve a
major role in LDD in the asymptomatic population has remained to be
determined and the influence of spinal morphology, including lumbar
spinal subtypes (LSS) on disc degeneration, also remains
controversial.
To systematically describe the normal sagittal
alignment of the lumbar spine in asymptomatic young adults,
Roussouly et al (13)
proposed a four-point classification system based on lumbar and
pelvic parameters. The lumbar postural subtypes were classified by
sacral slope (SS) and spinal morphology (13). Subsequently, according to the shape
of the lumbar spine, Roussouly and Pinheiro-Franco (14) proposed a different process of
degeneration. The classification is as follows: Type I: The SS is
<35˚ and the center of the L5 vertebral body is located at the
apex of lumbar lordosis (LL). The lower or upper arc angle is
minimal and the inflexion point is lower or posterior. Due to the
L4-5 hyperextension, it may induce a nutcracker L5 spondylolysis.
Type II: The SS is <35˚ and the base of the L4 vertebral body is
located in the apex of lumbar lordosis. The LL arc is flat. Type II
has a high risk of early disc herniation. Type III: The SS is
between 35˚ and 45˚. Type III: An average shape that does not hold
characteristics for a specific degeneration. Type IV: The SS is
>45˚. Type IV: Retains the lordosis curvature, which may result
in a degenerative L4/L5 spondylolisthesis. To the best of our
knowledge, the association between LSS and LDD has only been
investigated in young asymptomatic adults between 20 to 40 years of
age (4), while, the correlation in
asymptomatic middle-aged and aged adults has remained to be
determined. There are two major reasons. First, in this specific
asymptomatic population, the natural degeneration of intervertebral
disc was universally occurring; thus, investigating this population
was considered to be of high significance. Furthermore,
asymptomatic middle-aged and aged individuals were difficult to be
recruited. These are the reasons for the remaining lack of relevant
studies in this population. Based on the above reasons, this
specific population was examined in the present study.
The aim of the present study was to identify whether
lumbar subtypes and spinopelvic parameters are associated with LDD
in middle-aged and aged individuals. In the present study, it was
assumed that lumbar sagittal alignment and LSS do not have the
power to result in level-specific predilection for LDD; therefore,
it was expected that no difference would be observed.
Materials and methods
Study population
The present study was a single-center, retrospective
data analysis that aimed to investigate the correlation between LSS
and LDD in middle and old-aged asymptomatic volunteers. Following
approval by the institutional review board of The Second Affiliated
Hospital and Yuying Children's Hospital of Wenzhou Medical
University (Wenzhou, China), a cohort of 158 asymptomatic Chinese
adults aged >40 years encountered between May 2016 and November
2018 at the Second Affiliated Hospital and Yuying Children's
Hospital of Wenzhou Medical University (Wenzhou, China) was
recruited. All volunteers provided written informed consent prior
to enrollment.
The inclusion criteria were as follows: Volunteers
aged >40 years, with available standing lumbar plain film
radiographs. Once these volunteers met the inclusion criteria, an
MRI of the lumbar spine was performed using a 3T MR scanner
(Discovery 750; GE Healthcare). The exclusion criteria were as
follows (15): i) Lameness or
unequal length of the lower limbs; ii) significant scoliosis (Cobb
angle >10˚ in the coronal position) (16); iii) a history of trauma of the lower
extremities, pelvis or spine; iv) a history of hip or knee
arthroplasty and/or spinal, pelvic or lower-limb surgery; v)
complaints of back pain, neck pain or limb numbness caused by
degenerative diseases of the spine, including disc herniation,
spinal canal stenosis and lumbar spondylolisthesis; vi) strabismus
or torticollis affecting balance; vii) a history of neuromuscular
disorders or congenital abnormalities; viii) pregnancy or
preparation for pregnancy.
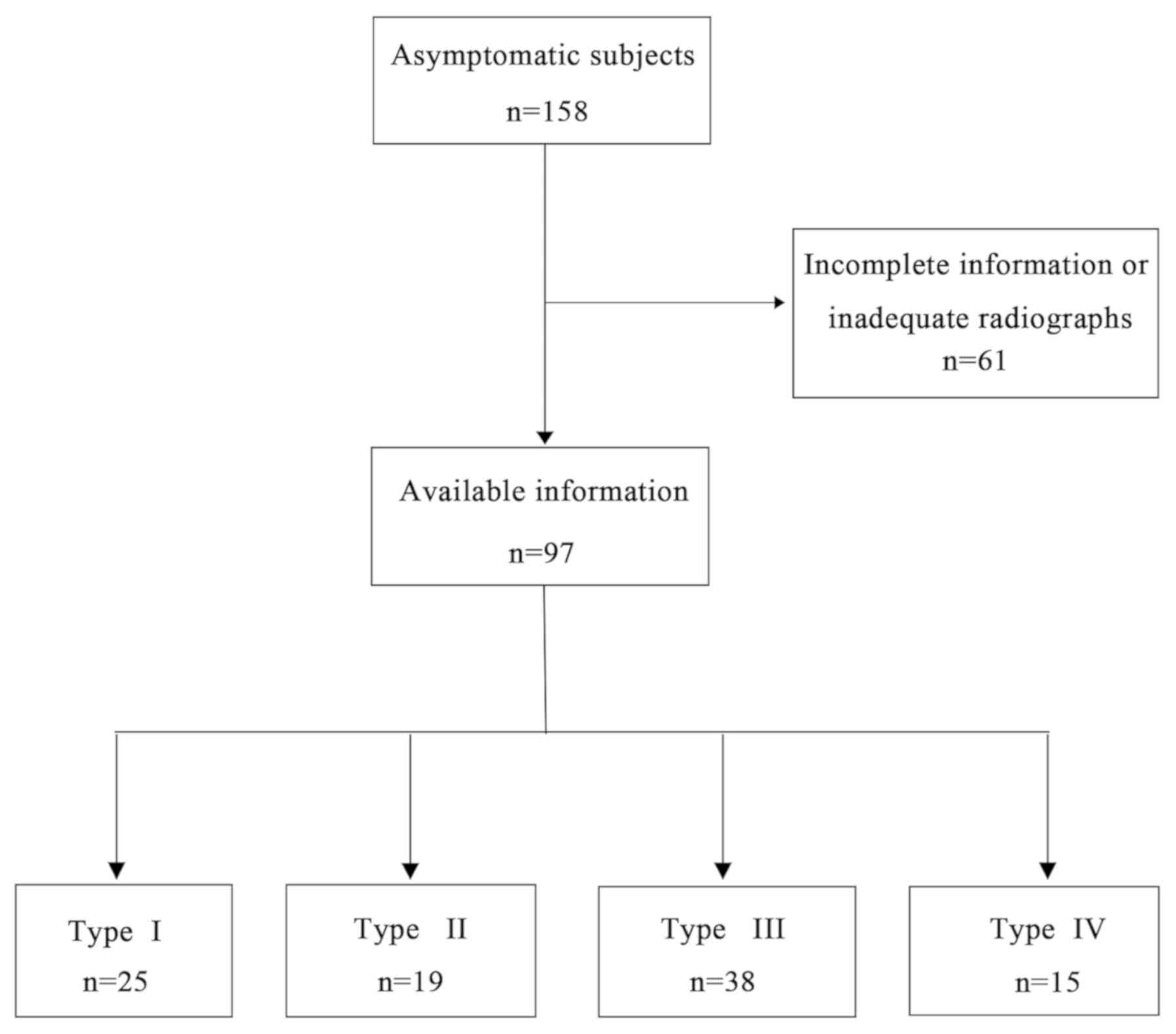
Of the 158 subjects, 61 volunteers who had
incomplete information or inadequate radiographs available, or
those who met the exclusion criteria were excluded. Subsequently, a
total of 97 asymptomatic volunteers were included in the present
study, and baseline information was recorded, including age,
weight, height and BMI. Finally, cases were classified into four
groups according to LSS after carefully evaluating standing lumbar
plain film radiographs: Type I, n=25; Type II, n=19; Type III, n=38
and Type IV, n=15 (Figs. 1 and
2).
Radiographic analysis and data
collection
Lumbar spinal standing anteroposterior and lateral
radiographs were acquired for all volunteers with their arms in the
fists-on-clavicles position (17).
The radiographs were examined by a spine surgeon who had
independently reviewed hundreds of images previously. Parameters
collected from plain film radiographs included LL, SS, pelvic tilt
(PT) and pelvic incidence (PI). The LL is defined as the subtended
angle between the upper endplate of L1 and the superior end plate
of S1. The SS is defined as the angle between the horizontal and
the upper sacral endplate. The PT is defined as the angle between
the vertical and the line through the midpoint of the sacral plate
to femoral head axis. The PI is defined as the angle perpendicular
to the upper sacral endplate at its midpoint and the line
connecting this point to the femoral head axis.
The degree of each intervertebral disc degeneration,
which was based on the classification of Pfirrmann, was evaluated
by two spinal surgeons (CAH and YZY) with >5 years of experience
using MRI (Fig. 3) (18). Controversial discs were then
subsequently presented to a third spinal surgeon (XYW) who provided
the final evaluation. According to the Pfirrmann grades (I-V)
(4), discs were then categorized as
two groups: Non-degenerated (Pfirrmann ≤II) and degenerated
(Pfirrmann ≥III).
Statistical analysis
Data in the present study were presented as either
the mean ± standard deviation or median (interquartile range).
Following distribution analysis according to the Shapiro-Wilk test,
baseline information and spinopelvic parameters were compared using
a Kruskal-Wallis, χ2 test or one-way analysis of
variance (ANOVA) to compare the variance of observed values among
the LSS types, including values for age, gender, body mass index
(BMI), SS, PI, PT and LL. The frequency of LDD among the four LSS
types were compared using a χ2 or Fisher's exact test.
All statistical analyses were performed using SPSS 22.0 (IBM
Corp.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline data and measurement of
parameters
For type I-IV LSS, there were differences in the
proportion of volunteers, where the number of individuals in the
type III subgroup was largest. As presented in Fig. 1, 25 (25.78%), 19 (19.59%), 38
(39.18%) and 15 subjects (15.46%) were included in group I-IV,
respectively. All baseline data and certain radiographic
parameters, including PI and PT, demonstrated a normal
distribution, which represented real-valued random variables and
they were compared between groups using one-way ANOVA. SS and LL
were compared using a Kruskal-Wallis test due to data having a
skewed distribution. As presented in Table I, baseline data were approximately
equal for all four groups and exhibited no statistically
significant difference, including for age, gender, body weight,
body height and BMI (all P>0.05). In terms of the lumbar spine
sagittal alignment, the values in type I were as follows: SS, 29.4˚
(25.6;33.5˚); PI, 39.1±5.6˚; PT, 9.9±5.0˚; LL, 38.5˚(31.9,46.1˚).
The average values in type II were as follows: SS, 31.8˚
(28.8;34.3˚); PI, 41.1±5.5˚; PT, 10.0±4.5˚; LL, 44.5˚(35.7;48.8˚).
Average values in type III were as follows: SS, 39.0˚ (37.2;41.0˚);
PI, 49.8±8.2˚; PT, 10.8±7.3˚; LL, 53.2˚ (47.7;56.5˚). Average
values in type IV were as follows: SS, 45.9˚ (45.2;48.3˚); PI,
58.4±7.3˚; PT, 11.4±5.2˚; LL, 63.8˚ (60.0;68.7˚). Statistically
significant differences among the four LSS groups were observed for
SS, PI and LL (P<0.001 for each), but no significant difference
was observed for PT (P=0.21).
 | Table IClinicopathological characteristics of
patients by Roussouly type. |
Table I
Clinicopathological characteristics of
patients by Roussouly type.
| Variable | Type I (n=25) | Type II (n=19) | Type III (n=38) | Type IV (n=15) | P-value |
|---|
| Sex
(male/female) | 4/21 | 10/9 | 13/25 | 5/10 | 0.079a |
| Age (years) | 52.16±8.68 | 53.11±7.87 | 53.87±7.50 | 53.67±7.86 | 0.861b |
| Body weight (kg) | 60.00±9.25 | 64.18±7.42 | 64.20±10.05 | 62.87±7.00 | 0.289b |
| Body height (m) | 1.62±0.067 | 1.66±0.065 | 1.63±0.065 | 1.64±0.067 | 0.160b |
| BMI
(kg/m2) | 22.83±3.06 | 23.13±1.44 | 24.18±3.09 | 23.44±2.48 | 0.142b |
| SS (degrees) | 29.4 (25.6,33.5) | 31.8 (28.8,34.3) | 39.0 (37.2,41.0) | 45.9 (45.2,48.3) |
<0.001c |
| PI (degrees) | 39.1±5.6 | 41.1±5.5 | 49.8±8.2 | 58.4±7.3 |
<0.001b |
| PT (degrees) | 9.9±5.0 | 10.0±4.5 | 10.8±7.3 | 11.4±5.2 | 0.210b |
| LL (degrees) | 38.5
(31.9,46.1) | 44.5
(35.7,48.8) | 53.2
(47.7,56.5) | 63.8
(60.0,68.7) |
<0.001c |
Assessment of LDD
To assess the correlation between LSS and LDD, the
degree of disc degeneration was assessed according to the Pfirrmann
classification. Among each LSS, the distribution of the degree of
the LDD at each sequential lumbar level from L1/L2 to L5/S1 is
presented in Table II. Across all
LSS groups, L1/L2, L2/L3 and L3/L4, LDD was mainly indicated to be
grade II and III, and L4/L5, L5/S1 was mainly indicated to be grade
III and IV, but grade I and V were rarely observed at all levels.
Furthermore, despite the different subgroups, the proportion of
disc degeneration increased from proximal toward distal (Table III and Fig. 4). The percentage of degenerated discs
in groups I-IV based on the Roussouly classification was ~50%,
accounting for 44, 52, 50 and 48%, respectively (Table III).
 | Table IIDistribution of the lumbar disc
degeneration degree in each lumbar spinal subgroup. |
Table II
Distribution of the lumbar disc
degeneration degree in each lumbar spinal subgroup.
| A, Roussouly type
I |
|---|
| Pfirrmann
classification | L1-L2 | L2-L3 | L3-L4 | L4-L5 | L5-S1 |
|---|
| I | 3 | 0 | 1 | 0 | 0 |
| II | 16 | 15 | 15 | 11 | 9 |
| III | 5 | 5 | 7 | 8 | 5 |
| IV | 1 | 4 | 2 | 6 | 10 |
| V | 0 | 1 | 0 | 0 | 1 |
| B, Roussouly type
II |
| Pfirrmann
classification | L1-L2 | L2-L3 | L3-L4 | L4-L5 | L5-S1 |
| I | 0 | 0 | 1 | 0 | 0 |
| II | 12 | 13 | 10 | 5 | 5 |
| III | 5 | 3 | 5 | 4 | 5 |
| IV | 1 | 3 | 3 | 9 | 6 |
| V | 1 | 0 | 0 | 1 | 3 |
| C, Roussouly type
III |
| Pfirrmann
classification | L1-L2 | L2-L3 | L3-L4 | L4-L5 | L5-S1 |
| I | 0 | 1 | 1 | 1 | 4 |
| II | 29 | 19 | 14 | 12 | 13 |
| III | 7 | 16 | 13 | 13 | 4 |
| IV | 2 | 2 | 9 | 11 | 16 |
| V | 0 | 0 | 1 | 1 | 1 |
| D, Roussouly type
IV |
| Pfirrmann
classification | L1-L2 | L2-L3 | L3-L4 | L4-L5 | L5-S1 |
| I | 0 | 0 | 0 | 2 | 2 |
| II | 12 | 11 | 6 | 1 | 5 |
| III | 3 | 4 | 7 | 4 | 1 |
| IV | 0 | 0 | 2 | 8 | 7 |
| V | 0 | 0 | 0 | 0 | 0 |
 | Table IIIDistribution of the lumbar disc
degeneration degree in each lumbar spinal subgroup. |
Table III
Distribution of the lumbar disc
degeneration degree in each lumbar spinal subgroup.
| | Type I | Type II | Type III | Type IV | |
|---|
| Level |
Non-degenerated | Degenerated |
Non-degenerated | Degenerated |
Non-degenerated | Degenerated |
Non-degenerated | Degenerated | χ2 | P-value |
|---|
| L1/L2 | 19 | 6 | 12 | 7 | 29 | 9 | 12 | 3 | NA | 0.706a |
| L2/L3 | 15 | 10 | 13 | 6 | 20 | 18 | 11 | 4 | 2.523 | 0.471b |
| L3/L4 | 16 | 9 | 11 | 8 | 16 | 22 | 6 | 9 | 3.971 | 0.265b |
| L4/L5 | 11 | 14 | 5 | 14 | 13 | 25 | 3 | 12 | NA | 0.440a |
| L5/S1 | 9 | 16 | 5 | 14 | 17 | 21 | 7 | 8 | 2.271 | 0.518b |
| Total | 70(56) | 55(44) | 46(48) | 49(52) | 95(50) | 95(50) | 39(52) | 36(48) | | |
No differences among LSS
Based on the evaluation of the proportion of disc
degeneration at each level, no statistically significant difference
among types I to IV was indicated (Table III). LDD was not indicated to be
significantly associated with lumbar spinal morphology, nor was
higher spinopelvic parameters protective against LDD among
asymptomatic middle-aged and aged adults (Table III).
Discussion
The present study included a total of 97 volunteers
aged >40 years and investigated the effect of LSS on the
prevalence of lumbar intervertebral disc degeneration. The
prevalence of LDD at the caudal lumbar intervertebral levels at
L4/5 and L5/S1 was significantly higher compared with the proximal
levels in all types. Contact forces and shear stress primarily act
on the caudal region of the lumbar spine due to body weight stress
(19); therefore, the increased
mechanical stress at L4/5 and L5/S1 may lead to the pathological
procress of degeneration.
Furthermore, it was demonstrated that the frequency
of disc degeneration from type I to IV was 44, 52, 50 and 48%,
respectively, in a population of middle-aged and aged asymptomatic
subjects, which was significantly higher compared with that in
young individuals in a previous study (4). It was clear that age was an important
and non-negligible factor that contributed to the increase of LDD.
For instance, disc degeneration in 88% of individuals aged >55
years was increased compared with 42% in subjects aged <30 years
(20).
The pelvic position and shape interact with the
spinal organization and regulate the balance between the spine and
pelvis. According to biomechanical analysis, spinopelvic sagittal
alignment was expected to explain intervertebral disc degeneration.
Therefore, with regard to spinopelvic parameters, Roussouly and
Pinheiro-Franco (14) categorized
LSS as type I to IV, which are all considered to be normal.
Excluding extrinsic triggers of intervertebral disc degeneration,
including physical activity (21,22), the
parameters of lumbar spine sagittal alignment, including LL and PI,
were considered to be strong predictors and serve a predisposing
role in the pathogenesis of LDD diseases (12,23). SS,
PI and LL have been observed to be different from type I to IV
(5,23), thus, it was possible that different
LSS (I-IV) may influence level-specific degeneration via specific
biomechanical stressors. The Roussouly type II subtype is a flat
lordosis that is characterized by mild thoracic and lumbar
curvatures, with an SS of <35˚. Therefore, stress is at its
maximum on the discs in type II, and therefore, this type is
associated with a higher risk of disc degeneration compared with
type IV. Although the higher prevalence of lower back pain has been
demonstrated in the type II subtype (14), the impact of LSS on the degeneration
of the intervertebral disc has remained to be identified. A number
of studies have refuted the theory regarding the influence of the
spinal structure on the progress of LDD. Battie et al
(24) reported that heredity, as
opposed to physical loading, has a relatively dominant role in the
progress of disc degeneration, which may explain the high
prevalence of up to 74% seen in the general population. Torrie and
Videman (5) suggested that LSS was
not statistically associated with LDD and a higher PI was not a
protective factor against LDD. Similarly, in the present study, the
difference of spinopelvic parameters was observed, including SS, PI
and LL, but level-specific degeneration was indicated to not be
significantly different among LSS.
Previous studies on the effect of genetic factors on
the LDD process also supported the influence of genetic defects on
the structural and functional changes in the intervertebral disc,
which may compromise the disc's mechanical properties and metabolic
activities (9,24-27).
Battie et al (24) considered
that genetic factors and not physical loading specific to
occupation and sport served the dominant role in disc degeneration
in a previous study involving twins. Based on the present results,
it was hypothesized that genetic heritability and not spinal
biomechanical differences result in the initialization of LDD in a
specific age group, particularly in older populations. Heritability
has previously been described as the proportion of phenotypic
variation within a population, where the trait of disc degeneration
has been suggested to be heritable in a previous study (28). Genetic factors may become major risk
factors and contribute to the patho-etiology of LDD, as described
by various studies (3,24,29). In
a previous study on spines of twins, genetic heritability was
demonstrated to be associated with lower back pain (3). Recently, a review also highlighted the
genetic basis of LDD and assessed how genetic variants influenced
IDD using cell biology (29).
The present study was not the first to identify the
association between lumbar spine sagittal alignment and disc
degeneration in an asymptomatic population. A previous study
reported that the subtype II was significantly associated with disc
degeneration at L4-L5 in asymptomatic young adults (4), which was in contradiction with the
results of the present study. It may be suggested that genetic
heritability may be a major factor in the process of intervertebral
disc degeneration in older individuals and lumbar spine sagittal
alignment may not influence disc degeneration. Genes were not
indicated to exhibit the power to cause disc degeneration and this
may have been the reason why the high prevalence was reduced to 42%
in the young population. Therefore, biomechanical factors may have
a weak role at one specific level. Future studies should focus on
biomechanical factors in the young asymptomatic population and
increase attention to other factors in the older population.
Regarding the clinical prevention of intervertebral disc
degeneration, the present study indicated that it may not be
necessary for spinal surgeons to evaluate LSS in asymptomatic
middle-aged and aged individuals.
A number of limitations were present in the current
study. First, genetic factors were not assessed, which may be a
major risk factor for this disorder. This was why only the
biomechanical factors were evaluated. Furthermore, the small number
of asymptomatic volunteers included in the present study may result
in a large error. In future studies, it is essential to assess more
volunteers in a larger cohort study. Finally, the study was
retrospective and was not a longitudinal cohort study. However, the
present study successfully demonstrated that structural differences
are not a risk factor in LSS and did not influence LDD in the study
on subjects aged >40 years.
In conclusion, in the present study, no correlation
between LSS and intervertebral disc degeneration was observed among
asymptomatic middle-aged and aged subjects. In addition, other risk
factors may serve a vital role in disc degeneration in asymptomatic
individuals of these age groups and this requires further
study.
Acknowledgements
Not applicable.
Funding
The current study was supported by grants from the
National Nature Foundation of China (grant no. 81871806) and the
Zhejiang Public Service Technology Research Program/Social
Development (grant no. LGF18H060008).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW, YZY and XYW designed the study. BW, JLL, LJC,
YZY, XYW and YFS recruited volunteers. BW, CAH, YZY, XYW and YFS
collected the data. BW, BDC and ZXS analyzed the data. CAH, YZY and
XYW performed the radiological analysis and interpreted the
results. BW, LJC and ZXS organized the manuscript. JLL reviewed the
papers and revised the manuscript. All authors have read and
approved the final version of the manuscript. All authors
contributed toward data analysis, drafting and revising the paper
and agree to be accountable for all aspects of the work.
Ethics approval and consent to
participate
The present study was approved by the Ethics Board
of the Second Affiliated Hospital and Yuying Children's Hospital of
Wenzhou Medical University (2016 number 10; Wenzhou, China).
Written informed consent for publication was obtained from all
individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luoma K, Riihimaki H, Luukkonen R,
Raininko R, Viikari-Juntura E and Lamminen A: Low back pain in
relation to lumbar disc degeneration. Spine (Phila Pa 1976).
25:487–492. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Murray CJ, Vos T, Lozano R, Naghavi M,
Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S,
et al: Disability-adjusted life years (DALYs) for 291 diseases and
injuries in 21 regions, 1990-2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2197–2223.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Livshits G, Popham M, Malkin I, Sambrook
PN, Macgregor AJ, Spector T and Williams FM: Lumbar disc
degeneration and genetic factors are the main risk factors for low
back pain in women: The UK Twin Spine Study. Ann Rheum Dis.
70:1740–1745. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Menezes-Reis R, Bonugli GP, Dalto VF, da
Silva Herrero CF, Defino HL and Nogueira-Barbosa MH: Association
between lumbar spine sagittal alignment and L4-L5 Disc degeneration
among asymptomatic young adults. Spine (Phila Pa 1976).
41:E1081–E1087. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Torrie PA, McKay G, Byrne R, Morris SA and
Harding IJ: The influence of lumbar spinal subtype on lumbar
intervertebral disc degeneration in young and Middle-aged adults.
Spine Deform. 3:172–179. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Battie MC, Videman T, Gill K, Moneta GB,
Nyman R, Kaprio J and Koskenvuo M: 1991 Volvo Award in clinical
sciences. Smoking and lumbar intervertebral disc degeneration: An
MRI study of identical twins. Spine (Phila Pa 1976). 16:1015–1021.
1991.PubMed/NCBI
|
|
7
|
Hangai M, Kaneoka K, Kuno S, Hinotsu S,
Sakane M, Mamizuka N, Sakai S and Ochiai N: Factors associated with
lumbar intervertebral disc degeneration in the elderly. Spine J.
8:732–740. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leino-Arjas P, Kaila-Kangas L, Solovieva
S, Riihimaki H, Kirjonen J and Reunanen A: Serum lipids and low
back pain: An association? A follow-up study of a working
population sample. Spine (Phila Pa 1976). 31:1032–1037.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Feng Y, Egan B and Wang J: Genetic factors
in intervertebral disc degeneration. Genes Dis. 3:178–185.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xia DD, Lin SL, Wang XY, Wang YL, Xu HM,
Zhou F and Tan J: Effects of shear force on intervertebral disc: An
in vivo rabbit study. Eur Spine J. 24:1711–1719. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis Cartilage. 23:1057–1070.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ergun T, Lakadamyali H and Sahin MS: The
relation between sagittal morphology of the lumbosacral spine and
the degree of lumbar intervertebral disc degeneration. Acta Orthop
Traumatol Turc. 44:293–299. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Roussouly P, Gollogly S, Berthonnaud E and
Dimnet J: Classification of the normal variation in the sagittal
alignment of the human lumbar spine and pelvis in the standing
position. Spine (Phila Pa 1976). 30:346–353. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Roussouly P and Pinheiro-Franco JL:
Biomechanical analysis of the spino-pelvic organization and
adaptation in pathology. Eur Spine J. 20 (Suppl 5):S609–S618.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yan YZ, Shao ZX, Pan XX, Chen SQ, Wu AM,
Tian NF, Wu YS and Wang XY: Acceptable Chin-brow vertical angle for
neutral position radiography: Preliminary analyses based on
parameters of the whole sagittal spine of an asymptomatic chinese
population. World Neurosurg. 120:e488–e496. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Glassman SD, Berven S, Bridwell K, Horton
W and Dimar JR: Correlation of radiographic parameters and clinical
symptoms in adult scoliosis. Spine (Phila Pa 1976). 30:682–688.
2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aota Y, Saito T, Uesugi M, Ishida K,
Shinoda K and Mizuma K: Does the fists-on-clavicles position
represent a functional standing position? Spine (Phila Pa 1976).
34:808–812. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Keller TS, Colloca CJ, Harrison DE,
Harrison DD and Janik TJ: Influence of spine morphology on
intervertebral disc loads and stresses in asymptomatic adults:
Implications for the ideal spine. Spine J. 5:297–309.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cheung KM, Chan D, Karppinen J, Chen Y,
Jim JJ, Yip SP, Ott J, Wong KK, Sham P, Luk KD, et al: Association
of the Taq I allele in vitamin D receptor with degenerative disc
disease and disc bulge in a Chinese population. Spine (Phila Pa
1976). 31:1143–1148. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Elfering A, Semmer N, Birkhofer D, Zanetti
M, Hodler J and Boos N: Risk factors for lumbar disc degeneration:
A 5-year prospective MRI study in asymptomatic individuals. Spine
(Phila Pa 1976). 27:125–134. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Samartzis D, Karppinen J, Mok F, Fong DY,
Luk KD and Cheung KM: A population-based study of juvenile disc
degeneration and its association with overweight and obesity, low
back pain, and diminished functional status. J Bone Joint Surg Am.
93:662–670. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang X, Kong Q, Song Y, Liu L, Zeng J and
Xing R: The characteristics of spinopelvic sagittal alignment in
patients with lumbar disc degenerative diseases. Eur Spine J.
23:569–575. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Battie MC and Videman T: Lumbar disc
degeneration: Epidemiology and genetics. J Bone Joint Surg Am. 88
(Suppl 2):S3–S9. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chan D, Song Y, Sham P and Cheung KM:
Genetics of disc degeneration. Eur Spine J. 15 (Suppl 3):S317–S325.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cheung KM: The relationship between disc
degeneration, low back pain, and human pain genetics. Spine J.
10:958–960. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kalichman L and Hunter DJ: The genetics of
intervertebral disc degeneration. Associated genes. Joint Bone
Spine. 75:388–396. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Williams FM, Popham M, Sambrook PN, Jones
AF, Spector TD and MacGregor AJ: Progression of lumbar disc
degeneration over a decade: A heritability study. Ann Rheum Dis.
70:1203–1207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Munir S, Rade M, Maatta JH, Freidin MB and
Williams FMK: Intervertebral disc biology: Genetic basis of disc
degeneration. Curr Mol Biol Rep. 4:143–150. 2018.PubMed/NCBI View Article : Google Scholar
|