Introduction
Prostate cancer (PCa) remains the most prevalent
cancer in men in North America and the second leading cause of
cancer-related mortality in males. The majority of PCa-related
deaths are due to metastases rather than primary tumour burden
(1,2). PCa can metastasize to the bone, lymph
nodes, liver, adrenal glands or lungs (3). The 5-year survival rate of patients
with non-metastatic PCa has been reported to be 98.9% but that of
patients with metastatic PCa is only 28.2% (4).
Epithelial-mesenchymal transition (EMT) is a
differentiation program where cells switch from epithelial to
mesenchymal phenotypes, which serves a key role in the early stage
of cancer cell metastasis (5). EMT
is initiated following the dissolution of tight junctions resulting
in the loss of apical-basal cell polarity. The characteristics of
EMT include altered expression of proteins, coupled with the
upregulation of mesenchymal markers, such as N-cadherin and
vimentin, and loss of epithelial markers, such as E-cadherin
(6). The Wnt/β-catenin signalling
pathway has an essential role in EMT, in which β-catenin functions
as a key signalling mediator. In epithelial tissues under
non-cancerous conditions, β-catenin participates in the linking of
E-cadherin and contributes to cell-cell adhesion (7). During EMT, β-catenin dissociates from
the E-cadherin/β-catenin complex at the cell membrane, accumulates
in the cytoplasm and translocates into the nucleus where it acts as
a transcriptional activator to promote the transcription of
EMT-related genes, such as Snail, vimentin and matrix
metalloproteinase-7(8). Numerous
studies have demonstrated that EMT is a critical process in the
invasion and metastasis of PCa (9,10). EMT
has been shown to occur after androgen withdrawal therapy in PCa
and is associated with a poor clinical prognosis (11). Therefore, it is important to identify
the molecular triggers of EMT in order to decrease metastasis and
improve the survival rates of patients with PCa.
CD147, also named extracellular matrix
metalloproteinase inducer, is a glycosylated transmembrane member
of the immunoglobulin superfamily. CD147 is highly expressed on the
cell surface of most cancer cells, including PCa cells (12). During tumorigenesis, CD147
contributes to cell metastasis, drug resistance and angiogenesis
(13-15).
A previous study demonstrated that CD147 plays a vital role in the
invasion and metastasis of PCa cells (13). However, the relationship between
CD147 and EMT in PCa cells remains elusive. The aim of the present
study was to evaluate the role of CD147 in induction of EMT and to
decipher the underlying molecular mechanisms.
Materials and methods
Cell culture
Androgen-sensitive LNCaP cells, (provided by the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences) infected with the pGV248 lentiviral vector as the
backbone for the CD147 short hairpin (sh)RNA construct (Shanghai
GeneChem Co., Ltd) were termed LNCaP/shCD147 cells. LNCaP/Scramble
cells (negative control) were established by infecting LNCaP cells
with the pGV248 lentiviral vector containing a control shRNA
sequence. The target sequences for the CD147 and control shRNA
duplexes were 5'-GTCGTCAGAACACATCAACT-3' and
5'-CAGTCGCGTTTGCGACTGG-3', respectively (16). Lentiviral infection was performed
following the manufacturer's protocol (Shanghai GeneChem Co., Ltd).
Briefly, 1x106 cells/well were seeded in 12-well plates
24 h prior to the experiment. The cells were infected at 30
multiplicity of infection. Lentiviral vectors were added in the
presence of polybrene (5 µg/ml) and the supernatant was removed 24
h post-infection. The cells were maintained in RPMI 1640 medim
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in an atmosphere of
95% air and 5% CO2. At 3 days after lentivirus
infection, cells were digested by trypsin and collected by
centrifugation (1,200 x g at room temperature for 10 min), and
reverse transcription-quantitative PCR (RT-qPCR) was used to
determine the expression of CD147 gene levels in the cells of the
two groups. For western blotting experiments, cells were treated
with 20 mM lithium chloride (LiCl; cat. no. L9650; Sigma-Aldrich;
Merck KGaA) for 3 h at 37˚C.
RT-qPCR
Total RNA was extracted with TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was
synthesized using 1 µl RNA in 30 µl of reaction buffer by RT using
an All-in-One First-Strand cDNA Synthesis kit (cat. no. QP006;
GeneCopoeia Inc.) and oligo(dT) 18 primers at 42˚C for 90 min
according to the manufacturer's instructions. RT-qPCR was performed
using an UltraSYBR Mixture with ROX (CoWin Biosciences) according
to the manufacturer's instructions on the ABI 7500 fluorescence
qPCR instrument (Applied Biosystems). The following primer pairs
were used for the qPCR: CD147 forward, 5'-CAGAGTGAAGGCTGTGAAGTCG-3'
and reverse, 5'-TGCGAGGAACTCACGAAGAA-3' and β-actin forward,
5'-CACTGTGCCCATCTACGAGG-3' and reverse, 5'-TAATGTCACGCACGATTTCC-3'.
The reaction conditions were as follows: 2 min at 50˚C and 10 min
at 95˚C, followed by 40 cycles (15 sec at 95˚C, 30 sec at 60˚C and
30 sec at 70˚C) and final extension at 72˚C for 30 sec. All values
were normalized to β-actin expression. Relative quantification was
performed using the ΔΔCq method, and the results are
expressed in a linear form using the formula 2-ΔΔCq
(17).
Cell Counting Kit-8 (CCK-8) assay
The cells (1x104/well) were seeded in
96-well plates and cultured at 37˚C with 5% CO2 for 2,
4, 6 and 8 days. Cell viability was assessed using CCK-8 (Beyotime
Institute of Biotechnology). After 2, 4, 6 and 8 days, 10 µl CCK-8
solution was added to the medium, the supernatants were removed and
the absorbance of the sample was measured at 450 nm. Cell viability
(%)=(experimental group/control group) x100.
Transwell assay
The cell migration and invasion assays were
performed in 24-well plates with a filter chamber (pore size, 8 µm;
Corning Inc.). For the migration assays, the cells
(5x104/well, 200 µl) were seeded into the upper chamber
without a Matrigel-coated membrane with RPMI-1640 medium containing
0.1% FBS. The bottom chambers were filled with 600 µl RPMI-1640
medium containing 10% FBS. For the invasion assay, the cells
(1x105/well, 200 µl) were seeded into the upper chamber
on top of a Matrigel-coated membrane with RPMI-1640 medium
containing 0.1% FBS. Following incubation for 48 h at 37˚C, the
remaining cells in the upper chamber were gently removed with a
cotton swab. The cells on the lower surface of the membrane filter
were stained with 0.1% crystal violet for 20 min at room
temperature and observed by light microscopy (magnification, x200;
Olympus Corporation).
Western blotting
The cells were washed twice with PBS and then lysed
with lysis buffer (Beyotime Institute of Biotechnology) containing
a cocktail of protease inhibitors (Roche Diagnostics GmbH). To
extract specific protein compartments, the Compartmental Protein
Extraction kit (Beyotime Institute of Biotechnology) was used,
according to the manufacturer's protocol. Protein concentration was
determined using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Equal amounts of protein (30 µg) were
separated on 10% sodium dodecyl sulphate-polyacrylamide gels and
transferred onto polyvinylidene fluoride membranes (EMD Millipore).
The membranes were then blocked with 5% nonfat milk in PBS
containing 0.05% Tween-20 at room temperature for 2 h. The
membranes were incubated with rabbit monoclonal antibodies against
CD147 (cat. no. 13287), human p-glycogen synthase kinase (GSK)-3β
(Ser 9; cat. no. 9322), GSK-3β (cat. no. 9315), E-cadherin (cat.
no. 3195), N-cadherin (cat. no. 13116), vimentin (cat. no. 5741),
β-catenin (cat. no. 8480), p-β-catenin (Ser 33/37/Thr 41; cat. no.
9561) and Snail (cat. no. 3879) all at 1:1,000 dilution (Cell
Signaling Technology, Inc.) overnight at 4˚C. β-actin (cat. no.
4970) and lamin B (cat. no. 13435) were used as loading controls
(both 1:2,000; Cell Signaling Technology, Inc.). Subsequently, the
membranes were incubated with a horseradish peroxidase-conjugated
anti-rabbit IgG antibody (1:3,000; cat. no. A0208; Beyotime
Institute of Biotechnology) and was visualized and quantified using
an enhanced chemiluminescence detection system (iBright CL1500;
Invitrogen; Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation
of three experiments. Differences between two groups were assessed
using the Mann-Whitney U test and comparisons between multiple
groups were performed using the Kruskal-Wallis test followed by
Dunn's multiple comparisons post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
CD147 expression in LNCaP/Scramble and
LNCaP/shCD147 cells
To study the function of CD147, the LNCaP cell line,
which has cytological features of prostate cancer cells, was chosen
to knock down CD147 expression. RT-qPCR and western blotting were
performed to evaluate the relative mRNA and protein expression
levels. As shown in Fig. 1, the mRNA
and protein expression levels of CD147 were depleted in LNCaP cells
following infection with lentiviruses to deplete CD147 expression
(LNCaP/shCD147) compared with LNCaP cells infected with the control
lentivirus (LNCaP/Scramble).
CD147 promotes the growth of LNCaP
cells
The cells were cultured for 2, 4, 6 and 8 days with
cell growth measured using the CCK-8 assay. As shown in Fig. 2, knockdown of CD147 expression in
LNCaP cells inhibited cell growth compared with LNCaP cells
infected with the scramble lentivirus. LNCaP cells were infected
with the lentiviral shCD147 or scramble vector for 3 days and
further experiments were conducted 3 days after infection in order
to reduce the influence of depletion of CD147-induced cell growth
inhibition.
CD147 promotes the migration and
invasion of LNCaP cells
To investigate whether CD147 inhibits the migration
and invasion of LNCaP cells, Transwell assays were performed with
un-coated and Matrigel-coated membranes to determine the effects of
CD147 expression on cell migration (Fig.
3A) and invasion (Fig. 3B). The
results showed that the cells which had been depleted of CD147
expression were significantly less migratory or invasive than the
LNCaP cells that had been infected with the Scramble
lentivirus.
CD147 induces EMT in LNCaP cells
To assess whether CD147 affects the EMT of PCa
cells, the expression of key markers of EMT was detected. The
results demonstrated that the knockdown of CD147 in LNCaP cells led
to an increase in the expression of the epithelial marker
E-cadherin, and a decrease in the expression of mesenchymal markers
N-cadherin and vimentin compared with the LNCaP/Scramble cells
(Fig. 4). These results suggested
that CD147 could promote the EMT of LNCaP cells.
CD147-induced EMT is associated with
activation of the Wnt/β-catenin pathway
The Wnt/β-catenin pathway is the one of the key
pathways that induce EMT. Therefore, the expression levels of the
Wnt pathway components β-catenin and GSK-3β were analysed in LNCaP
cells. It was revealed that knockdown of CD147 significantly
inhibited the nuclear expression of β-catenin and Snail (P<0.05;
Fig. 5A). Knockdown of CD147 in
LNCaP cells also reduced the expression of p-GSK-3β (Ser9)
(P<0.05; Fig. 5B). Subsequently,
the present study sought to verify whether CD147 participated in
the EMT process through the Wnt/β-catenin signalling pathway. To
address this question, LNCaP/Scramble and LNCaP/shCD147 cells were
treated with lithium chloride (LiCl) for 3 h. LiCl, as an agonist
of the Wnt/β-catenin signalling pathway and a GSK-3β inhibitor, did
not affect the expression of CD147(data not shown). The
expression levels of β-catenin, p-β-catenin (Ser 33/37/Thr 41),
E-cadherin and β-catenin-targeted proteins, including Snail and
vimentin, were subsequently examined. The expression of p-β-catenin
(Ser33/37/Thr41) was upregulated in LNCaP/shCD147 cells, as
expected, which was consistent with the downregulation of
β-catenin, Snail and vimentin. Treatment with LiCl significantly
attenuated the upregulation of p-β-catenin (Ser33/37/Thr41)
expression that followed knockdown of CD147, leading to an increase
in β-catenin, Snail, and vimentin expression. As E-cadherin is
downstream of Snail, the change in E-cadherin expression was
consistent with the change in Snail expression (Fig. 5C and D).
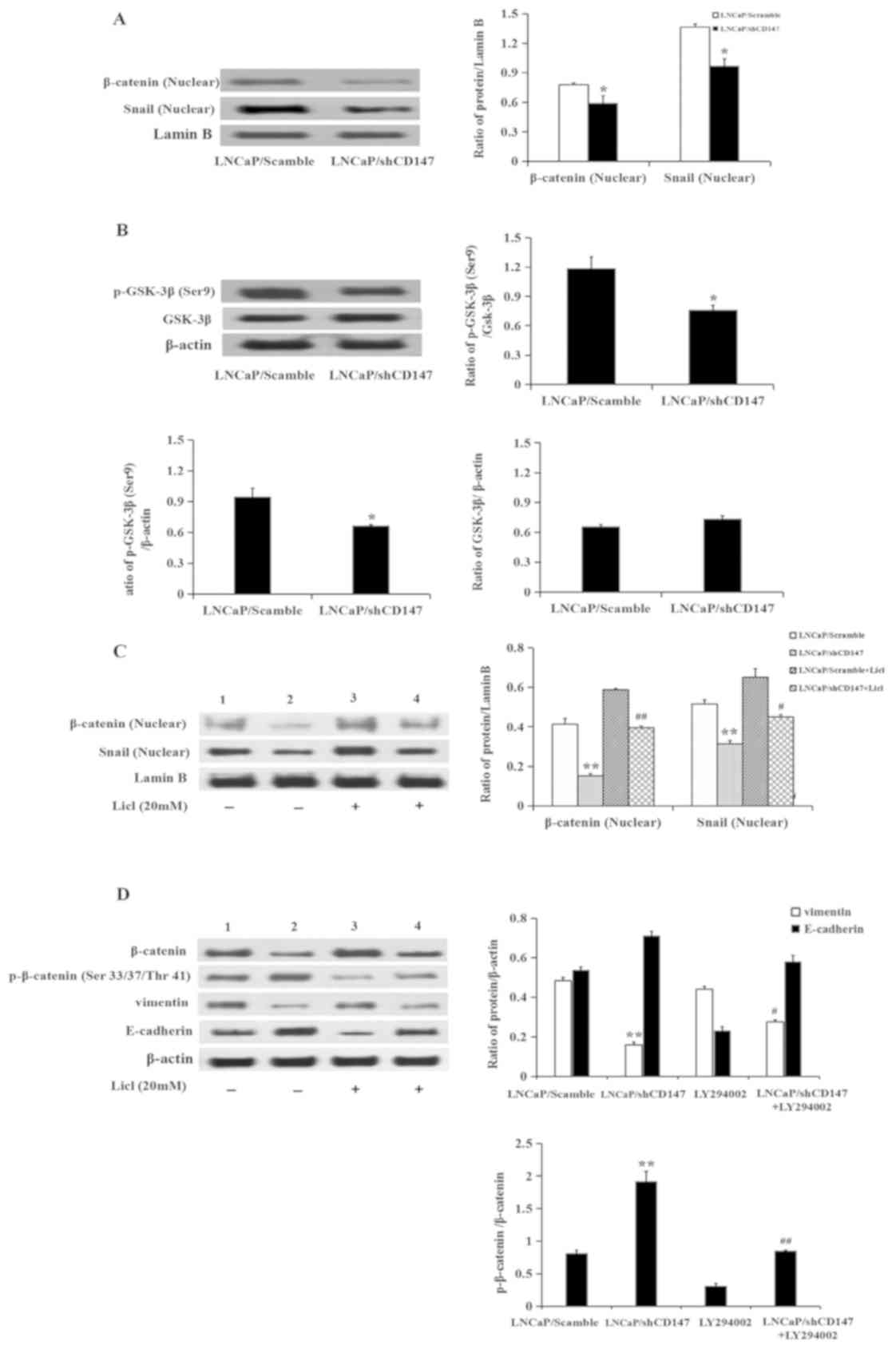 | Figure 5CD147 regulates epithelial-mesenchymal
transition via the Wnt/β-catenin pathway. (A and B) Expression of
β-catenin, Snail and p-GSK-3β (Ser9) was determined in
LNCaP/Scramble and LNCaP/shCD147 cells by western blotting. (C and
D) Expression of β-catenin, Snail, p-β-catenin (Ser33/37/Thr41),
vimentin and E-cadherin following treatment with or without LiCl in
LNCaP/Scramble and LNCaP/shCD147 cells by western blotting. Values
are presented as the mean ± standard deviation of three
experiments. Lanes 1 and 3, LNCaP/Scramble group; Lanes 2 and 4,
LNCaP/shCD147 group. *P<0.05, **P<0.01
vs. LNCaP/Scramble; #P<0.05, ##P<0.01
vs. LNCaP/shCD147. LNCaP, lymph node carcinoma of the prostate;
LiCl, lithium chloride; sh, short hairpin RNA; p-, phosphorylated;
GSK-3β, glycogen synthase kinase-3β. |
Taken together, these data suggested that knockdown
of CD147 may induce inhibition of the Wnt/β-catenin pathway,
leading to the activation of GSK-3β, degradation of β-catenin, and
subsequent downregulation of Snail and vimentin.
Discussion
Tumour metastasis is a crucial cause of treatment
failure and mortality in patients with cancer. Cancer morbidity and
mortality are largely related to the spread of the primary,
localized tumour to adjacent and distant sites, which result in the
arrival of malignant cells to their growth and proliferation in the
host organ (18). Some studies have
shown that tumour epithelial cells lose epithelial cell polarity
and gain mesenchymal morphology, promoting tumour metastasis
(6,19). CD147 serves as a pro-survival and
pro-migration factor in physiological and pathological processes,
and it has been reported to promote invasion and EMT in colorectal
cancer by regulating the MAPK/ERK signalling pathway (20). Moreover, CD147 was able to increase
hypoxia-induced metastasis and EMT in oesophageal cancer cells by
regulating hypoxia-inducible factor-1α (21), and promoted transforming growth
factor-β-induced EMT and invasion via Snail and Slug in
hepatocellular carcinoma (22). In
this study, it was found that downregulation of CD147 inhibited the
invasion and migration of PCa cells. The study then sought to
characterize the contribution of CD147 in the control of EMT-driven
metastasis in PCa cells. One of the most distinctive features of
EMT is the loss of E-cadherin expression. In this study, the
knockdown of CD147 upregulated the expression of the epithelial
marker E-cadherin, and downregulated the expression of mesenchymal
markers N-cadherin and vimentin in LNCaP cells, suggesting that
CD147 may promote EMT in LNCaP cells.
The Wnt/β-catenin signalling pathway is also
associated with the EMT process. The increased levels of β-catenin
lead to its nuclear translocation and activation of its target
genes, such as the EMT-related genes Snail and vimentin. Snail, a
target of β-catenin, is a critical transcription factor in the
regulation of EMT. In this process, Snail causes the
transcriptional repression of E-cadherin by the assembly of the
repressor complex at the E-cadherin promoter (23). In this study, one of the significant
findings was that of the role of CD147 in EMT was dependent on the
Wnt/β-catenin signalling pathway. The study revealed that knockdown
of CD147 led to β-catenin downregulation in the nucleus, and
decreased expression of Snail and vimentin. In the Wnt/β-catenin
pathway, GSK-3β is an upstream factor of β-catenin, which promotes
its phosphorylation at Ser33/37and Thr41. The phosphorylated form
of β-catenin is ubiquitinated through E3 ubiquitin ligase and
consequently targeted for ubiquitin-mediated degradation, which
maintained low levels of β-catenin in the cytoplasm (24). GSK-3β has four different
phosphorylation regions, and the phosphorylation of the regulatory
serine residue 9 in GSK-3β is associated with the inhibition of its
kinase activity (25). The present
results demonstrated that knockdown of CD147 expression inhibited
the phosphorylation of GSK-3β on Ser 9. To confirm that the
Wnt/GSK-3β/β-catenin pathway was involved in CD147-induced
promotion of EMT in LNCaP cells, LiCl, an activator of the Wnt
signalling pathway, was used. LiCl treatment attenuated CD147
knockdown-induced p-β-catenin (Ser33/37/Thr41) expression, which
resulted in the upregulation of β-catenin in the nucleus. The
expression of Snail and vimentin, as β-catenin targets, was
increased by LiCl treatment in LNCaP/shCD147 cells.
In summary, the present study described a novel role
of CD147 in the migration of PCa cells. These results demonstrated
that CD147 promoted the migration and invasion of PCa cells by
suppressing EMT. In addition, CD147 affected the expression of
proteins involved in EMT regulation via the Wnt/β-catenin
signalling pathway.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant no. 81202031), the Scientific
Research Project of Jilin Province Science and Technology
Department (grant no. 20160204033YY), the Technical Innovation
Project of Jilin Province Health Department (grant no. 2016J100),
the Scientific Research Project of Jilin City (grant no. 20163306)
and National Training Programs of Innovation and Entrepreneurship
for Undergraduates (grant no. 201813743011).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW conceived and designed the study. FF and QI
contributed to data acquisition and analysis and drafted the
manuscript. MW, CN and HX were involved in data acquisition. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ibrahim T, Flamini E, Mercatali L, Sacanna
E, Serra P and Amadori D: Pathogenesis of osteoblastic bone
metastases from prostate cancer. Cancer. 116:1406–1418.
2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bubendorf L, Schopfer A, Waqner U, Sauter
G, Moch H, Willi N, Gasser TC and Mihastsch MJ: Metastatic patterns
of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol.
31:578–583. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Seton-Roqers S: Epithelial-mesenchymal
transition: Untangling EMT's functions. Nat Rev Cancer.
16(1)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Coopman P and Djiane A: Adherens Junction
and E-Cadherin complex regulation by epithelial polarity. Cell Mol
Life Sci. 73:3535–3553. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Serrano-Gomez SJ, Maziveyi M and Alahari
SK: Regulation of epithelial-mesenchymal transition through
epigenetic and post-translational modifications. Mol Cancer.
15(18)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chien MH, Lin YW, Wen YC, Yang YC, Hsiao
M, Chang JL, Huang HC and Lee WJ: Targeting the SPOCK1-snail/slug
axis-mediated epithelial-to-mesenchymal transition by apigenin
contributes to repression of prostate cancer metastasis. J Exp Clin
Cancer Res. 38(246)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Seol MA, Kim JH, Oh K, Kim G, Seo MW, Shin
YK, Sim JH, Shin HM, Seo BY, Lee DS, et al: Interleukin-7
contributes to the invasiveness of prostate cancer cells by
promoting epithelial-mesenchymal transition. Sci Rep.
9(6917)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun Y, Wang BE, Leong KG, Yue P, Li L,
Jhumjhunwala S, Chen D, Seo K, Modrusan Z, Gao WQ, et al: Androgen
deprivation causes epithelial-mesenchymal transition in the
prostate: Implications for androgen-deprivation therapy. Cancer
Res. 72:527–536. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bi XC, Liu JM, Zheng XG, Xian ZY, Feng ZW,
Lou YX, Zhong WD and Wu CL: Over-expression of extracellular matrix
metalloproteinase inducer in prostate cancer is associated with
high risk of prostate-specific antigen relapse after radical
prostatectomy. Clin Invest Med. 34(358)2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang L, Wu G, Yu L, Yuan J, Fang F, Zhai
Z, Wang F and Wang H: Inhibition of CD147 expression reduces tumor
cell invasion inhuman prostate cancer cell line via RNA
interference. Cancer Biol Ther. 5:608–614. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Grass GD, Dai L, Qin Z, Parsons C and
Toole BP: CD147: Regulator of hyaluronan signaling in invasiveness
and chemoresistance. Adv Cancer Res. 123:51–73. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Szubert S, Szpurek D, Moszynski R, Nowicki
M, Frankowski A, Sajdak S and Michalak S: Extracellular matrix
metalloproteinase inducer (EMMPRIN) expression correlates
positively with active angiogenesis and negatively with basic
fibroblast growth factor expression in epithelial ovarian cancer. J
Cancer Res Clin Oncol. 140:361–369. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fang F, Qin Y, Hao F, Li Q, Zhang W, Zhao
C, Chen S, Zhao L, Wang L and Cai J: CD147 modulates androgen
receptor activity through the Akt/Gsk-3β/β-catenin/AR pathway in
prostate cancer cells. Oncol Lett. 12:1124–1128. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Arvelo F, Sojo F and Cotte C: Tumor
progression and metastasis. Ecancermedicalscience.
10(617)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu Q, Deng F, Qin Y, Zhao Z, Wu Z, Xing Z,
Ji A and Wang QJ: Long no-coding RNA regulation of
epithelial-mesenchymal transitiion in cancer metastasis. Cell Death
Dis. 7(e2254)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu T, Zhou M, Peng L, Kong S, Miao R, Shi
Y, Sheng H and Li L: Upregulation of CD147 promotes cell invasion,
epithelial-to-mesenchymal transition and activates MAPK/ERK
signaling pathway in colorectal cancer. Int J Exp Pathol.
15:7432–7441. 2014.PubMed/NCBI
|
|
21
|
Wu X, Qiao B, Liu Q and Zhang W:
Upregulation of extracellular matrix metalloproteinase inducer
promotes hypoxia-induced epithelial-mesenchymal transition in
esophageal cancer. Mol Med Rep. 12:7419–7424. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ru NY, Wu J, Chen ZN and Bian H:
HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal
transition and hepatocellular carcinoma invasion. Cell Biol Int.
39:44–51. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin Y, Dong C and Zhou BP: Epigenetic
regulation of EMT: The snail story. Curr Pharm Des. 20:1698–1705.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wu G, Xu G, Schulman BA, Jeffirey PD,
Harper JW and Pavletich NP: Structure of a
beta-TrCP1-Skp1-beta-catenin complex: Destruction motif binding and
lysine specificity of the SCF (beta-TrCP1) ubiquitin ligase. Mol
Cell. 11:1445–1456. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang QM, Fiol CJ, Depaoli-Roach AA and
Roach PJ: Glycogen synthase kinase-3 beta is a dual specificity
kinase differentially regulated by tyrosine and serine/threonine
phosphorylation. J Biol Chem. 269:14566–14574. 1994.PubMed/NCBI
|



















