Introduction
Cisplatin (CP) is a chemotherapeutic drug that has
been used clinically for decades in patients with malignant tumors;
however, nephrotoxicity, which is the major side effect of CP
treatment, has greatly limited its application as a treatment
(1,2). Thus, there is an urgent requirement to
develop a novel therapeutic agent that protects against CP-induced
renal injury and obtain novel insights into the treatment of
patients with cancer undergoing CP-based chemotherapy regimens
(3,4).
The molecular mechanisms underlying CP-induced
nephrotoxicity are complex (5). It
has been reported that inflammation and apoptosis are associated
with the development of nephrotoxicity (6,7);
proinflammatory factors stimulated by CP, including tumor necrosis
factor α (TNF-α), interleukin (IL)-1β, IL-6 and IL-8 have been
observed to serve essential roles in the pathogenesis of CP-induced
renal injury (8,9). Thus, the inhibition of the inflammatory
response and apoptosis could be a promising therapeutic strategy
for attenuating CP-induced nephrotoxicity. NF-κB, an important
regulator of cytokine induction, promotes the expression of
multiple proinflammatory genes (10). NF-κB has been demonstrated to have a
vital role in the progression of CP-induced renal injury (11). Previous studies have found that the
severity of CP-induced nephrotoxicity is related to the activation
of NF-κB, and conversely, the inflammatory response and severity of
nephrotoxicity can be attenuated through the inhibition of NF-κB
activity (12,13). In addition, numerous studies have
suggested a role for nuclear factor E2-related factor 2 (Nrf2) in
the regulation of physiological processes that serve to inhibit the
development and progression of CP-induced renal damage (14,15). It
has been reported that the absence of Nrf2 exacerbates CP-induced
nephrotoxicity, whilst the pharmacological activation of Nrf2 has
been observed to inhibit CP-mediated nephrotoxicity (8). Thus, the pharmacological activation of
Nrf2 is considered to be an important molecular target to prevent
CP-induced renal damage.
Rehmannia glutinosa is a traditional herbal
medicine that has been used to enhance the functions of the liver,
kidney and heart (16). In certain
cases, Rehmannia glutinosa has also been used to treat
diabetes, anemia and urinary tract infections (17). A previous study extracted the
effective component in Rehmannia glutinosa and identified it
as catalpol, whose molecular formula is
C15H22O10 (18). Studies have since revealed that
catalpol exhibits anti-inflammatory and anti-apoptotic effects
(19,20). In addition, catalpol was found to
have important functions in protecting against
lipopolysaccharide-induced acute lung injury through suppressing
the inflammatory response (21). In
other studies, catalpol inhibited apoptosis in hydrogen
peroxide-induced cardiac myocytes through the caspase pathway and
ameliorated hepatic insulin resistance in type 2 diabetes by acting
through the 5'-AMP-activated protein kinase/NADPH oxidase
4/PI3K/AKT signaling pathway (22,23).
However, to the best of our knowledge, no previous study has been
conducted to examine the inhibitory roles of catalpol in CP-induced
renal injury. In the present study, the potential protective
effects of catalpol in CP-induced kidney injury were investigated
in an in vivo rat model and the underlying molecular mechanisms
were subsequently investigated. The results suggested that catalpol
may protect against CP-induced apoptosis and inflammation in
tubular cells by inhibiting and activating the NF-κB and Nrf-2
signaling pathway, respectively.
Materials and methods
Reagents
Catalpol was purchased from Sigma-Aldrich; Merck
KGaA; the Urea Nitrogen Diacetylmonoxime Test kit and the
Creatinine LiquiColor Test (Kinetic) kit were obtained from Tiangen
Biotech Co., Ltd. TNF-α (cat. no. ZB-10764C-R9648), IL-1β (cat. no.
ZB-10119C-R9648), IL-6 (cat. no. ZB-10135C-R9648), IL-8 (cat. no.
ZB-11167C-R9648), IL-10 (cat. no. ZB-10108C-R9648) and iNOS (cat.
no. ZB-10740C-R9648) ELISA kits were purchased from ZellBio GmbH.
TRIzol® reagent was obtained from Invitrogen; Thermo
Fisher Scientific, Inc. Primary antibodies against cleaved
caspase-3 (cat. no. 9661; 1:1,000), Nrf2 (cat. no. 12721; 1:1,000),
heme oxygenase-1 (HO-1; cat. no. 86806; 1:1,000), inhibitory κB
(IκB; cat. no. 76041; 1:100), ECH-associated protein 1 (Keap1; cat.
no. 8047; 1:2,000) and NF-κB p65 (cat. no. 8242; 1:2,000) were
purchased from Cell Signaling Technology, Inc.
Animal studies
A total of 40 male Sprague-Dawley rats (age, 8
weeks; mean body weight, 392±15 g) were purchased from the Animal
Center of the Military Medical University (Chongqing, China) and
were housed at 2 rats/cage in a light-controlled environment at
24±1˚C, 40-80% humidity, with 12-h light/dark cycles and with
access to food and water ad libitum throughout the
experimental period. The experiments were approved by the Animal
Care and Use Ethics Committee of the Military Medical
University.
Experimental design
Rats were randomly divided into five groups
(n=8/group): i) CP group, which was subjected to a single injection
of 20 mg/kg CP intraperitoneally on day 3; ii) control group, which
was administered intraperitoneally with an equal volume of the
saline solution (20 ml/kg) instead of CP or catalpol; and iii) CP
and catalpol (CP + cat) groups treated with 25, 50 or 100 mg/kg
catalpol for 2 days and 20 mg/kg CP and catalpol on day 3. A 2-ml
blood sample was collected on day 4 from the retroorbital venous
sinus following anesthetization by the intraperitoneal injection of
300 mg/kg 10% chloral hydrate aqueous solution. The blood samples
were centrifuged at 1,509 x g for 15 min at 4˚C and stored at -80˚C
until further use. The rats were sacrificed by decapitation
following the drawing of blood. The dose of catalpol was chosen
based on a previous study (24).
Renal function analysis
To examine the renal injury, the expression levels
of blood urea nitrogen (BUN) and creatinine in the serum were
analyzed using a biochemical AutoAnalyzer (Cobas® 8000;
Roche Diagnostics GmbH), according to the manufacturer's
protocol.
Histological analysis using
hematoxylin & eosin (H&E) staining
For histopathological evaluation of the renal
injury, kidney tissues were obtained from the rats and were
subsequently fixed in 10% formaldehyde at room temperature for 24 h
and embedded in paraffin. The 5-µm sections were heated at 60˚C for
1 h, before being dewaxed in xylene and rehydrated using a
descending ethanol series. Hematoxylin and eosin (H&E) staining
was then performed on sections, with hematoxylin for 10 min room
temperature and eosin for 5 min at room temperature. Stained
sections were visualized using a light microscope (magnification,
x200) by a pathologist in a blinded manner. Renal histopathological
changes including cellular necrosis, loss of brush border,
interstitial edema and tubule dilatation were evaluated using the
following criteria: i) 0=none; ii) 1=≤25%; iii) 2=25-49%; iv)
3=50-74%; and v) 4=≥75% (6).
TUNEL assay
To evaluate the apoptotic ability of the cells in
the cortex following CP-induced renal injury, a TUNEL assay was
performed using the TUNEL apoptosis detection kit (cat. no. C1098;
Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. Apoptotic cells were observed and counted
in five randomly selected fields using a light optical microscope
(magnification, x200). Cells with a brown nucleus were positive
cells. The number of apoptotic cells in proportion to the total
number of cells was used to calculate the apoptotic index.
Evaluation of inflammatory
cytokines
To determine the expression levels of TNF-α, IL-1β,
IL-6, IL-8, IL-10 and iNOS in renal tissues, The renal tissues (100
mg) were homogenized with 1 ml PBS (pH 7.4, 100 mM) and
PathScan® Sandwich ELISA lysis buffer (cat. no. 7018;
Cell Signaling Technology, Inc.) was used to lyse the tissues
before centrifugation at 3,660 x g for 10 min at 4˚C. TNF-α (cat.
no. ZB-10764C-R9648), IL-1β (cat. no. ZB-10119C-R9648), IL-6 (cat.
no. ZB-10135C-R9648), IL-8 (cat. no. ZB-11167C-R9648), IL-10 (cat.
no. ZB-10108C-R9648) and iNOS (cat. no. ZB-10740C-R9648) ELISA kits
were used according to the manufacturer's protocol.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). Total RNA was reverse transcribed into cDNA using the Revert
Aid First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
temperature protocol for reverse transcription was as follows: 42˚C
for 45 min, 99˚C for 5 min and 5˚C for 5 min. qPCR was performed
using the SYBR® Green PCR Master Mix (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
The sequences of primers (Shanghai Institute of Biological
Sciences) are listed in Table I. The
following thermocycling conditions were used for qPCR: Initial
denaturation at 95˚C for 5 min, followed by 45 cycles at 95˚C for
15 sec, 60˚C for 20 sec and 72˚C for 10 sec. Relative mRNA
expression levels were calculated using the 2-∆∆Cq method (25) and normalized to the internal
reference gene GAPDH.
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| | Primer sequence
(5'-3') |
|---|
| Target gene | Forward | Reverse |
|---|
| NF-κB |
ACCTGCAGTTCGATGCTGAT |
CCTGTCACCAGGCGAGTTAT |
| Keap1 |
TGCAAATGGATTCTGCTTCACCTACTTTGCAGGAA |
TGAGCCCAGAACCTCCTTTTTCTCCAGTTTC |
| Nrf2 |
GCAACTCCAGAAGGAACAGG |
GGAATGTCTCTGCCAAAAGC |
| HO-1 |
CTTTCAGAAGGGTCAGGTGTC |
TGCTTGTTTCGCTCTATCTCC |
| IκB |
TGGCCAGTGTAGCAGTCTTG |
GACATCAGCACCCAAAGTCA |
| β-actin |
CCACTGCCGCATCCTCTT |
GCATCGGAACCGCTCATT |
Western blotting
Renal tissue samples were ground in liquid nitrogen
and lysed using RIPA lysis buffer (Beyotime Institute of
Biotechnology) for 30 min. Total protein was quantified using a
bicinchoninic acid assay and 50 µg protein was separated using 12%
SDS-PAGE for 90 min. The separated proteins were subsequently
transferred onto polyvinylidene difluoride membranes and blocked in
TBS with 5% skimmed milk for 2 h at room temperature. The membranes
were incubated with primary antibodies (all from Cell Signaling
Technology, Inc.) against GAPDH (cat. no. 5174; 1:1,000), cleaved
caspase-3 (cat. no. 9661; 1:1,000), Keap1 (cat. no. 8047; 1:2,000),
Nrf2 (cat. no. 12721; 1:1,000), HO-1 (cat. no. 86806; 1:1,000),
NF-κB p65 (cat. no. 8242; 1:2,000) and IκB (cat. no. 76041; 1:100)
overnight at 4˚C. Following the primary antibody incubation, the
membranes were subsequently incubated with a horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2370;
1:5,000; Santa Cruz Biotechnology Inc.) for 1 h at 37˚C. The
protein-antibody complexes were visualized using Pierce™ Fast
Western Blot Kit, ECL Substrate (cat. no. 35050; Thermo Fisher
Scientific, Inc.) with a chemiluminescence instrument (Tanon
Science and Technology Co., Ltd.). Protein expression was
quantified using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc.). Experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc.) and data are presented as the mean ± SD.
Statistical differences between groups were determined using
one-way ANOVA with a Tukey's post hoc test for multiple
comparisons. Histological scores were compared using Kruskal-Wallis
statistical test followed by Dunn's post hoc analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Catalpol prevents CP-induced renal
injury
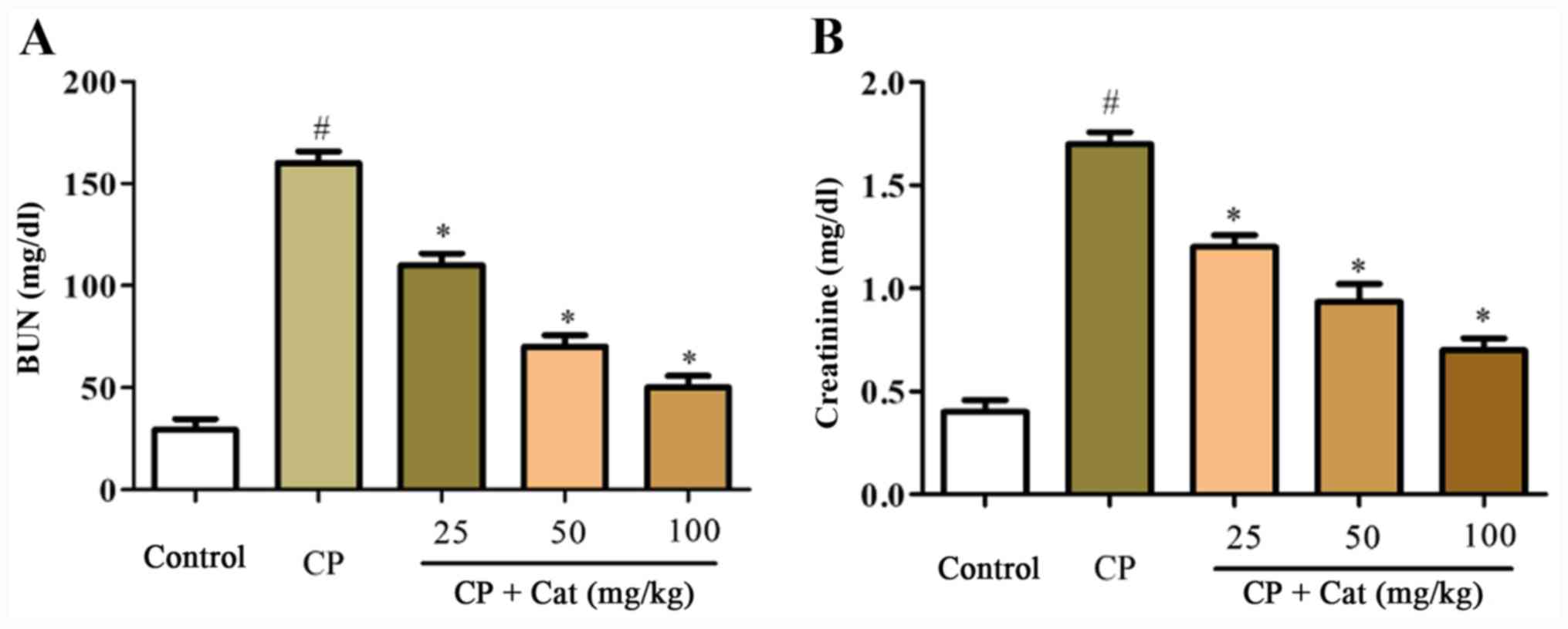
To analyze the protective roles of catalpol on
CP-induced renal injury, the expression levels of BUN and
creatinine in the serum were determined. The expression levels of
serum BUN and creatinine in the CP group were significantly
increased compared with the control group; however, serum BUN and
creatinine expression levels were significantly reduced following
the treatment with catalpol in a dose-dependent manner compared
with CP treatment alone (Fig. 1).
These results suggested that CP may stimulate renal injury, which
may be prevented by catalpol treatment.
Effects of catalpol on CP-induced
renal histopathological changes
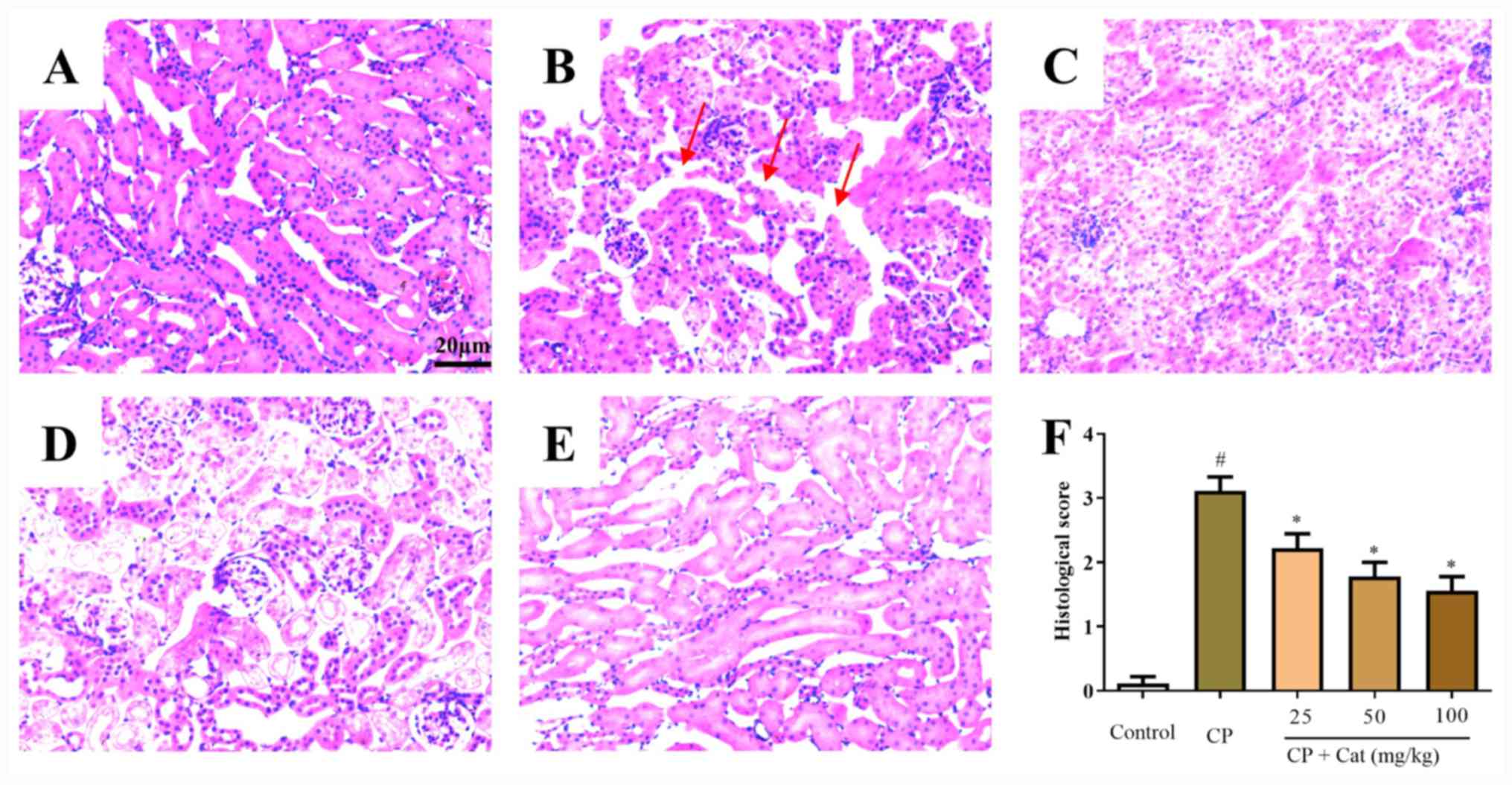
To investigate the functions of catalpol on
CP-induced renal injury, the histological appearance of kidney
tissues was analyzed. Normal morphology was observed in the control
group (Fig. 2A); however, tubular
epithelial damage, intratubular cast formation and tubular
dilatation were detected in CP-treated tissues (Fig. 2B). Catalpol was observed to prevent
the CP-induced histological disturbances in renal tissues in a
dose-dependent manner (Fig. 2C-F).
These results indicated that CP treatment may lead to the aberrant
morphological appearance of kidney tissues, and these impairments
may be rescued by catalpol treatment.
Catalpol inhibits the apoptosis of
tubular cells in the kidney tissues with CP-induced renal injury in
vivo
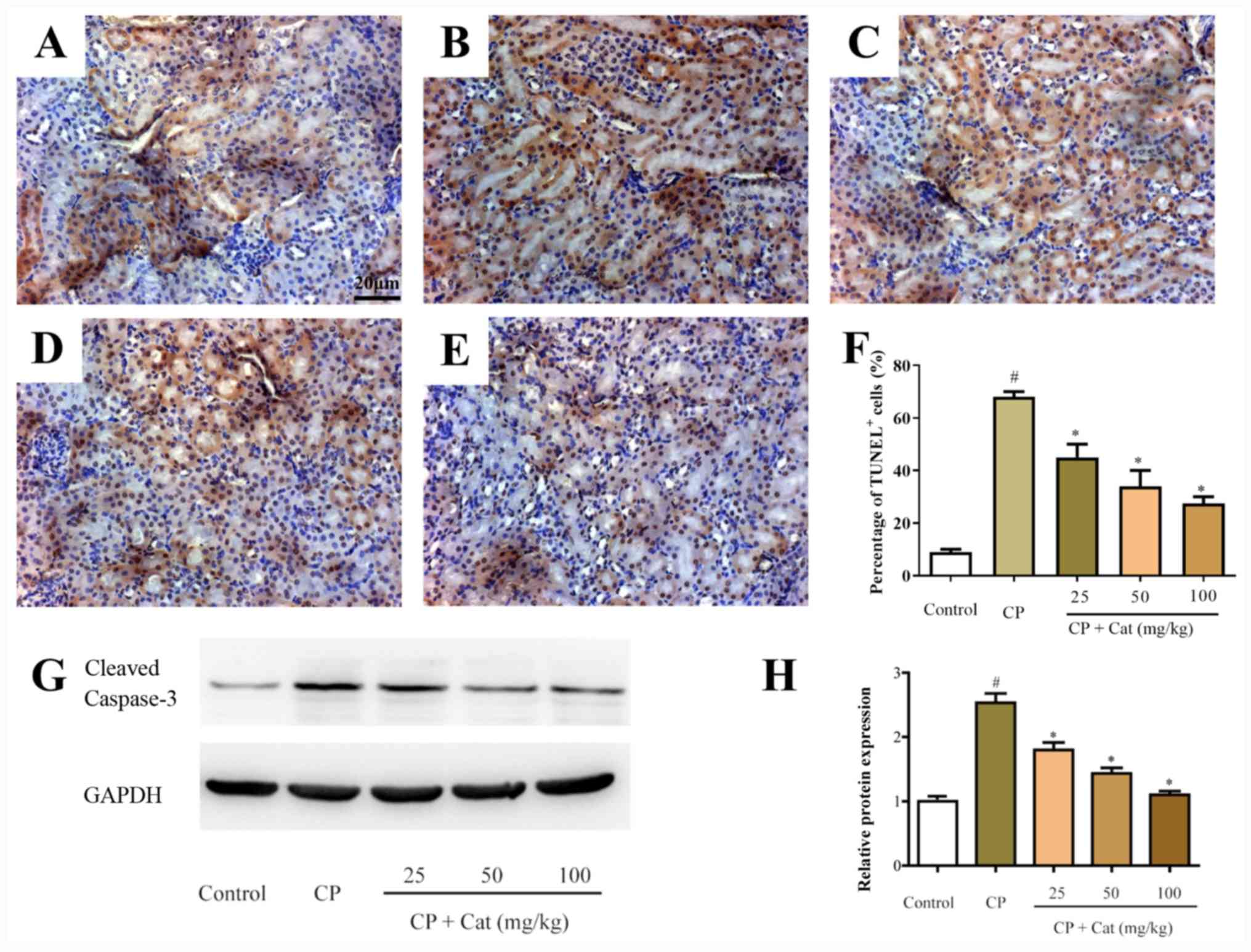
The apoptosis of tubular cells serves essential
pathogenic roles in CP-induced renal injury (6). In the present study, the apoptotic
ability of the cells was determined using TUNEL staining and
analyzing caspase-3 activity. Low numbers of apoptotic cells were
detected in the kidney tissues of the control group (Fig. 3A-F); however, following the treatment
with CP, the number of apoptotic cells in the kidney tissues was
significantly increased compared with the control group (Fig. 3B-F). Furthermore, catalpol treatment
significantly inhibited the number of apoptotic cells in the kidney
tissues compared with that in the CP group (Fig. 3C-F). In addition, cleaved caspase-3
expression levels were increased in the CP group compared with the
control group, whereas catalpol treatment significantly decreased
these levels (Fig. 3G and H). The
results demonstrated that the apoptotic rate of cells isolated from
the CP group was significantly increased compared with the control
group; however, this effect was significantly reversed following
the treatment with catalpol, suggesting that the increased cell
apoptotic ability and subsequent CP-induced renal injury may be
inhibited by catalpol treatment.
Catalpol inhibits the secretion of
cisplatin-induced inflammatory cytokines
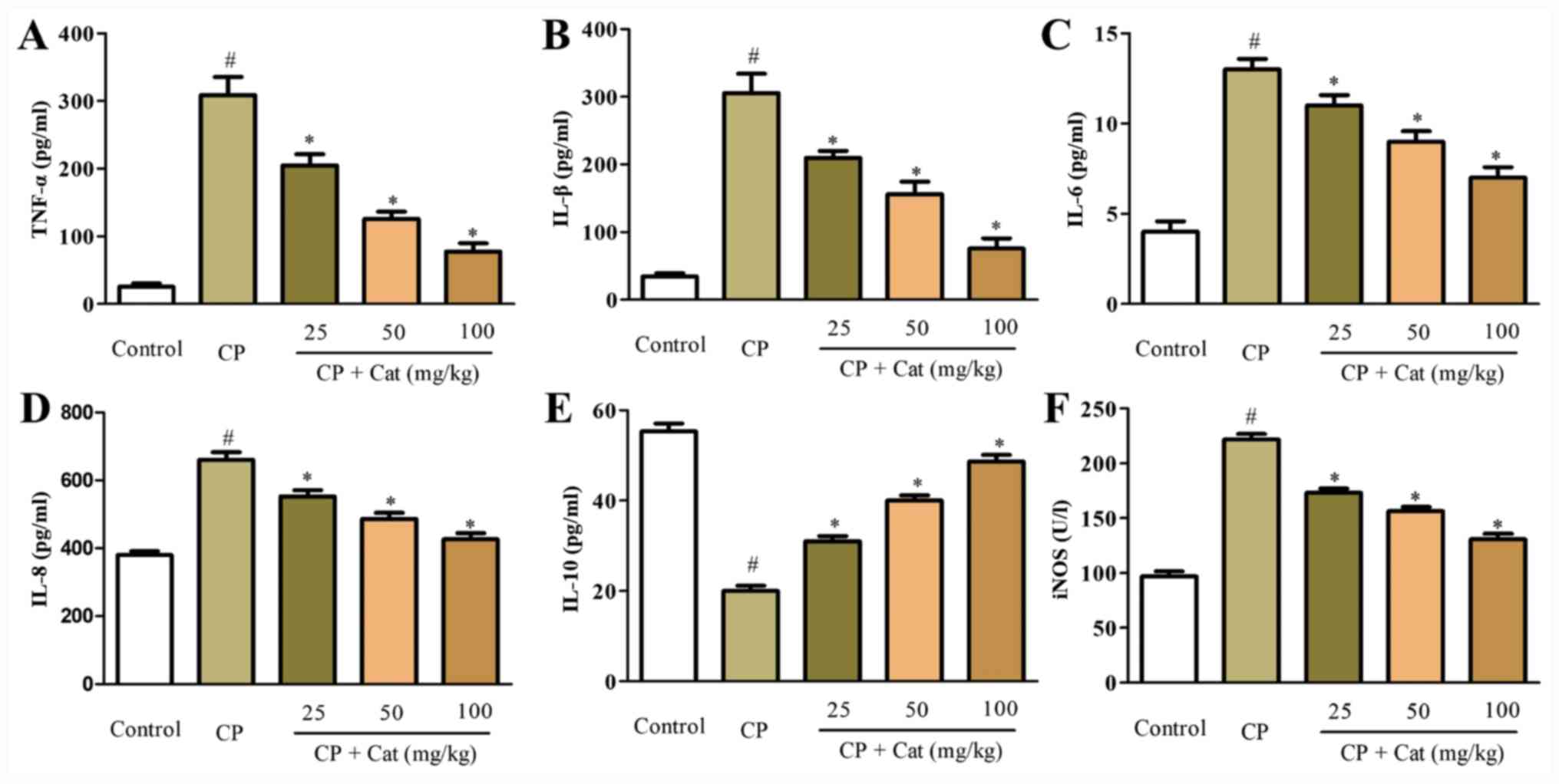
Previous studies have demonstrated that the
production of inflammatory cytokines, including TNF-α, IL-1β, IL-6,
IL-8, IL-10 and iNOS, served important roles in CP-induced kidney
injury (8,9). To investigate the anti-inflammatory
functions of catalpol, the effects of catalpol on the expression
levels of CP-induced inflammatory cytokines were determined. ELISAs
revealed that the production of pro-inflammatory cytokines, TNF-α,
IL-1β, IL-6, IL-8 and iNOS in kidney tissues were significantly
increased in the CP group compared with the control group, whereas
the secretion of the anti-inflammatory cytokine IL-10 was
significantly decreased compared with the control group (Fig. 4). The treatment with catalpol
significantly inhibited the production of CP-induced TNF-α, IL-1β,
IL-6, IL-8 and iNOS, whilst significantly increasing the expression
of IL-10 in a dose-dependent manner compared with the CP group
(Fig. 4). These results indicated
that the CP-induced inflammatory response may be suppressed by the
treatment with catalpol, which may represent a novel therapeutic
candidate for the treatment of CP-induced inflammation and renal
injury.
Effects of catalpol on the expression
levels of Nrf2, HO-1, IκB, Keap1 and NF-κB
The mRNA and protein expression levels of Nrf2, HO-1
and Keap1 were analyzed using RT-qPCR and western blotting,
respectively. The mRNA and protein expression levels of Nrf2 and
HO-1 were significantly reduced, whilst the expression levels of
Keap1 were significantly increased following the treatment with CP
compared with the control group (Fig.
5). Meanwhile, catalpol treatment significantly increased the
expression levels of Nrf2 and HO-1, whilst significantly decreasing
the expression levels of Keap1 compared with the CP group (Fig. 5). The activation of NF-κB serves an
important role in the induction of proinflammatory mediators and
nuclear translocation of the NF-κB transcription factor is preceded
by the degradation IκB-α (8). To
determine whether catalpol affected NF-κB activity, NF-κB p65 and
IκB-α expression levels were analyzed using RT-qPCR and western
blotting. Catalpol significantly increased IκB-α expression levels
and inhibited nuclear NF-κB p65 expression levels following
CP-induced renal injury (Fig.
5).
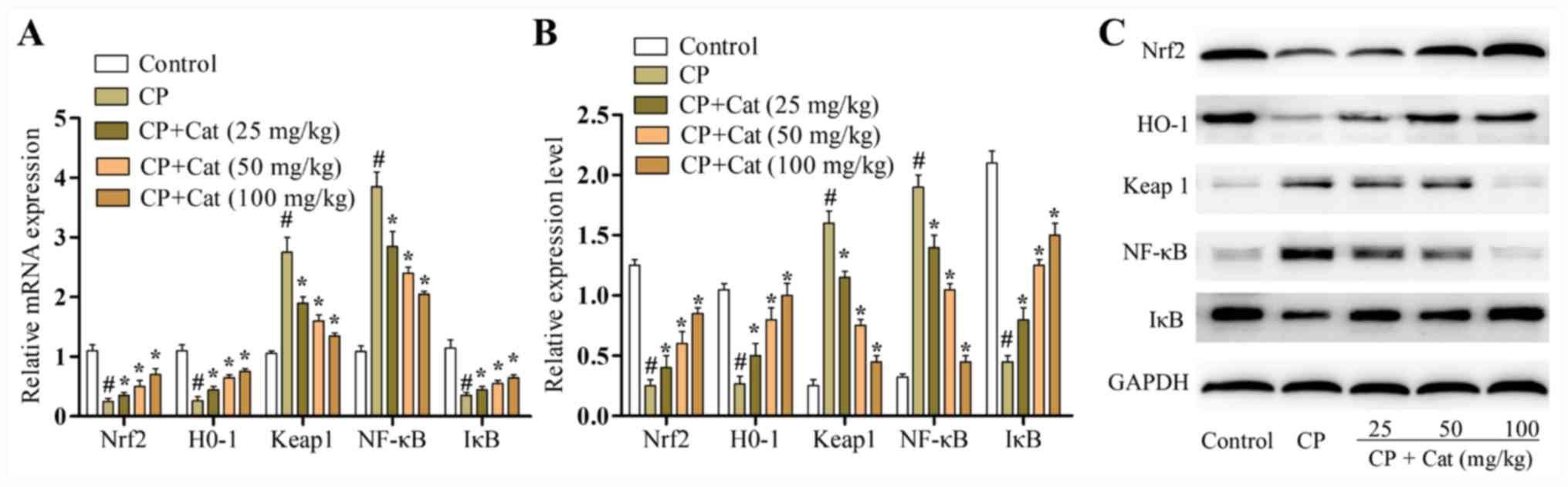 | Figure 5Effects of catalpol on the mRNA and
protein expression levels of Nrf2, HO-1, IκB, Keap1 and NF-κB in
rats treated with CP. (A) mRNA expression levels of Nrf2, HO-1,
IκB, Keap1 and NF-κB in the different treatment groups were
analyzed using reverse transcription-quantitative PCR. (B)
Densitometry analysis of western blotting results. (C) Protein
expression levels of Nrf2, HO-1, IκB, Keap1 and NF-κB in the
different treatment groups were analyzed using western blotting.
Results are presented as the mean ± SD (n=8). #P<0.01
vs. control group; *P<0.05 vs. CP group. CP,
cisplatin; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1,
heme oxygenase 1; Keap1, Kelch-like ECH-associated protein 1; IκB,
inhibitory κB; Cat, catalpol. |
Discussion
CP is one of the most effective chemotherapy drugs;
however, its therapeutic application is restricted by
nephrotoxicity, the major CP-induced side effect that consequently
leads to renal injury (26,27). It has been reported that
CP-stimulated injury in the kidney is closely associated with
inflammation and apoptosis (28).
For example, Mitazaki et al (29) demonstrated that IL-6 was involved in
the regulation of oxidative stress during the development of
CP-induced nephrotoxicity; Lee et al (30) reported that mice with an IL-1α
deficiency were more resistant to CP-induced acute renal failure
compared with the control group; and Kim et al (31) reported that the expression levels of
IL-10, an anti-inflammatory cytokine, were significantly decreased
following treatment with CP. Additionally, in another study, IL-10
was found to protect the kidney against renal ischemia and
CP-induced injury (32). In the
present study, the serum levels of BUN and creatinine in the CP
group were significantly increased, whereas the treatment with
catalpol reduced their expression levels in a dose-dependent
manner. These results suggested that CP may promote renal injury,
which may be subsequently prevented by catalpol treatment; this
conclusion could be further validated by analyzing the expression
levels of urinary microalbumin and β2-microglobulin in future
studies. Moreover, catalpol inhibited the production of
pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-8 and
iNOS and subsequently reduced the damage of CP-induced renal
dysfunction. Furthermore, the treatment with catalpol resulted in
the increased secretion of the anti-inflammatory cytokine, IL-10,
which may protect the normal function of kidney. Thus, catalpol may
be a promising therapeutic candidate for the treatment of
CP-induced kidney injury.
However, to the best of our knowledge, the
underlying molecular mechanisms of catalpol-mediated protection on
kidney function were unknown, so they were further investigated in
the present study. NF-κB is a pleiotropic transcription factor with
important functions in the intestinal immune system. NF-κB family
members control the transcriptional activities of various promoters
of proinflammatory cytokines, cell surface receptors, transcription
factors and adhesion molecules involved in intestinal inflammation
(10). NF-κB is located in the
cytoplasm of most cells as an inactive complex with unprocessed
precursor proteins or IκB. The degradation of these precursor
proteins enables NF-κB dimers to translocate to the nucleus and
induce the expression of specific target genes (13). Notably, the transcription factor has
an essential role in the transcriptional regulation of cytokines,
such as IL-1β, IL-6 and TNF-α, and the activation of the NF-κB
pathway promotes the expression of inflammatory parameters
associated with severe renal injury (6). The results of the present study
revealed that the activation of NF-κB was significantly inhibited
by catalpol, indicating that catalpol may attenuate the CP-induced
inflammation of renal injury through suppressing the NF-κB
signaling pathway.
In addition, the apoptosis of tubular cells is
considered as one of the pathogenic mechanisms that contributes to
diseases associated with renal injury (33). Nrf2 exerts cytoprotective and
antiapoptotic effects, and is found present as an inactive complex
in the cytoplasm with Keap1. In the nucleus, Nrf2 activates the
expression of the HO-1 gene, which subsequently alleviates
oxidative stress-induced cellular damage (8,14). In
addition, HO-1 is a phase II detoxifying enzyme and an
anti-inflammatory reactive protein (34). In the present study, catalpol
treatment significantly reduced the apoptotic ability of tubular
cells in vivo. Furthermore, as an important protein involved in the
regulation of antioxidant proteins and the inhibition of cell
apoptosis, the expression levels of Nrf2 and associated genes,
including Keap1 and HO-1, were evaluated following the treatment
with CP and catalpol. The results revealed that CP suppressed the
levels of genes involved in the Nrf2 axis, whereas catalpol
promoted the expression of the aforementioned genes, which
suggested that catalpol may inhibit the apoptosis of tubular cells
and subsequently protect the kidney against CP-induced renal
injury.
In conclusion, the results of the present study
demonstrated that catalpol was able to attenuate CP-induced
inflammation and apoptosis during renal injury through the
activation and inhibition of the Nrf2 and NF-κB signaling pathway,
respectively. These findings provided novel insights into the
potential protective roles of catalpol against kidney injury,
suggesting that catalpol may be a potential therapeutic candidate
in the treatment of CP-induced renal damage. However, as a widely
used chemotherapy drug, the anti-tumor functions of CP are mainly
attributed to its regulatory roles on cell apoptosis, thus, the
therapeutic outcome of combined CP and catalpol treatment for
patients with solid tumors remains unknown and requires further
investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Science Foundation of China (grant no. 81130011) and National
Institutes of Health (grant nos. DK064005 and DK091239).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JHZ and JZ conceived and designed the present study.
JZ performed the experiments; LL analyzed the data. FL and ZW
contributed to interpretation of data. JZ wrote the manuscript and
JHZ edited the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Animal Care and
Use Ethics Committee of the Military Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang Y, Jiang J, Jia H, Qiao Z and Zhang
J: Recovery of miR-139-5p in ovarian cancer reverses cisplatin
resistance by targeting c-Jun. Cell Physiol Biochem. 51:129–141.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Du A, Jiang Y and Fan C: NDRG1
downregulates ATF3 and inhibits cisplatin-induced cytotoxicity in
lung cancer A549 cells. Int J Med Sci. 15:1502–1507.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu B, Zeng M, Zeng J, Feng J and Yu L:
Meta-analysis of clinical trials comparing the efficacy and safety
of liposomal cisplatin versus conventional nonliposomal cisplatin
in nonsmall cell lung cancer (NSCLC) and squamous cell carcinoma of
the head and neck (SCCHN). Medicine (Baltimore).
97(e13169)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Anari F, O'Neill J, Choi W, Chen DYT,
Haseebuddin M, Kutikov A, Dulaimi E, Alpaugh RK, Devarajan K,
Greenberg RE, et al: Neoadjuvant dose-dense gemcitabine and
cisplatin in muscle-invasive bladder cancer: results of a phase 2
trial. Eur Urol Oncol. 1:54–60. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Santiago MJ, Fernández SN, Lázaro A,
González R, Urbano J, López J, Solana MJ, Toledo B, Del Castillo J,
Tejedor A, et al: Correction: Cisplatin-induced non-oliguric acute
kidney injury in a pediatric experimental animal model in piglets.
PLoS One. 13(e0207547)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang W, Hou J, Yan X, Leng J, Li R, Zhang
J, Xing J, Chen C, Wang Z and Li W: Platycodon grandiflorum
saponins ameliorate cisplatin-induced acute nephrotoxicity through
the NF-κB-mediated inflammation and PI3K/Akt/apoptosis signaling
pathways. Nutrients. 10(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ye J, Ren Y, Wei Z, Peng J, Chen C, Song
W, Tan M, He Y and Yuan Y: Nephrotoxicity and long-term survival
investigations for patients with peritoneal carcinomatosis using
hyperthermic intraperitoneal chemotherapy with cisplatin: A
retrospective cohort study. Surg Oncol. 27:456–461. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li F, Yao Y, Huang H, Hao H and Ying M:
Xanthohumol attenuates cisplatin-induced nephrotoxicity through
inhibiting NF-κB and activating Nrf2 signaling pathways. Int
Immunopharmacol. 61:277–282. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Alibakhshi T, Khodayar MJ, Khorsandi L,
Rashno M and Zeidooni L: Protective effects of zingerone on
oxidative stress and inflammation in cisplatin-induced rat
nephrotoxicity. Biomed Pharmacother. 105:225–232. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yenuganti VR, Ravinder R and Singh D:
Conjugated linoleic acids attenuate LPS-induced pro-inflammatory
gene expression by inhibiting the NF-κB translocation through PPARγ
in buffalo granulosa cells. Am J Reprod Immunol. 72:296–304.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Potočnjak I, Broznić D, Kindl M, Kropek M,
Vladimir-Knežević S and Domitrović R: Stevia and stevioside protect
against cisplatin nephrotoxicity through inhibition of ERK1/2,
STAT3, and NF-κB activation. Food Chem Toxicol. 107 (Pt A):215–225.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim IH, Kwon MJ, Jung JH and Nam TJ:
Protein extracted from Porphyra yezoensis prevents
cisplatin-induced nephrotoxicity by downregulating the MAPK and
NF-κB pathways. Int J Mol Med. 41:511–520. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yu X, Meng X, Xu M, Zhang X, Zhang Y, Ding
G, Huang S, Zhang A and Jia Z: Celastrol ameliorates cisplatin
nephrotoxicity by inhibiting NF-κB and improving mitochondrial
function. EBioMedicine. 36:266–280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Polat EC, Besiroglu H, Ozcan L, Otunctemur
A, Eruyar AT, Somay A, Ozbay N, Cekmen M, Eraldemir C and Ozbek E:
Beneficial effects of Oltipraz, nuclear factor - erythroid - 2 -
related factor 2 (Nrf2), on renal damage in unilateral ureteral
obstruction rat model. Int Braz J Urol. 44:1243–1251.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zúñiga-Toalá A, Zatarain-Barrón ZL,
Hernández-Pando R, Negrette-Guzmán M, Huerta-Yepez S, Torres I,
Pinzón E, Tapia E and Pedraza-Chaverri J: Nordihydroguaiaretic acid
induces Nrf2 nuclear translocation in vivo and attenuates renal
damage and apoptosis in the ischemia and reperfusion model.
Phytomedicine. 20:775–779. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kwak M, Yu K, Lee PC and Jin JO:
Rehmannia glutinosa polysaccharide functions as a mucosal
adjuvant to induce dendritic cell activation in mediastinal lymph
node. Int J Biol Macromol. 120 (Pt B):1618–1623. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dai X, Su S, Cai H, Wei D, Yan H, Zheng T,
Zhu Z, Shang EX, Guo S, Qian D, et al: Protective effects of total
glycoside from Rehmannia glutinosa leaves on diabetic
nephropathy rats via regulating the metabolic profiling and
modulating the TGF-β1 and Wnt/β-catenin signaling pathway. Front
Pharmacol. 9(1012)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou Y, Yang K, Zhang D, Duan H, Liu Y and
Guo M: Metabolite accumulation and metabolic network in developing
roots of Rehmannia glutinosa reveals its root developmental
mechanism and quality. Sci Rep. 8(14127)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang H, Jia R, Wang F, Qiu G, Qiao P, Xu
X and Wu D: Catalpol protects mice against
Lipopolysaccharide/D-galactosamine-induced acute liver injury
through inhibiting inflammatory and oxidative response. Oncotarget.
9:3887–3894. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Z, Zhu P, Zhang L, Xiong B, Tao J,
Guan W, Li C, Chen C, Gu J, Duanmu J, et al: Autophagy inhibition
attenuates the induction of anti-inflammatory effect of catalpol in
liver fibrosis. Biomed Pharmacother. 103:1262–1271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Han Y, Shen M, Tang LY, Tan G, Yang QC, Ye
L, Ye LH, Jiang N, Gao GP and Shao Y: Antiangiogenic effects of
catalpol on rat corneal neovascularization. Mol Med Rep.
17:2187–2194. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hu LA, Sun YK, Zhang HS, Zhang JG and Hu
J: Catalpol inhibits apoptosis in hydrogen peroxide-induced cardiac
myocytes through a mitochondrial-dependent caspase pathway. Biosci
Rep. 36(36)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan J, Wang C, Jin Y, Meng Q, Liu Q, Liu
Z, Liu K and Sun H: Catalpol ameliorates hepatic insulin resistance
in type 2 diabetes through acting on AMPK/NOX4/PI3K/AKT pathway.
Pharmacol Res. 130:466–480. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xiao WQ, Yin GJ, Fan YT, Qiu L, Cang XF,
Yu G, Hu YL, Xing M, Wu DQ, Wang XP, et al: Catalpol ameliorates
sodium taurocholate-induced acute pancreatitis in rats via
inhibiting activation of nuclear factor kappa B. Int J Mol Sci.
15:11957–11972. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mohri J, Katada C, Ueda M, Sugawara M,
Yamashita K, Moriya H, Komori S, Hayakawa K, Koizumi W and Atsuda
K: Predisposing factors for chemotherapy-induced nephrotoxicity in
patients with advanced esophageal cancer who received combination
chemotherapy with docetaxel, cisplatin, and 5-fluorouracil. J
Transl Int Med. 6:32–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee D, Yu JS, Lee SR, Hwang GS, Kang KS,
Park JG, Kim HY, Kim KH and Yamabe N: Beneficial Effects of
Bioactive Compounds in Mulberry Fruits against Cisplatin-Induced
Nephrotoxicity. Int J Mol Sci. 19(19)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang SW, Xu Y, Weng YY, Fan XY, Bai YF,
Zheng XY, Lou LJ and Zhang F: Astilbin ameliorates
cisplatin-induced nephrotoxicity through reducing oxidative stress
and inflammation. Food Chem Toxicol. 114:227–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mitazaki S, Hashimoto M, Matsuhashi Y,
Honma S, Suto M, Kato N, Nakagawasai O, Tan-No K, Hiraiwa K,
Yoshida M, et al: Interleukin-6 modulates oxidative stress produced
during the development of cisplatin nephrotoxicity. Life Sci.
92:694–700. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee JW, Nam WJ, Han MJ, Shin JH, Kim JG,
Kim SH, Kim HR and Oh DJ: Role of IL-1α in cisplatin-induced acute
renal failure in mice. Korean J Intern Med. 26:187–194.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim WS, Kim H, Kwon KW, Im SH, Lee BR, Ha
SJ and Shin SJ: Cisplatin induces tolerogenic dendritic cells in
response to TLR agonists via the abundant production of IL-10,
thereby promoting Th2- and Tr1-biased T-cell immunity. Oncotarget.
7:33765–33782. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tadagavadi RK and Reeves WB: Endogenous
IL-10 attenuates cisplatin nephrotoxicity: Role of dendritic cells.
J Immunol. 185:4904–4911. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee D, Lee DS, Jung K, Hwang GS, Lee HL,
Yamabe N, Lee HJ, Eom DW, Kim KH and Kang KS: Protective effect of
ginsenoside Rb1 against tacrolimus-induced apoptosis in renal
proximal tubular LLC-PK1 cells. J Ginseng Res. 42:75–80.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mahran YF: New insights into the
protection of growth hormone in cisplatin-induced nephrotoxicity:
The impact of IGF-1 on the Keap1-Nrf2/HO-1 signaling. Life Sci.
253(117581)2020.PubMed/NCBI View Article : Google Scholar
|